Abstract
We tested the hypothesis that spontaneous release of vasoactive intestinal peptide (VIP) from enteric neurons maintains homeostasis in smooth muscle function in mild inflammatory insults and that infusion of exogenous VIP has therapeutic effects on colonic smooth muscle dysfunction in inflammation. In vitro experiments were performed on human colonic circular smooth muscle tissues and in vivo on rats. The incubation of human colonic circular smooth muscle strips with TNF-α suppressed their contractile response to ACh and the expression of the pore-forming α1C subunit of Cav1.2 channels. VIP reversed both effects by blocking the translocation of NF-κB to the nucleus and its binding to the κB recognition sites on hα1C1b promoter. The translocation of NF-κB was inhibited by blocking the degradation of IκBβ. Induction of inflammation by a subthreshold dose of 17 mg/kg trinitrobenzene sulfonic acid (TNBS) in rats moderately decreased muscularis externa concentration of VIP, and it had little effect on the contractile response of circular smooth muscle strips to ACh. The blockade of VIP and pituitary adenylate cyclase-activating peptide receptors 1/2 during mild inflammatory insult significantly worsened the suppression of contractility and the inflammatory response. The induction of more severe inflammation by 68 mg/kg TNBS induced marked suppression of colonic circular muscle contractility and decrease in serum VIP. Exogenous infusion of VIP by an osmotic pump reversed these effects. We conclude that the spontaneous release of VIP from the enteric motor neurons maintains homeostasis in smooth muscle function in mild inflammation by blocking the activation of NF-κB. The infusion of exogenous VIP mitigates colonic inflammatory response and smooth muscle dysfunction.
Keywords: inflammation, vasoactive intestinal peptide receptors, NF-κB, inflammatory bowel disease
the neuronal and immune cells belong to the category of secretory cell types. They secrete reactive peptides and inflammatory mediators in their microenvironment. In addition, each cell type usually expresses the receptors of reactive molecules secreted by the other cell type. In organs, such as the gut, the microenvironments of these two cell types overlap (18). Therefore, the molecules secreted by one cell type influence the other cell type, resulting in neuroimmune interactions. Several neuropeptides modulate the activation of immune cells, whereas the inflammatory mediators suppress expression/release of neuropeptides or degenerate neuronal cells and their axons (24, 26, 37, 38). The exact biological effects of this bidirectional interaction are incompletely understood.
In the gut wall, the smooth muscle cells are in the microenvironments of both the enteric neurons and the immune cells. Therefore, during inflammation, the smooth muscle cells are exposed to both the neuropeptides and inflammatory mediators concurrently. We asked the question whether the mediators of the two cell types acting concurrently on a third cell type interact with each other in the target cell. If so, what is the biological role of this interaction?
To focus the study, we chose vasoactive intestinal peptide (VIP) as representative of the enteric neuropeptides secreted by enteric motor neurons and TNF-α as representative of the inflammatory mediators. Recent studies show that the enteric inhibitory motor neurons spontaneously release VIP (31, 35). The immune system in the gut wall is in an active state of alert because of the pathogenic intraluminal environment. We tested the hypothesis that the spontaneous release of VIP from enteric neurons maintains homeostasis in smooth muscle function when challenged by mild inflammatory insults. We also investigated whether infusion of exogenous VIP has parallel effects in mitigating colonic inflammatory response and smooth muscle dysfunction in colonic inflammation. The experiments were performed on circular smooth muscle strips obtained from human and rat colons as well as on primary cultures of human and rat colonic circular smooth muscle cells. We established the translational relevance of our cellular findings by using the trinitrobenzene sulfonic acid (TNBS) model of experimental inflammation in adult Sprague-Dawley rats.
MATERIALS AND METHODS
Primary cultures of human colonic circular smooth muscle cells.
Human tissues were obtained from the descending and sigmoid colons of patients undergoing surgery for colon cancer with approval of the University of Texas Medical Branch Institutional Review Board, as described previously (31, 33). Full-thickness tissues were dissected from disease-free margins of resected colon segments. The microdissected circular muscle layer was collected in ice-cold HEPES buffer (in mmol/L: 120 NaCl, 2.6 KH2SO4, 4 KCl, 2 CaCl2, 0.6 MgCl2, 25 HEPES, 14 glucose, and 2.1% essential amino acid mixture, pH 7.4). Two successive digestions with papain and collagenase dispersed smooth muscle cells. The cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum in the presence of 100 U/ml of penicillin G, 100 μg/ml of streptomycin sulfate, and 0.25 μg/ml of amphoteracin B. The cells in passages 3–5 were used in experiments. All cells were cultured in low serum (1%) for 24 h before experiments to minimize the potential effects of growth factors.
Induction of colonic inflammation by TNBS.
Sprague-Dawley rats weighing 180–280 g (Harlan Laboratories, Indianapolis, IN) were used. Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston, Texas approved all procedures. Colonic inflammation was induced by intracolonic administration of 17-mg/kg or 68-mg/kg (0.25 ml in 40% ethanol) doses of TNBS (Sigma Chemical, St. Louis, MO) under 2% isoflurane anesthesia, as described elsewhere (6). Twenty-four hours before infusion of TNBS, drinking water was replaced with Colyte, and rats were fasted overnight to cleanse the colon. This step minimized variable absorption of TNBS by different quantities of fecal pellets among rats and allowed consistent exposure of a given dose of TNBS to mucosal surface. The control rats received 0.25 ml of saline. The animals were euthanized 7 days later to obtain tissues from the distal colon.
We used a previously described and modified macroscopic scoring system to assess mucosal damage (3, 22, 34) as follows: 0 = normal mucosa; 1 = localized hyperemia but no erosions, ulcers, or scars; 2 = linear ulcer or scar with inflammation at one site >2 mm but <5 mm; 3 = two or more sites of ulceration and/or inflammation, each up to 5 mm; and 4 = two or more major sites of inflammation and ulcerations >5 mm each or one major site of inflammation extending >1 cm along the length of the mucosa.
Systemic administration of VIP with osmotic pump.
A 7-day osmotic pump filled with 90 μl of saline or human-, rat-, porcine-, and bovine-specific VIP or VIP and pituitary adenylate cyclase-activating peptide receptor (VPAC1/2) antagonist [(p-chloro-d-Phe6, Leu17)-VIP; Bachem, King of Prussia, PA] was implanted subcutaneously in the thigh, as previously described (35), 4 h before the induction of inflammation. The infusion rate was 0.5 μl/h.
Human and rat tissue preparation for muscle bath studies.
Freshly obtained full-thickness human colon tissues were stored on ice for no longer than 1 h in carbogenated Krebs solution (in mmol/L: 118 NaCl, 4.7 KCl, 2.5 CaC2, 1 NaH2PO4, 1.2 MgCl2, 11 d-glucose, and 25 NaHCO3). The mucosal layer, submucosal layer, and taenia coli were removed under magnifying glass. Circular muscle strips (2 mm × 10 mm) were cut along the circumferential axis and placed in DMEM containing test agents or vehicle control at 37°C in a CO2 incubator for 24 h (33). The circular muscle strips were mounted in 5-ml muscle baths (Radnoti Glass, Monrovia, CA) filled with carbogenated Krebs solution at 37°C (6, 28, 31, 34, 35). A similar procedure was used to obtain circular muscle strips from the rat colon (35). The contractile activity of colonic circular muscle strips was recorded with Grass isometric force transducers and amplifiers connected to Biopac data acquisition system (Goleta, CA). The contractile response to ACh was obtained at concentrations of 10−6 M to 10−2 M, after equilibrating the muscle strips under 1 g of tension for 1 h. The bathing solution was replaced every 15 min during equilibration and about 5 min after obtaining data with each new concentration of ACh. After wash, the tension was readjusted to 1 g and the muscle strips left to equilibrate for at least 15 min before adding the next concentration of ACh. At this time, the baseline tension was similar to that before administration of the previous concentration of ACh. The contractile response of circular muscle was quantified as increase in area under contractions during 4 min after addition of ACh to the bath over the baseline area under contractions during 4 min before addition of ACh.
Western blotting.
Whole cell extracts and cytoplasmic and nuclear proteins were processed, as described previously (32). The proteins in the samples were resolved by standard immunoblotting method using equal quantities (10 μg or 20 μg) in each lane. β-Actin was used as internal control. The antibody (1:200 to 1:400) for α1C subunit of Cav1.2 channels was purchased from Alomone Laboratories, Jerusalem, Israel (ACC-003). The p65, p50, IκBα, and IκBβ antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Agarose-oligonucleotide pull down assay.
The agarose-oligonucleotide pull down assay was performed, as described previously (33). The oligonucleotides containing NF-κB-like sequences in the hα1C1b promoter (RE1: 5′-GGGGAGCCCG-3′ at −1,499/−1,490 and RE2: 5′- GGGGGTGCCG-3′ at −1,481/−1,472) with 3′-terminal biotinylation and its complementary strand were synthesized by BioSource International (Camarillo, CA).
Luciferase promoter-reporter assay.
Human colonic circular smooth muscle cells (HCCSMCs) in passage 4 were seeded into 12-well plates (Costar, Acton, MA) and grown to 70–80% confluence. Transfection was carried out by incubation of cells in each well with 1.5 μl Lipofectamine 2000, 0.5 μg pGL2 construct (31, 33), and 0.125 μg phospho-secreted embryonic alkaline phosphatase 2 (pSEAP2) control vector (BD Biosciences Clontech, Palo Alto, CA) for 24 h. The pSEAP2 control vector was cotransfected to normalize data. The transfected cells were treated with VIP or control medium and collected after 24 h. Twenty microliters of supernatant from cell lysates were used to measure luciferase activity by a luminometer (Promega, Madison, WI). SEAP activity was measured with a Clontech SEAP detection kit (Clontech, Palo Alto, CA).
Multiplex immunoassay of cytokines and chemokines.
Rat colonic muscularis externa tissues were homogenized in cold PBS supplemented with protease inhibitors for protein extraction. LINCO rat cytokine/chemokine multiplex immunoassay kit (LINCO, St. Charles, MO) was used to quantitate IL-1β and regulated on activation, normal T cell expressed and secreted (RANTES) concentrations in the homogenates. The assay results were read and analyzed by a Bio-Rad BioPlex System powered by Luminex xMAP Technology (Bio-Rad Laboratories, Hercules, CA).
Measurement of myeloperoxidase.
Myeloperoxidase (MPO) in colonic muscularis externa was measured using the rat MPO ELISA kit from HyCult (Uden, Netherlands).
Materials and reagents.
Collagenase type II was purchased from Worthington Biochemical (Freehold, NJ), VIP from Bachem (Torrance, CA), RNeasy kit from Qiagen (Stanford, CA), antibodies to phospho-IKK-α/β from Cell Signaling (Beverly, MA), Hanks buffer, DMEM, Opti-MEM, and Lipofectamine 2000 from Invitrogen, and LINCO rat cytokine/chemokine multiplex immunoassay kit from LINCO Research. All other chemicals were purchased from Sigma.
Statistical analysis.
All data are expressed as means ± SE. Statistical analysis was performed by analysis of variance with nonrepeated measures. Multiple comparisons were made with Student-Newman-Keuls test. The difference between two means was tested by t-test. P < 0.05 was considered statistically significant.
RESULTS
VIP reverses TNF-α-induced suppression of α1C gene expression in primary cultures of HCCSMCs.
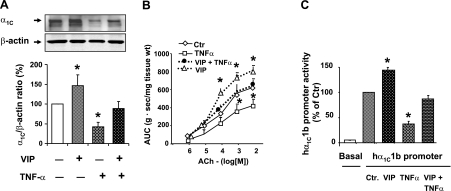
The incubation of human colonic circular smooth muscle strips with 20 ng/ml TNF-α for 24 h significantly suppressed expression of the pore-forming α1C subunit of Cav1.2 channels (Fig. 1A) and the contractile response to ACh (Fig. 1B). The incubation of these strips with 10−7 M VIP and TNF-α together for 24 h almost completely reversed the suppression of α1C subunit and suppression of contractile response to ACh (Fig. 1, A and B). Note that incubation with VIP alone enhanced the expression of α1C subunit and contractility of smooth muscle strips as reported previously (31). Circular smooth muscle strips incubated with vehicle (sterile PBS) served as controls.
Fig. 1.
A and B: in vitro effects of vasoactive intestinal peptide (VIP) and TNF-α on the expression of l-type Ca2+ channel α1C subunit and contractility in human colonic circular smooth muscle strips. C: effects of incubation of primary cultures of human colonic circular smooth muscle cells (HCCSMCs) transfected with the hα1C1b promoter-luciferase gene construct with 10−7 M VIP and 20 ng/ml TNF-α. N = 3 or 4 independent experiments, *P < 0.05 vs. control (Ctr). AUC, area under contractions.
The above effects of TNF-α and VIP were mediated at the level of α1C gene transcription. The incubation of primary cultures of HCCSMCs transfected with the hα1C1b promoter-luciferase gene construct (33) for 24 h with TNF-α significantly suppressed the promoter activity (Fig. 1C). The inclusion of VIP in incubation medium significantly reduced the suppression of promoter activity by TNF-α. VIP alone enhanced hα1C1b promoter activity. Cell cultures incubated with vehicle served as controls.
The role of transcription factor NF-κB in maintaining homeostasis in smooth muscle function by VIP in response to TNF-α.
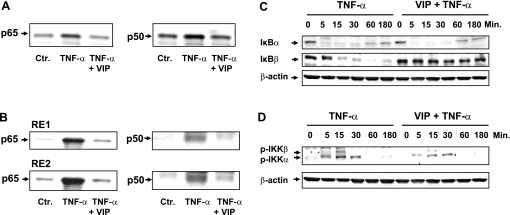
The human α1C1b promoter (hα1C1b) has two κB recognition sites, GGGGAGCCCG at −1,499/−1,490 and GGGGGTGCCG at −1,481/−1,472, that mediate the suppression of α1C gene by TNF-α (33). We found that TNF-α treatment of HCCSMCs for 60 min significantly increases the translocation of p50 and p65 subunits of NF-κB to the nucleus (Fig. 2A) as well as their bindings to both NF-κB recognition sites (RE1 and RE2) (Fig. 2B). However, the incubation of the HCCSMCs with TNF-α in the presence of VIP significantly reduces the nuclear translocation of both NF-κB subunits as well as their binding to the κB recognition sequences.
Fig. 2.
TNF-α induced the translocation of NF-κB p65 and p50 to the nucleus (A) and their nuclear binding to the RE1 and RE2 κB sites in the hα1C1b promoter (B) in primary culture of HCCSMCs. Cotreatment with VIP suppressed both effects of TNF-α. Cells were treated with 20 ng/ml TNF-α for 60 min in the absence or presence of 10−7 M VIP. 10 μg of nuclear extracts were used for each lane. VIP alone did not induce the translocation of NF-κB p65 and p50 to the nucleus or their binding to RE1 and RE2 (NF-κB recognition sites) (data not shown). C and D: TNF-α induced IκBα and IκBβ degradation (C) and IKKα and IKKβ phosphorylation (D) in primary cultures of HCCSMCs. VIP had little effect on the degradation of IκBα , but it blocked the degradation of IκBβ (C). VIP also blocked the phosphorylation of IKKβ and decreased the phosphorylation of IKKα (D). The primary cultures of HCCSMCs were treated with 20 ng/ml TNF-α for various durations in the absence or presence of 10−7 M VIP. Whole cell lyses (10 μg) were used in these experiments. Blots are representatives of 3 independent experiments.
The above findings suggest that VIP counters the adverse effects of TNF-α on smooth muscle cells by suppressing the translocation of NF-κB to the nucleus. NF-κB is retained in the nucleus by inhibitory proteins of the IκB family (2). The cell-signaling pathways for the activation of NF-κB stimulated by most extracellular stimuli converge on the family of IκB kinases, IKKα, and IKKβ. The activation of these enzymes phosphorylates IκBs (predominantly IκBα and IκBβ), which dissociates them from the cytoplasmic NF-κB, resulting in their ubiquitination and proteasomal degradation. This unmasks the nuclear localization sequence of NF-κB, and it translocates to the nucleus. We found that TNF-α induces degradation of both IκBs within a few minutes of its exposure to HCCSMCs (Fig. 2C). Concurrent incubation with VIP has no effect on the degradation of IκBα, but it blocks the degradation of IκBβ (Fig. 2C). TNF-α, by itself, phosphorylates both IKKα and IKKβ (Fig. 2D). However, in the presence of VIP, it is unable to phosphorylate IKKβ (Fig. 2D). These data suggest that VIP inhibits NF-κB activation by TNF-α by blocking the cell signaling pathways leading to the phosphorylation of IKKβ.
Homeostatic role of endogenous VIP in preserving smooth muscle function during mild inflammatory insult.
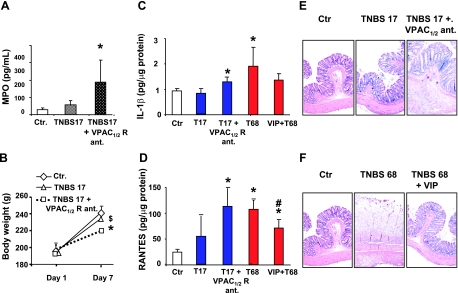
Our experiments indicated that 17 mg/kg TNBS is a subthreshold dose that does not significantly increase MPO in the muscularis externa (Fig. 3A), and it has no significant effect on weight gain over a 7-day postinflammation period, when compared with control rats (Fig. 3B). This dose of TNBS also does not significantly increase the concentrations of cytokines, such as IL-1β, and chemokines, such as RANTES, in the muscularis externa tissues of the distal colon (Fig. 3, C and D). In addition, there is little damage to the mucosal surface visualized by hematoxylin and eosin staining (Fig. 3E, left and middle). This dose of TNBS has marginal effect on the reactivity of colonic circular smooth muscle strips to ACh (Figs. 4A and 5). Finally, a 17-mg/kg dose of TNBS has no significant effect on the expression of α1C subunit (Fig. 4B) or the nuclear binding of p65 (Fig. 4C) and p50 (Fig. 4D) subunits of NF-κB.
Fig. 3.
A: subthreshold 17-mg/kg dose of trinitrobenzene sulfonic acid (TNBS) had no significant effect on myeloperoxidase (MPO) in the muscularis externa on day 7 of inflammation. However, the blockade of VIP and pituitary adenylate cyclase-activating peptide (VPAC)1/2 receptors by systemic infusion VPAC1/2 receptor antagonist (R ant.) for 7 days, starting before the application of the subthreshold dose of TNBS, significantly increased MPO in the muscularis externa. B: subthreshold dose of TNBS had no significant effect on weight gain over a 7-day postinflammation period. However, the same subthreshold dose of TNBS during the blockade of VPAC1/2 receptors, as described above, resulted in a significant reduction in weight gain over the same period. C and D: subthreshold dose of TNBS had no significant effect on the muscularis externa concentration of IL-1β (C) or regulated on activation, normal T cell expressed and secreted (RANTES) (D). However, the blockade of VPAC1/2 receptors, as described above, significantly increased the concentrations of both inflammatory mediators in the muscularis externa. On the other hand, the 68-mg/kg dose of VIP significantly increased the concentrations of IL-1β (C) and RANTES (D) in the muscularis externa on day 7 of inflammation. However, VIP infusion by an osmotic pump, starting before the induction of inflammation, blunted the increase of both inflammatory mediators in the muscularis externa. E and F: representative microscopic views (x20) of the effects of 2 doses of TNBS on colonic structures and the effects of concurrent infusions of VPAC1/2 receptor antagonist (E) and VIP (F) on colonic inflammation. T17 and TNBS17, TNBS 17-mg/kg dose; T68 and TNBS68, TNBS 68-mg/kg dose. Saline-treated rats served as controls. N = 4 to 6 rats in each group. *P < 0.05 vs. control; $P < 0.05 vs. TNBS 17 + VPAC1/2 receptor antagonist group; #P < 0.05 vs. TNBS 68 group.
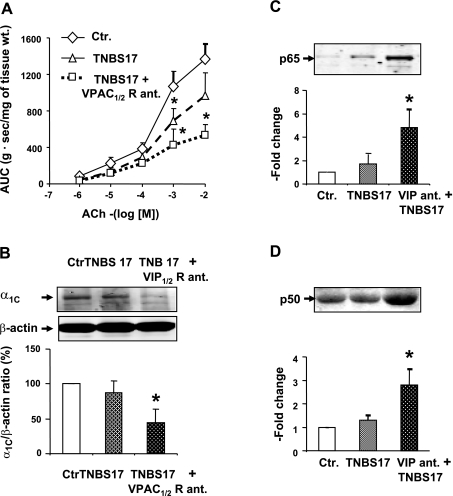
Fig. 4.
A: subthreshold dose of 17 mg/kg (TNBS17) marginally suppressed the contractile response of colonic circular muscle strips to ACh on day 7 of inflammation. However, during the blockade of VPAC1/2 receptors by systemic infusion of their antagonist by an osmotic pump, as described in the legend of Fig. 5, the same dose of TNBS increased the suppression of reactivity to ACh. B: subthreshold dose of TNBS had no significant effect on the expression of the α1C subunit of Cav1.2 channels. However, during the blockade of VPAC1/2 receptors, as described above, the subthreshold dose significantly suppressed the expression of α1C subunit on day 7 of inflammation. C and D: subthreshold dose of TNBS had no significant effect on the nuclear abundance of p65 (C) or p50 (D). However, during the blockade of VPAC1/2 receptors, as described above, the same subthreshold dose significantly increased the nuclear translocation of NF-κB p65 and p50 subunits (C) and (D), respectively, in colonic muscularis externa. Rats treated with saline served as controls. N = 3 to 5 rats in each group. Blots are representative of 3 to 5 independent experiments. *P < 0.05 vs. control.
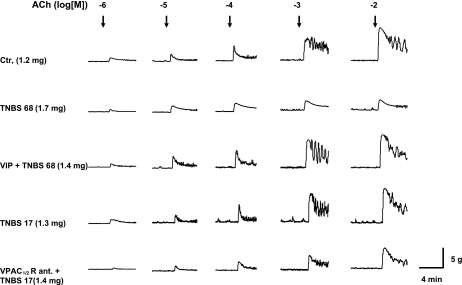
Fig. 5.
Representative tracing of contractile activity of colonic circular muscle strips isolated from rats administered with saline (Ctr.), TNBS 65 mg/kg (TNBS 65), VIP + TNBS 65 mg/kg, TNBS 17 mg/kg (TNBS 17), and VPAC1/2 receptor antagonist (VPAC1/2 R) + TNBS 17 mg/kg for 7 days by an osmotic pump. All strips were cut into 10-mm-long and 3-mm-wide strips. The dry weight of each muscle strip is indicated in parenthesis.
We tested the hypothesis that spontaneous release of endogenous VIP maintains homeostasis in smooth muscle function and restrains the induction of inflammation by the subthreshold inflammatory insult. We applied the subthreshold insult during blockade of VPAC1/2 receptors by a 7-day systemic infusion of 20 nmol/day (p-chloro-d-Phe6, Leu17)-VIP with a surgically implanted osmotic pump. The infusion started 4 h before the application of subthreshold inflammatory insult. During the blockade of VPAC1/2 receptors, the subthreshold insult significantly increases MPO (Fig. 3A), the concentrations of IL-1β (Fig. 3C), and RANTES (Fig. 3D) in the muscularis externa, and it significantly blunts the weight gain of rats (Fig. 3B) during the 7-day postinsult period. The visual macroscopic damage score by subthreshold inflammatory insult during the blockade of VPAC1/2 receptors, 2.7 ± 0.2, is significantly greater than that in control rats infused with vehicle and subjected to the same insult, 1.8 ± 0.2. This score is 0 ± 0 in naive control rats. Hematoxylin and eosin staining supports these findings (Fig. 3E).
The blockade of VPAC1/2 receptors during mild inflammatory insult also significantly worsens the suppression of contractility (Figs. 4A and 5) and the suppression of α1C subunit (Fig. 4B), and it increases the nuclear binding of p65 and p50 subunits of NF-κB (Fig. 4, C and D), respectively.
Effect of neuroimmune interactions on VIP concentration in muscularis externa.
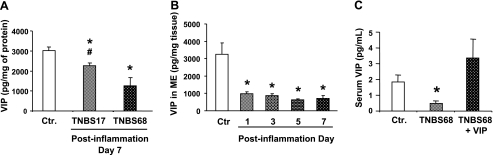
The nerves and immune cells interact to regulate the functions of each other. We found that subthreshold insult in naive rats significantly, but moderately, reduces the tissue concentration of VIP in the muscularis externa on day 7 of inflammation (Fig. 6A). The intensity of TNBS-induced inflammation depends on the magnitude of the insult (6). Therefore, we investigated whether a larger dose of TNBS, 68 mg/kg, would further depress the concentration of VIP in the muscularis externa. Indeed, the tissue concentration of VIP in the muscularis externa with this dose of TNBS was significantly less than that with the subthreshold dose of TNBS (Fig. 6A). Further examination showed that the suppression of VIP concentration in the muscularis externa occurs within 24 h after the induction of inflammation, and it remains suppressed for at least 7 days (Fig. 6B). The 68-mg/kg dose of TNBS also significantly suppressed the serum concentration of VIP (Fig. 6C).
Fig. 6.
A: decrease in the muscularis externa concentration of VIP by inflammation induced with 68-mg/kg dose of TNBS (TNBS68) was significantly greater than that induced with the 17-mg/kg dose (TNBS17) on postinflammation day 7. N = 3 rats for each group. *P < 0.05 vs. control; #P < 0.05 vs. TNBS68 group. B: 68-mg/kg dose of TNBS reduced the muscularis externa (ME) concentration of VIP by day 1 postinflammation, and it remained at the reduced level at least until day 7. C: serum VIP concentration on day 7 of TNBS68 inflammation was also significantly lower than that in controls. However, the infusion of exogenous VIP by an osmotic pump significantly elevated the serum VIP concentration that was sustained on day 7 of inflammation. Rats infused with an identical daily volume of saline by an osmotic pump served as controls. N = 4 rats for each group. *P < 0.05 vs. control, P < 0.05 vs. TNBS68 group.
Therapeutic potential of VIP in maintaining homeostasis of smooth muscle function during severe inflammatory response.
We reported recently that the systemic infusion of VIP by an osmotic pump elevates the serum concentration of VIP that is sustained throughout the period of infusion (35). Therefore, we investigated whether reestablishing serum concentration of VIP by an osmotic pump would compensate for the decrease in muscularis externa VIP by the inflammatory response and prevent smooth muscle dysfunction.
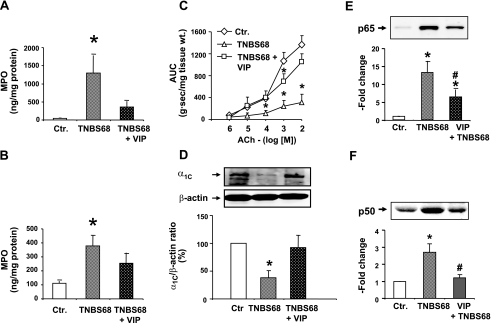
We found that inflammation induced with 68 mg/kg TNBS significantly increases the tissue concentrations of MPO in muscularis externa and in mucosal/submucosal tissue (Fig. 7, A and B). It also significantly suppresses the contractile response to ACh (Figs. 5 and 7C) and the expression of α1C subunit (Fig. 7D), and it increases the nuclear abundance of p65 and p50 NF-κB subunits in the muscularis externa (Fig. 7, E and F). Systemic infusion of 20 nmol/day VIP for 7 days by an osmotic pump, starting 4 h before the induction of inflammation with 68 mg/kg TNBS, significantly reverses all the adverse effects of the inflammatory response, including the nuclear translocation of p65 and p50 (Fig. 7). It reduced the visual macroscopic score from 3.4 ± 0.4 to 1.5 ± 0.2. Hematoxylin and eosin staining confirmed the reduction in inflammatory damage (Fig. 3F). The plasma VIP concentration on day 7 of infusion was about twofold greater than that before the start of infusion (Fig. 6C).
Fig. 7.
Therapeutic effects of exogenous infusion of VIP (20 nmol/day) by a 7-day osmotic pump on colonic inflammation induced by 68 mg/kg of TNBS (TNBS68). A and B: TNBS68 inflammation significantly increased the MPO levels in the colonic muscularis externa (A) and mucosa/submucosa (B). VIP infusion blunted the increase of MPO in both tissues. C: TNBS68 significantly suppressed the colonic circular smooth muscle reactivity to ACh; VIP infusion blunted this suppression. D: TNBS68 significantly suppressed expression of the α1C subunit of Cav1.2 channels in muscularis externa on day 7 of inflammation; VIP infusion blocked this suppression. E and F: TNBS68 inflammation significantly increased the nuclear translocation of NF-κB p65 (E) and NF-κB p50 (F) subunits in colonic muscularis externa. Rats treated with saline served as controls. N = 3 to 5 in each group. *P < 0.05 vs. control; #P < 0.05 vs. TNBS68 group. Blots are representative of 3 to 5 independent experiments.
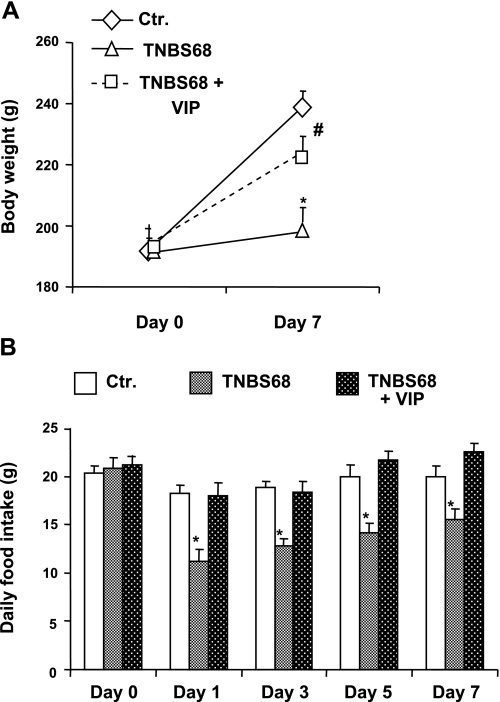
The gain in body weight of rats receiving 68 mg/kg TNBS was significantly stunted over the 7-day postinflammation period, when compared with age-matched vehicle-treated animals (Fig. 8A). The rats ate significantly less food per day starting from day 1 of inflammation (Fig. 8B). The food intake improved gradually, but it was still significantly less on day 7, when compared with control food intake before the induction of inflammation (Fig. 8B). There was no significant decrease in food intake in rats subjected to severe inflammatory insult while receiving 20 nmol/day VIP with an osmotic pump (Fig. 8B).
Fig. 8.
Therapeutic effects of exogenous infusion of VIP (20 nmol/day) by a 7-day osmotic pump on colonic inflammation induced by 68 mg/kg of TNBS (TNBS68). A: TNBS68 inflammation significantly reduced the rate of increase in weight gain during the 7-day postinflammation period. VIP infusion for 7 days by an osmotic pump, as described above, significantly improved the gain in weight, when compared with that attributable to TNBS68 inflammation. B: induction of TNBS68 inflammation significantly reduced food intake, starting with day 1 of inflammation and lasting at least until day 7. VIP infusion ,as described above, significantly reversed the decrease in food intake. *P < 0.05 vs. control; #P < 0.05 vs. TNBS68 group. N = 3 to 5 in each group.
DISCUSSION
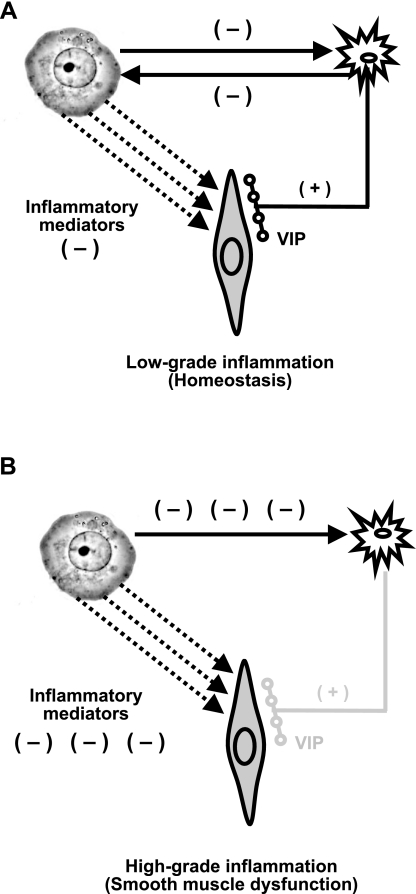
Our findings show a three-way interaction between the enteric VIPergic neurons, circular smooth muscle cells, and immunocytes in the muscularis externa (Fig. 9, A and B). Under low-grade inflammatory conditions, the proinflammatory cytokines, such as TNF-α and IL-1β, attempt to suppress circular smooth muscle contractility, but the spontaneous release of endogenous VIP from the enteric VIPergic neurons opposes this effect and maintains homeostasis (Fig. 9A). At the same time, bilateral interaction between VIPergic neurons and immune cells results in small, but significant, suppression of VIP expression and the release of inflammatory mediators from activated immune cells in the muscularis externa. The residual VIP under these conditions is sufficient to prevent a significant increase of inflammatory mediators and MPO in the muscularis externa. As a result, the colonic circular smooth muscle contractility maintains nearly normal function. However, if the protective effects of endogenous VIP on immunocytes and smooth muscle cells are precluded by blocking VPAC1/2 receptors, the inflammatory response in the muscularis externa increases and smooth muscle function is impaired.
Fig. 9.
Diagrammatic illustration of three-way interaction among the enteric VIPergic neurons, immunocytes, and smooth muscle cells. A: in low-grade inflammation, the spontaneous release of VIP from the enteric neuronal varicosities maintains homeostasis in circular smooth muscle function by countering the deleterious effects of low-level increase in proinflammatory mediators. B: in high-grade inflammation, the inflammatory mediators significantly suppress the synthesis/release of VIP from the enteric neurons, and the higher levels of proinflammatory mediators suppress circular smooth muscle contractility.
The suppression of neural VIP by immune cells increases with the intensity of inflammation. At higher intensities of inflammation, the inflammatory mediators reduce the concentrations of VIP in muscularis externa to about one-third of their basal levels. Under these conditions, the concentrations of the inflammatory mediators in the muscularis externa and MPO increase significantly, and they suppress smooth muscle contractility in the absence of basal protection by endogenous VIP. The suppression of VIP expression in the muscularis externa by inflammatory response seems to be a critical step in smooth muscle dysfunction. If the depletion of endogenous VIP is compensated by its exogenous infusion, the inflammatory response is suppressed and smooth muscle function is restored to near normal conditions. Concurrently, the exogenous VIP partially suppresses the inflammatory response in the mucosal/submucosal tissue. Therefore, the restoration of smooth muscle function by exogenous VIP is partially attributable to suppression of the inflammatory response and partially attributable to its direct effect on smooth muscle cells to block the deleterious effect of the residual inflammatory response.
Inflammatory insult rapidly suppresses the total VIP content in the muscularis externa (Fig. 6). Our findings and those of others show that the amplitude of VIP suppression correlates with the degree of inflammation (5, 18, 29). Immunohistochemical findings on the effects of inflammation on VIPergic neurons are divergent (5, 17–20, 25, 29, 30, 36). However, the consensus is that inflammation damages morphology of the enteric nervous system, including a reduction in the number of cell bodies and VIP release in Crohn's disease, ulcerative colitis, and in experimental models of inflammation (4, 18, 25, 36). Taken together, these data show that, in severe inflammatory response, very little endogenous VIP may be available to counter inflammation and to protect the function of neighboring nonimmune cells in the muscularis externa and in mucosal/submucosal layers. Although the inflammatory response and VIP counter each other successfully in low-grade inflammation, the inflammatory response gains upper hand in severe inflammation by suppressing the expression of VIP and damaging axons (Fig. 9).
Severe inflammation stunts weight gain, and it reduces food intake. The exogenous administration of VIP reverses both effects. These data suggest that basal plasma circulation of VIP may play a critical role in maintaining appetite. This regulation may be a protective mechanism to prevent loading of the gut with food under conditions where its digestive functions are impaired by inflammation.
NF-κB is a ubiquitous transcription factor that induces transcription of genes encoding inflammatory mediators in immune as well as in nonimmune cells (15, 27, 28). In nonimmune cells, such as CCSMCs, NF-κB activation suppresses the transcription of genes encoding the cell-signaling proteins of the excitation-contraction coupling (33). The enteric VIPergic neurons directly innervate the circular smooth muscle cells as well as the immune cells of the gut lymphoid tissue (12, 39). Previous in vitro studies found that VIP suppresses inflammatory response in immune cells of the lamina propria by inhibiting translocation of NF-κB to the nucleus and its subsequent binding to κB recognition sites on the promoters of genes encoding the inflammatory mediators (8–10).
Our findings show that VIP also inhibits the translocation of NF-κB in circular smooth muscle cells to prevent suppression of contractility by proinflammatory cytokines. VIP inhibits the phosphorylation of IKKβ in response to TNF-α, which prevents the degradation of IκBβ, but not that of IκBα. The degradation of IKKβ is associated with longer-term activation of NF-κB, whereas the degradation of IκBα results in transient activation of NF-κB (16, 40). By contrast, stimulation of immune cells by LPS degrades IκBα to induce the expression of inflammatory mediators (7). Therefore, it seems that VIP inhibits activation of the canonical pathway of activation of NF-κB regardless of the cell type and the involvement of species of IκB proteins and their kinases.
The signaling pathways for the activation of NF-κB and the affinities of NF-κB dimmers to bind to their variant κB recognition sites on target promoters are cell, stimulus, and promoter specific (15). We found that both endogenous and exogenous VIP inhibit the translocation of NF-κB subunits p50 and p65 to the nucleus and their subsequent binding to promoter of the gene encoding the α1C subunit of Cav1.2 channels in smooth muscle cells of the muscularis externa in vivo and in vitro. On the other hand, in immune cells, LPS translocates homodimers of p65 to induce the transcription of inflammatory mediators (7). These differences may contribute to the differential effects of NF-κB activation; in immune cells, the activation of this transcription factor enhances the transcription of genes, whereas, in smooth muscle cells, it suppresses the transcription of genes encoding proteins of the excitation-contraction coupling.
Two other groups previously investigated the therapeutic potential of exogenous administration of VIP in a murine model of TNBS inflammation and reported conflicting outcomes (1, 23). Both studies investigated only the therapeutic potential of VIP on inflammatory response and physical symptoms. Abad et. al (1) reported that one or more intraperitoneal bolus injections of VIP block the inflammatory response to TNBS and development of physical symptoms, such as mucosal damage and weight loss. Newman et al. (23) found that similar bolus injections of VIP or its constant infusion by an osmotic pump block chemokine-induced chemotaxis of mononuclear cells, but it fails to modify histological damages, cytokine markers of disease, or clinical symptoms, including weight loss. By contrast, we found that constant infusion of exogenous VIP by an osmotic pump in rats diminishes the inflammatory response, improves clinical symptoms, and growth in weight, and it restores suppression of smooth muscle contractility. Abad et al. (1) and Newman et. al (23) used much higher doses of TNBS, 142 mg/kg and 118 mg/kg, respectively, compared with 68 mg/kg used by our laboratory. Their doses produced massive inflammation, resulting in dramatic weight loss of about 20%, mortality of about 30%, bloody diarrhea, and rectal prolapse. Our dose of 68 mg/kg TNBS suppressed smooth muscle contractility, resulting in loose stools. This dose of TNBS stunted growth, but it did not cause weight loss or mortality. The inflammatory and clinical symptoms in our study are closer to those seen in acute and chronic inflammation in humans. The exogenous administration of VIP may not be able to overcome massive wasting disease.
VIP, injected as a bolus, is eliminated from the plasma within a few minutes (11, 13, 14, 21). It is not clear how such a short duration of VIP pulse could alter longer-term transcription of genes encoding inflammatory mediators (1). It is noteworthy that the severity of inflammatory response and its duration varied dramatically in the study of Abad et al. (1). This may be due to the variable amount of fecal pellets present in the colon at the time of administration of TNBS. Different quantities of fecal pellets may absorb different amounts of TNBS. In our study, we cleansed the colon by replacing drinking water with Colyte 24 h before TNBS administration followed by overnight fasting. Abad et al. (1) did not demonstrate the inhibition of NF-κB activation by bolus doses of VIP in their model of TNBS inflammation.
In conclusion, the gut wall is persistently exposed to hostile pathogenic environment, and the VIPergic neurons emanating from the myenteric and submucosal plexi densely innervate most immune and nonimmune cells in muscularis externa and mucosal/submucosal tissues. Our findings show that spontaneous release of VIP from these neurons may be one of the first lines of defense against mild/moderate inflammatory insults. The second line of defense, of course, is the generation of anti-inflammatory cytokines, but that happens after the inflammatory response has matured. The spontaneous release of VIP in the gut wall maintains homeostasis in both immune and nonimmune cells. Homeostasis in smooth muscle function is maintained by inhibiting the translocation of NF-κB to the nucleus attributable to stabilization of IκBβ. A severe inflammatory insult suppresses the total content of VIP as well as its release from nerve varicosities, which allows the inflammatory response to mature and adversely affect the function of nonimmune cells, such as smooth muscle cells. The suppression of VIP in gut tissues is critical for the inflammatory response to mature. The compensation for loss of neuronal VIP by its exogenous administration protects smooth muscle function directly by inhibiting the activation of NF-κB and indirectly by partially suppressing the inflammatory response.
GRANTS
This work was supported in part by NIDDK Grants DK 072414 (to S. Sarna) and DK 32346 (to S. Sarna and X.-Z. Shi).
REFERENCES
- 1.Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology 124: 961–971, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14: 649–683, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol Gastrointest Liver Physiol 268: G622–G630, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bishop AE, Polak JM, Bryant MG, Bloom SR, Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology 79: 853–860, 1980 [PubMed] [Google Scholar]
- 5.Boyer L, Ghoreishi M, Templeman V, Vallance BA, Buchan AM, Jevon G, Jacobson K. Myenteric plexus injury and apoptosis in experimental colitis. Auton Neurosci 117: 41–53, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Choudhury BK, Shi XZ, Sarna SK. Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS. Am J Physiol Gastrointest Liver Physiol 296: G632–G642, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 7: 563–568, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit interleukin-12 transcription by regulating nuclear factor kappaB and Ets activation. J Biol Chem 274: 31930–31940, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-kappa B-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem 276: 369–380, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J Immunol 162: 4685–4696, 1999 [PubMed] [Google Scholar]
- 11.Domschke S, Domschke W, Bloom SR, Mitznegg P, Mitchell SJ, Lux G, Strunz U. Vasoactive intestinal peptide in man: pharmacokinetics, metabolic and circulatory effects. Gut 19: 1049–1053, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furness JB, Lloyd KC, Sternini C, Walsh JH. Projections of substance P, vasoactive intestinal peptide and tyrosine hydroxylase immunoreactive nerve fibres in the canine intestine, with special reference to the innervation of the circular muscle. Arch Histol Cytol 53: 129–140, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Gourlet P, Vandermeers A, Robberecht P, Deschodt-Lanckman M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP-27, but not PACAP-38) degradation by the neutral endopeptidase EC 342411. Biochem Pharmacol 54: 509–515, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Hassan M, Refai E, Andersson M, Schnell PO, Jacobsson H. In vivo dynamical distribution of 131I-VIP in the rat studied by gamma-camera. Nucl Med Biol 21: 865–872, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 25: 6706–6716, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Johnson DR, Douglas I, Jahnke A, Ghosh S, Pober JS. A sustained reduction in IkappaB-beta may contribute to persistent NF-kappaB activation in human endothelial cells. J Biol Chem 271: 16317–16322, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Koch TR, Carney JA, Go VL. Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel diseases. Dig Dis Sci 32: 369–376, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Kubota Y, Petras RE, Ottaway CA, Tubbs RR, Farmer RG, Fiocchi C. Colonic vasoactive intestinal peptide nerves in inflammatory bowel disease. Gastroenterology 102: 1242–1251, 1992 [PubMed] [Google Scholar]
- 19.Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil 17: 751–760, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Miampamba M, Sharkey KA. Distribution of calcitonin gene-related peptide, somatostatin, substance P and vasoactive intestinal polypeptide in experimental colitis in rats. Neurogastroenterol Motil 10: 315–329, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SJ, Bloom SR. Measurement of fasting and postprandial plasma VIP in man. Gut 19: 1043–1048, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers BS, Martin JS, Dempsey DT, Parkman HP, Thomas RM, Ryan JP. Acute experimental colitis decreases colonic circular smooth muscle contractility in rats. Am J Physiol Gastrointest Liver Physiol 273: G928–G936, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Newman R, Cuan N, Hampartzoumian T, Connor SJ, Lloyd AR, Grimm MC. Vasoactive intestinal peptide impairs leukocyte migration but fails to modify experimental murine colitis. Clin Exp Immunol 139: 411–420, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Dorisio MS. Neuropeptides and gastrointestinal immunity. Am J Med 81: 74–82, 1986 [DOI] [PubMed] [Google Scholar]
- 25.O'Morain C, Bishop AE, McGregor GP, Levi AJ, Bloom SR, Polak JM, Peters TJ. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut 25: 57–61, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottaway CA. Vasoactive intestinal peptide as a modulator of lymphocyte and immune function. Ann NY Acad Sci 527: 486–500, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18: 6853–6866, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Pazdrak K, Shi XZ, Sarna SK. TNFalpha suppresses human colonic circular smooth muscle cell contractility by SP1- and NF-kappaB-mediated induction of ICAM-1. Gastroenterology 127: 1096–1109, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol 155: 1051–1057, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, Schemann M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn's disease. Neurogastroenterol Motil 13: 255–264, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Shi XZ, Choudhury BK, Pasricha PJ, Sarna SK. A novel role of VIP in colonic motility function: induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology 132: 1388–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 124: 1369–1380, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology 129: 1518–1532, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Shi XZ, Sarna SK. Differential inflammatory modulation of canine ileal longitudinal and circular muscle cells. Am J Physiol Gastrointest Liver Physiol 277: G341–G350, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Shi XZ, Sarna SK. Gene therapy of Cav1.2 channel with VIP and VIP receptor agonists and antagonists: a novel approach to designing promotility and antimotility agents. Am J Physiol Gastrointest Liver Physiol 295: G187–G196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjolund K, Schaffalitzky OB, Muckadell DE, Fahrenkrug J, Hakanson R, Peterson BG, Sundler F. Peptide-containing nerve fibres in the gut wall in Crohn's disease. Gut 24: 724–733, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storsteen KA, Kernohan JW, Bargen JA. The myenteric plexus in chronic ulcerative colitis. Surg Gynecol Obstet 97: 335–343, 1953 [PubMed] [Google Scholar]
- 38.Strobach RS, Ross AH, Markin RS, Zetterman RK, Linder J. Neural patterns in inflammatory bowel disease: an immunohistochemical survey. Mod Pathol 3: 488–493, 1990 [PubMed] [Google Scholar]
- 39.Sundler F, Ekblad E, Grunditz T, Hakanson R, Uddman R. Vasoactive intestinal peptide in the peripheral nervous system. Ann NY Acad Sci 527: 143–167, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell 80: 573–582, 1995 [DOI] [PubMed] [Google Scholar]