Abstract
Infection with the gram-negative enteropathogenic Escherichia coli (EPEC), a food-borne pathogen, represents a significant risk to human health. Whereas diarrhea is a major consequence of this infection, malnutrition also occurs especially in severe and prolonged cases, which may aggravate the health status of the infected hosts. Here we examined the effect of EPEC infection on the intestinal uptake of the water-soluble vitamin B1 (thiamin) using an established human intestinal epithelial Caco-2 cell model. The results showed that infecting Caco-2 cells with wild-type EPEC (but not with nonpathogenic E. coli, killed EPEC, or filtered supernatant) leads to a significant (P < 0.01) inhibition in thiamin uptake. Kinetic parameters of both the nanomolar (mediated by THTR-2) and the micromolar (mediated by THTR-1) saturable thiamin uptake processes were affected by EPEC infection. Cell surface expression of hTHTR-1 and -2 proteins, (determined by the biotinylation method) showed a significantly (P < 0.01) lower expression in EPEC-treated cells compared with controls. EPEC infection also affected the steady-state mRNA levels as well as promoter activity of the SLC19A2 and SLC19A3 genes. Infecting Caco-2 cells with EPEC mutants that harbor mutations in the escN gene (which encodes a putative ATPase for the EPEC type III secretion system, TTSS) or the espA, espB, or espD genes (which encode structural components of the TTSS) did not affect thiamin uptake. On the other hand, mutations in espF and espH genes (which encode effector proteins) exhibited partial inhibition in thiamin uptake. These results demonstrate for the first time that EPEC infection of human intestinal epithelial cells leads to inhibition in thiamin uptake via effects on physiological and molecular parameters of hTHTR-1 and -2. Furthermore, the inhibition appears to be dependent on a functional TTSS of EPEC.
Keywords: thiamin transport, hTHTR-1, hTHTR-2
vitamin B1 (thiamin), a member of the water-soluble family of micronutrients, is essential for normal human health and well-being. This vitamin acts as a cofactor in a variety of critical metabolic reactions involved in energy metabolism (2). Furthermore, because thiamin bridges the glycolytic and the pentose phosphate metabolic pathway that is critical for creating chemical reducing power in cells, thiamin is also considered as having a role in reducing oxidative stress (4, 13). Thus thiamin deficiency at the cellular level leads to impairment in energy metabolism and oxidative stress (4, 13); it has also been reported to lead to apoptosis (23, 33). At the clinical level, thiamin deficiency leads to a variety of abnormalities that include neurological and cardiovascular disorders (2, 36, 39). In contrast to the negative effects of thiamin deficiency and suboptimal levels, optimizing thiamin body homeostasis may have beneficial effects in preventing diabetic retinopathy and nephropathy as well as tissue damage caused by hyperglycemia of diabetes (1, 17); it also reverses many of the clinical symptoms associated with the recessive disorder thiamin-responsive megaloblastic anemia, a disease caused by mutation in human thiamin transporter-1 (hTHTR-1) (7, 12).
Humans (and all other mammals) cannot synthesize thiamin and thus must obtain the vitamin from exogenous sources via intestinal absorption. The intestine, therefore, plays central role in regulating normal thiamin body homeostasis. Studies from our laboratory and others have characterized many physiological and biological aspects of the human intestinal thiamin uptake process and have shown the involvement of a specialized carrier-mediated process (9, 21, 29, 31). We have also shown that both the human thiamin transporter-1 and -2 (hTHTR-1 and hTHTR-2; products of the SLC19A2 and SLC19A3 genes, respectively) are expressed in the intestine at the RNA and protein levels (30, 31). Furthermore, we have shown that whereas the hTHTR-1 is expressed at both the apical and basolateral membrane domains of the polarized enterocytes, expression of the hTHTR-2 is restricted to the apical membrane domain of these cells (30, 35).
EPEC is a gram-negative food-borne pathogen that infects the human intestine. This infection leads to a significant morbidity and mortality, especially in infants (22). Infection with EPEC is common in developing countries, but it also occurs in developed countries as a result of food contamination and improper hygiene. Whereas diarrhea is a major consequence of EPEC infection, malnutrition also occurs especially in severe and prolonged cases (10, 11, 16). The latter may lead to clinical complications and aggravation of the health status of the infected individuals, many of whom are already nutritionally compromised (10, 11, 16).
The pathogenicity of EPEC involves attachment of the bacteria with human intestinal epithelial cells, causing effacement in the microvilli through the formation of actin-rich pedestals (8, 24). EPEC also delivers effector molecules into host intestinal epithelial cells via a syringelike type III secretion system (TTSS; reviewed in Ref. 8). These effector proteins (e.g., EspF, EspG, EspH, Tir, and Map) then interact with various intracellular targets in the infected intestinal epithelial cells thereby affecting different biological (e.g., stability of the microtubule network, RNA levels; Refs. 37, 41) and physiological (e.g., electrolyte and nutrient transport; Refs. 3, 5, 6, 18, 19), paracellular permeability (32) processes.
Significant progress has been made in recent years in the understanding of EPEC effects on electrolyte transport in intestinal epithelial cells and the cellular mechanisms involved (5, 18, 19). Nothing, however, is known about the effect of EPEC infection on intestinal absorption of thiamin. Given the knowledge that thiamin plays an important role in normal cellular functions and that the human body has a limited capability to store sufficient amounts of this essential water-soluble micronutrient, prolonged and severe infection with EPEC may negatively impact the normal body homeostasis of thiamin, leading to further aggravation of the health status of the infected hosts (many of whom are already nutritionally compromised). For this reason and because an effect(s) of this pathogen on the function of the hTHTR-1 and/or hTHTR-2 will provide an excellent opportunity to further our understanding of these two recently identified but little characterized intestinal transporters, we undertook these investigations and have examined the effect of EPEC infection of thiamin uptake by human intestinal epithelial cells. We used the human-derived intestinal epithelial Caco-2 cells as the model, since previous investigations have proven their suitability in similar type of investigations on the effect of EPEC on intestinal function (3, 5, 18). Our results showed that EPEC infection causes a significant inhibition in intestinal thiamin uptake and that the effect is mediated via alteration in physiological and molecular parameters of the hTHTR-1 and -2. Furthermore, the inhibition appears to be dependent on a functional TTSS of the pathogen.
MATERIALS AND METHODS
Materials
Radiolabeled [3H]thiamin (specific activity 555 GBq/mmol; radiochemical purity >98%) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Routine biochemicals, enzymes, dialyzed fetal bovine serum, and cell culture reagents were all of molecular biology quality and were purchased from either Fisher Scientific (Tustin, CA) or Sigma (St. Louis, MO). Caco-2 cells were obtained from ATCC (American Type Culture Collection, Manassas, VA).
Cell Culture
The human-derived intestinal epithelial Caco-2 cells (passage of 10 through 30) were grown and maintained in DMEM containing 4.5 g/l glucose, 10% FBS (Hyclone), glutamine (0.29 g/l), sodium bicarbonate (3.7 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l) in an atmosphere of 5% CO2-95% air at 37°C. Monolayers of Caco-2 cells were used for examining bacterial infection on thiamin uptake 5–6 days postconfluence. The night before infection, cells were grown overnight in a serum-free and antibiotic-free T84 medium consisting of a 1:1 mixture of Ham's F12 and DMEM supplemented with 0.5% mannose.
Bacterial Culture and Infection of Cells
The EPEC strains used in this study were the wild-type EPEC strain E2348/69 and the nonpathogenic isolate HS4. Overnight cultures of EPEC strain, grown in Luria-Bertani broth, were diluted (1:33) in an appropriate volume of serum and antibiotic-free T84 medium supplemented with 0.5% mannose. Bacteria were grown at 37°C to midlog phase (optical density at 600 nm = 0.4). The culture was spun down and resuspended in the same volume of fresh T84 media. Cell monolayers were then infected at a multiplicity of infection (MOI) of 100 for 1 h (unless otherwise stated). After infection, media were removed, and cell monolayers were washed with fresh medium containing gentamicin (50 μg/ml) for 30 min to completely remove the unbound EPEC. After gentamicin treatment, cells were washed with medium, further incubated at 37°C in a 5% CO2 water-jacketed incubator for 6 h (unless otherwise stated), and used for thiamin uptake studies.
Bacterial Supernatants
For the use of bacterial preparations, bacteria grown to mid-log growth phase in serum-free, antibiotic-free media were centrifuged at 5,000 rpm for 10 min. The extracellular culture supernatant was harvested and sterilized by passing through a 0.22-μm sterile syringe filter and used directly to treat the cells for 1 h at 37°C in a 5% CO2 water-jacketed incubator. Heat-killed EPEC cells were obtained by boiling for 30 min.
Thiamin Uptake
Thiamin uptake by Caco-2 cells treated with EPEC was performed as described previously (31). Briefly, cells were incubated with Krebs-Ringer (K-R) buffer, which contained (in mM) 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES, pH 7.4. Unless otherwise stated, all incubations were performed at 37°C for 5 min. Labeled and unlabeled thiamin were added to the incubation medium at the onset of uptake experiment and the reaction was terminated by the addition of 2 ml of ice-cold K-R buffer followed by immediate aspiration. Cells were then rinsed twice with ice-cold buffer, lysed with 1 ml of 1 N NaOH, neutralized with 10 N HCl, and then counted for radioactivity. The protein content of cell digests was measured from the experimental and control wells using a Bio-Rad kit (Bio-Rad, Richmond, VA).
Real-Time PCR Analysis
Total RNA was isolated from confluent Caco-2 cells, those treated with EPEC and those treated with nonpathogenic E. coli using TRIzol (Life Technologies). Three micrograms of total RNA were reverse transcribed with oligo(dT) primers by using Superscript II (Life Technologies) according to the manufacturer's procedures. After the reverse transcription, all samples were diluted with sterile water and three different dilutions were used for each real-time PCR assay (QuantiTect SYBRgreen PCR Kit, Qiagen, Valencia, CA). Real-time PCR was carried out based on Light Cycler technology to accurately determine the level of expression of SLC19A2 and SLC19A3 in the Caco-2 cells treated with EPEC or nonpathogenic E. coli. Gene-specific primers corresponding to the PCR targets were designed by using the specifications given by the vendors (Bio-Rad). For the SLC19A2, the primers were forward, 5′-AGCCAGACCGTCTCCTTGTA-3′; reverse, 5′-TAGAGAGGGCCCACCACAC-3′. For the SLC19A3, the primers were forward, 5′-TTCCTGGATTTACCCCACTG-3′; reverse, 5′-GTATGTCCAAACGGGGAAGA-3′. For 18S rRNA, the primers were forward, 5′-GGGAGGTAGTGACGAAAAATAACAAT-3′; reverse, 5′-TTGCCCTCCAATGGATCCTC-3′. Each SYBRgreen reaction (20 μl total volume) contained 9 μl of diluted cDNA as template. The amplification program consisted of 1 cycle of 95°C with a 30-s hold (“hot start”) followed by 40 cycles of 95°C for 1 min, specified annealing temperature with 15-s hold, 72°C with 30-s hold for extension, and data acquisition. A melting curve analysis program run for one cycle at 95°C with 0-s hold, 65°C with 10-s hold, and 95°C with 0-s hold at the step acquisition mode followed amplification. A negative control without cDNA template was run with every assay to assess specificity. Data were normalized relative to 18S rRNA.
Promoter Activity: Transfection and Reporter Gene Assay
The full-length SLC19A2 and SLC19A3 promoter-luciferase reporter constructs generated and characterized previously in our laboratory (26, 28) were used in the present investigations. The human intestinal epithelial Caco-2 cells were cotransfected with 2 μg of each test construct and 100 ng of the Renilla transfection control plasmid Renilla luciferase-thymidine kinase (pRL-TK) (Promega, Madison, WI). After 24 h of transfection, cells were infected with EPEC as described above. Cells were then harvested and Renilla-normalized firefly luciferase activity was determined by using the Dual Luciferase Assay system (Promega).
Biotinylation of Cell Surface Proteins and Western Blot Analysis
Biotinylation of cell surface proteins was performed essentially as described by Zimnicka et al. (40) with some minor modifications. Briefly, Caco-2 monolayers infected with EPEC were exposed from apical surface with sulfo-NHS-SS-biotin (no. 21331, Pierce Biotechnology, Rockford, IL) diluted into a biotinylation buffer (PBS, pH 7.5, supplemented with 10 mM triethanolamine, 2 mM CaCl2, and 150 mM NaCl) to the working concentration of 1 mg/ml and then incubated for 1 h at 4°C in horizontal motion. Cells were then quenched with PBS containing CaCl2, MgCl2, and 100 mM glycine for 20 min at 4°C. Cells were lysed in lysis buffer (150 mM NaCl, 50 mM Tris·HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100). After centrifugation, the supernatant was incubated overnight in streptavidin agarose and then washed three times with lysis buffer. The streptavidin agarose beads were spun down, added with sample loading buffer for SDS-PAGE, and resolved on a 10% SDS-polyacrylamide premade gel (Invitrogen, Carlsbad, CA). After electrophoresis, the proteins were electroblotted onto an immunoblot polyvinylidene difluoride membrane (Bio-Rad) overnight, washed twice with PBS-Tween 20 for 10 min, and blocked with 5% dried milk in PBS-Tween 20. The membrane samples were then probed with either anti-human hTHTR-1-specific polyclonal antibodies (1:5,000 diluted in PBS-Tween 20) or anti-human hTHTR-2-specific polyclonal antibodies (1:5,000 diluted in PBS-Tween 20) as described by us previously (30). Blots were then washed twice with PBS-Tween 20 buffer (Sigma) and reacted with goat anti-rabbit IgG conjugated to horseradish peroxidase (1:2,500 diluted in PBS-Tween 20) for 1 h at room temperature. The blots were finally washed twice with PBS-Tween 20 for 10 min each time and developed by use of an enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ).
Statistical Analysis
Transport data are presented as means ± SE of multiple separate uptake determinations, which are expressed in terms of either femtomoles or picomoles per milligram protein per 5 min. Kinetic parameters of the saturable thiamin uptake processes [determined by subtracting the diffusing component (determined from the slope of the uptake line between a high pharmacological concentration of thiamin of 1 mM and the point of origin) from total uptake at each concentration] were calculated by using a computerized model of the Michaelis-Menten equation. Statistical analysis was performed by the Student's t-test or one-way ANOVA with statistical significance being set at 0.05. All real-time PCR and biotinylation-Western blot analyses were performed on at least three separate occasions with comparable results. Data presented are from a representative set of experiments. Promoter activity data are presented as means ± SE of at least three independent experiments and given as fold over pGL3-Basic expression that was set arbitrarily at 1.
RESULTS
Effect of Infecting Caco-2 cells With EPEC on Thiamin Uptake
We examined the effect of infecting Caco-2 cells with wild-type EPEC or with nonpathogenic E. coli HS4 (for 1 h at 100 MOI) on initial rate of thiamin (30 nM) uptake. In these studies, the infected Caco-2 cells were washed and either used directly for [3H]thiamin uptake investigations or incubated for an additional 6 h in the absence of EPEC and then used for uptake studies. When uptake was performed immediately after infection, significant (P < 0.01) inhibition (∼35%) in the initial rate of thiamin uptake was observed in Caco-2 cells treated with wild-type EPEC but not those treated with nonpathogenic E. coli (Fig. 1A). When thiamin uptake was assayed 6 h postinfection, a slightly higher degree of inhibition (∼48%; P < 0.01) was observed by the wild-type EPEC (but not with the nonpathogenic E. coli) (Fig. 1B), demonstrating persistency of the inhibition in postinfection.
Fig. 1.
Enteropathogenic Escherichia coli (EPEC) infection inhibits thiamin uptake in Caco-2 cells. Caco-2 cells at 5–6 days postplating were serum starved overnight and then infected with wild-type EPEC (EPEC-WT) or nonpathogenic E. coli (HS4) at 100 multiplicity of infection (MOI) for 1 h alone (A) or 1 h infection along with 6 h postincubation after EPEC removal (B). [3H]thiamin uptake was subsequently measured as described in methods. *P < 0.01 compared with control. When not shown, error bars are smaller than symbols.
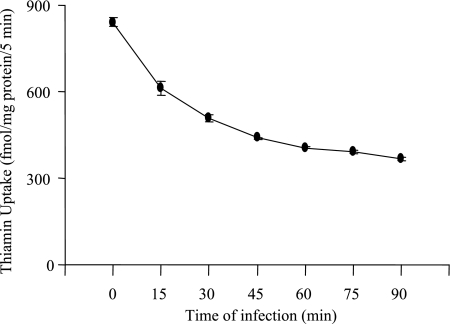
In another study, we investigated the effect of different treatment times (15, 30, 45, 60, 75, and 90 min) of Caco-2 cells with EPEC (100 MOI) on thiamin uptake. The results (Fig. 2) showed a progressive increase in the inhibitory effect of EPEC on thiamin uptake as a function of treatment time with the inhibition reaching a significant level as early as 15 min. Thiamin uptake was inhibited by ∼55% in Caco-2 cells treated for 90 min with EPEC.
Fig. 2.
Time course of EPEC effects on thiamin uptake. Caco-2 monolayers were infected with EPEC for 15, 30, 45, 60, 75, and 90 min, and [3H]thiamin uptake was measured. Values are means ± SE of 3–4 separate uptake determinations. When not shown, error bars are smaller than symbols.
Direct Contact of Live EPEC Is Necessary for the Inhibition of Thiamin Uptake
In these studies, Caco-2 cells were treated (for 1 h, 100 MOI) with wild-type EPEC, with heat-killed (boiled) EPEC, or with culture supernatant of EPEC on thiamin (30 nM) uptake. Uptake was examined 6 h postinfection. The results showed that whereas the wild-type EPEC significantly (P < 0.01) inhibit thiamin uptake, uptake was not affected in Caco-2 cells treated with boiled EPEC or with culture supernatant (Fig. 3). These results indicate that direct contact with live EPEC is needed for the inhibition in thiamin uptake to occur.
Fig. 3.
Direct contact of live EPEC is necessary for the inhibition of thiamin uptake by Caco-2 cells. Confluent monolayers of Caco-2 were treated (for 1 h) with wild-type EPEC, boiled EPEC, or culture supernatant of EPEC, and initial rate of thiamin was then examined. Results represent means ± SE of at least 3 separate experiments. *P < 0.01 compared with control.
Effect of EPEC Infection on Kinetic Parameters of Thiamin Uptake
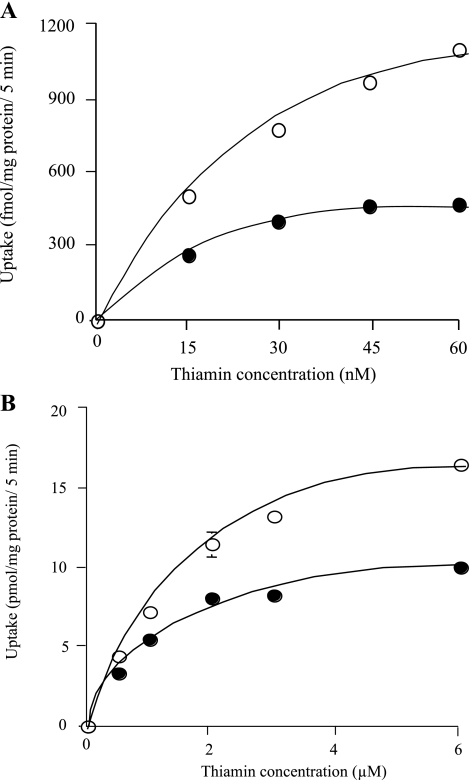
We have previously shown the existence of two thiamin-saturable uptake processes in intestinal epithelial Caco-2 cells; one operates at the nanomolar range and the other operates at the micromolar range (30, 31). In this study, we examined the effect of EPEC infection of Caco-2 cells on kinetic parameters of both of the thiamin uptake processes. The results showed that EPEC causes a considerable decrease in both the maximal velocity (Vmax) and apparent Michaelis constant (Km) of the nanomolar component (Vmax: 2,096 ± 779 and 460 ± 33 fmol·mg protein−1·5 min−1; apparent Km: 66 ± 22 and 7.82 ± 0.92 nM for control and EPEC-treated cells, respectively) (Fig. 4A). With regard to the micromolar saturable component, EPEC infection decreased the Vmax but not the apparent Km of the thiamin uptake process (Vmax: 23.86 ± 0.61 and 13.17 ± 1.14 pmol·mg protein−1·5 min−1; apparent Km: 2.14 ± 0.28 and 1.21 ± 0.05 μM, control and EPEC-treated cells, respectively; Fig. 4B).
Fig. 4.
EPEC infection inhibits thiamin uptake system that operates at the nanomolar (A) and micromolar (B) ranges. Confluent monolayer of Caco-2 cells was infected with wild-type EPEC (100 MOI for 1 h along with 6 h postincubation) followed by determination of initial rate of thiamin uptake at nanomolar and micromolar concentrations of thiamin. Each uptake data is the result of at least 3 separate experiments and is presented as means ± SE. Kinetic parameters of the saturable processes were determined by using a computerized model of the Michaelis-Menten equation. When not shown, error bars are smaller than symbols.
Effect of EPEC Infection on Cell Surface Expression of hTHTR-1 and hTHTR-2 Proteins
Studies from our laboratory have shown that both hTHTR-1 and hTHTR-2 are involved in the thiamin uptake by Caco-2 cells and both are involved in uptake across the apical membrane (30). Thus we examined the effect of EPEC infection on the levels of cell surface expression of hTHTR-1 and hTHTR-2 protein in Caco-2 cells by membrane biotinylation. Cell surface proteins extracted after biotinylation from control and infected cells were then analyzed by Western blotting using specific anti-hTHTR-1 and anti-hTHTR-2 polyclonal antibodies. As shown in Fig. 5, expression of hTHTR-1 and hTHTR-2 proteins in the membranous fraction of EPEC infected cells were significantly (P < 0.01) lower than that of uninfected controls or those infected with nonpathogenic E. coli (HS4). Densitometric scanning of the band intensities revealed a ∼55% decrease in the amount of hTHTR-1 (Fig. 5A) and ∼60% decrease in the amount of hTHTR-2 (Fig. 5B) in EPEC-infected cells compared with hTHTR-1 and hTHTR-2 in control and cells treated with nonpathogenic E. coli.
Fig. 5.
Effect of EPEC infection on human thiamin transporter-1 (hTHTR-1; A) and hTHTR-2 (B) protein levels in Caco-2 cells. Confluent monolayer of Caco-2 cells was infected with wild-type EPEC (100 MOI for 1 h along with 6 h postincubation) and subjected to surface biotinylation with sulfo-NHS-biotin at 4°C. Western blot analysis was performed with surface and total intracellular fractions by use of specific anti-hTHTR-1 and anti-hTHTR-2 polyclonal antibodies. Image and data shown are representative of 3 separate sets of experiments. *P < 0.01 compared with control.
Effect of EPEC Infection on mRNA Levels of hTHTR-1 and hTHTR-2
The effect of EPEC infection (100 MOI for 1 h followed by 6 h incubation in the absence of the bacteria) on hTHTR-1 and hTHTR-2 expression at mRNA levels in Caco-2 cells was also examined by real-time PCR analysis with the use of gene-specific primers corresponding to the sequences of hTHTR-1 and hTHTR-2. 18S rRNA was used as controls. The results showed the mRNA levels of both hTHTR-1 and hTHTR-2 were significantly (P < 0.01 for both) lower in EPEC-treated cells compared with controls (Fig. 6). It is interesting to note that the decrease in the level of hTHTR-2 was greater (∼3-fold) than that of hTHTR-1 (∼1.5-fold). No significant changes were observed in the levels of 18S rRNA in EPEC-treated and control cells.
Fig. 6.
Effect of EPEC infection on hTHTR-1 (A) and hTHTR-2 (B) mRNA levels in Caco-2 cells. Real-time PCR analysis was performed with the use of mRNA from Caco-2 cells treated with EPEC-WT and nonpathogenic E. coli (100 MOI for 1 h along with 6 h postincubation) and the hTHTR-1 and hTHTR-2 specific primers as described in methods. All experiments were run on at least 3 separate occasions. Results of a representative experiment are shown. Values are means ± SE. *P < 0.01, **P < 0.05 compared with control.
Effect of EPEC Infection on Activity of the SLC19A2 and SLC19A3 Promoters
The effect of EPEC infection (100 MOI for 1 h followed by 6 h incubation in the absence of the bacteria) on the activity of the SLC19A2 and SLC19A3 full-length promoters fused to luciferase reporter gene transfected into Caco-2 cells was examined in this study. The results showed the relative luciferase activity driven by both SLC19A2 and SLC19A3 promoters to be significantly (P < 0.05 for both) lower in EPEC-treated cells compared with controls as well as to those were treated with nonpathogenic E. coli (Fig. 7).
Fig. 7.
Effect of EPEC infection on SLC19A2 (left) and SLC19A3 (right) promoter levels in Caco-2 cells. Cells were transfected with SLC19A2 and SLC19A3-luciferase reporter plasmid constructs and a control pGL3-basic vector by use of Lipofectamine 2000. Cells were then infected (1 h) with wild-type EPEC and nonpathogenic E. coli (100 MOI) followed 6 h postincubation. Luciferase assay was performed as described in methods. Firefly luciferase activity was normalized relative to the activity of simultaneously expressed Renilla luciferase. Values represent means ± SE of a representative experiment performed in triplicate. *P < 0.05 compared with control.
Role of Specific EPEC Components in Causing the Inhibition in Intestinal Thiamin Uptake
As stated earlier, EPEC delivers several effector proteins into the host cell via a TTSS, which is comprised of a number of structural proteins that form molecular syringes for the translocation of the effector proteins into the host cells (8, 14). Here we examined the role of different structural components of the TTSS as well as the role of specific effector proteins in causing the inhibition in intestinal thiamin uptake using specific EPEC mutants.
Role of specific TTSS components.
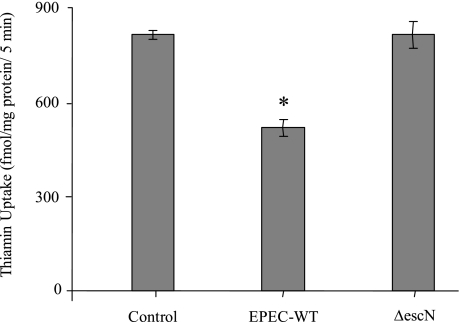
The functional contribution of TTSS and various EPEC structural proteins in the EPEC-induced inhibition in thiamin uptake by Caco-2 cells was investigated by using specific mutant strains. First, the requirement for TTSS (needed for the delivery of effector proteins into the host cells) was examined by use of the ΔescN mutant. EscN is a component of TTSS that encodes a putative ATPase that drives the entire type III secretion process. ΔescN mutant has a defective putative ATPase that cannot drive the secretion of various virulence proteins of type III secretion into host cells (14). As shown in Fig. 8, there was no inhibition in thiamin uptake by Caco-2 cells infected with the ΔescN mutant compared with those treated with wild-type EPEC.
Fig. 8.
Involvement of a functional type III secretion system (TTSS) of EPEC in the inhibitory effects of thiamin uptake. Caco-2 cells were infected with EPEC or ΔescN mutant strain at 100 MOI for 1 h followed by 6 h postincubation, and then [3H]thiamin uptake was measured. Results represent means ± SE of 3 independent experiments performed in triplicate. *P < 0.05 compared with control.
We further analyzed the role of several TTSS structural components in forming translocation apparatus (EspA and EspB) and forming pores (EspD) on host cell for injecting effector proteins in causing the inhibition in thiamin uptake by Caco-2 cells. For this, individual EPEC mutant carrying defect in particular structural component of TTSS (ΔespA, ΔespB, and ΔespD) was used. The results (Fig. 9) showed that infection of cells with these mutants had no effect on thiamin uptake compared with wild-type EPEC, suggesting that these structural components of the translocation apparatus are required for causing the inhibition in thiamin uptake.
Fig. 9.
Effect of EPEC type III structural proteins on thiamin uptake. Caco-2 cells were infected with EPEC or with one of the structural protein mutant strains of ΔespA, ΔespB, and ΔespD (100 MOI for 1 h along with 6 h postincubation) followed by measurement of [3H]thiamin uptake. Values are means ± SE of 3–4 separate uptake determinations. *P < 0.05 compared with control.
Role of specific effector proteins.
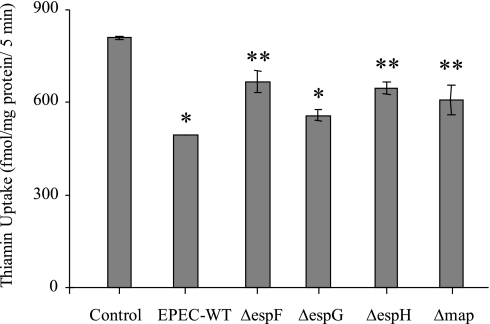
We also examined the role of EPEC effector proteins EspF, EspG, EspH, and Map in mediating the inhibitory effect on thiamin uptake by Caco-2 cells in these studies using specific mutant strains. The results (Fig. 10) showed that infecting Caco-2 cells with individual EPEC mutants caused different degrees of inhibition in thiamin uptake, although the effect was slightly less than that of wild-type EPEC.
Fig. 10.
Effect of EPEC type III effector proteins on thiamin uptake. Caco-2 cells were infected with EPEC or with one of the effector protein mutant strains of ΔespF, ΔespG, ΔespH, and Δmap (100 MOI for 1 h followed by 6 h postincubation) followed by measurement of [3H]thiamin uptake. Values are means ± SE of 3–4 separate uptake determinations. *P < 0.01, **P < 0.05 compared with control.
DISCUSSION
EPEC is a major cause of food-borne disease worldwide, causing severe diarrhea. EPEC infection modifies intestinal electrolyte and water transport (5, 15, 18), but its effect on absorption of essential micronutrients is not well defined. In this study we focused our attention on examining the effect of EPEC infection on intestinal uptake of the water-soluble vitamin thiamin. We used a well-validated human-derived intestinal epithelial cell model in our investigations. Our results showed that EPEC infection of the intestinal epithelial Caco-2 cells to lead a significant inhibition in thiamin uptake. This inhibition was increased with increasing the exposure time of Caco-2 cells to EPEC. More interestingly, the inhibition in thiamin uptake was found to be persistent (and actually increased) with time following removal of the bacteria and lasted for at least 6 h postinfection (the maximum time examined in this study). No inhibition in thiamin uptake was observed in Caco-2 cells that were exposed to the nonpathogenic E. coli (HS4). Furthermore, the inhibitory effect of wild-type EPEC on thiamin uptake required direct contact of live bacteria with intestinal epithelial Caco-2 cells as indicated by lack of inhibition in thiamin uptake by Caco-2 cells treated with boiled EPEC or with filtered bacterial culture supernatant. These findings demonstrate the specificity of the inhibitory effect on thiamin uptake to live pathogenic E. coli (EPEC) and shows that the inhibitory effect is persistent even after removal of the bacteria.
Previous studies from our laboratory have shown that intestinal thiamin uptake involves two saturable processes, one that operates at the nanomolar range (most likely mediated by hTHTR-2) and the other at the micromolar range (most likely mediated by hTHTR-1) (30, 31). EPEC infection appears to affect both these processes as indicated by the changes that occur in their transport kinetic parameters. In the case of the nanomolar uptake process, EPEC infection affected both the Vmax and the apparent Km of the uptake event. In the case of the micromolar uptake process, EPEC infection appeared to affect mainly the Vmax, with a lesser effect on apparent Km, of the uptake event.
With both hTHTR-1 and hTHTR-2 proteins being expressed in the human intestine at the apical membrane domain (30, 35), and with both being functionally involved in carrier-mediated thiamin uptake (30), we examined the effect of EPEC infection on level of expression of the hTHTR-1 and hTHTR-2 proteins at the cell surface using a membrane biotinylation approach followed by Western blotting. Our findings showed a marked decrease in expression of both hTHTR-1 and hTHTR-2 proteins at the apical cell membrane of confluent Caco-2 cells infected with EPEC compared with control cells and those cells that were exposed to the nonpathogenic E. coli. The latter finding may be the cause of the inhibition in thiamin uptake observed in the current investigation. Recent findings by others have shown that EPEC may also affect steady-state mRNA levels of proteins (27, 41). For this reason we also sought to investigate the effect of EPEC infection on mRNA levels of hTHTR-1 and hTHTR-2 using real-time PCR. Our results showed significant reduction in mRNA levels of both hTHTR-1 and hTHTR-2 as result of EPEC infection. The degree of inhibition in hTHTR-2 mRNA level was found to be greater than the inhibition in hTHTR-1 mRNA level, suggesting differential effect of EPEC on cellular RNA levels. Obviously a reduction in transcription rate could be one of the mechanisms that lead to a decrease in mRNA levels of a given protein, and thus we also examined the effect of EPEC infection on activity of SLC19A2 and SLC19A3 promoters (transfected into Caco-2 cells) and obtained evidence to show that EPEC infection does indeed inhibit the activity of both of these promoters. This inhibitory effect of EPEC on promoter activity of the thiamin transporters is in contrast to its stimulatory effect on the activity of the PepT1 promoter (27). From the above discussion, it appears that EPEC affect intestinal thiamin uptake process at different levels. First the bacteria appear to act at the level expression of the thiamin transporters at the apical membrane domain of intestinal epithelial cells, an effect that appears to persist even after removal of the bacteria. This inhibitory effect could then be prolonged (and compounded) by inhibition in mRNA levels of the thiamin uptake systems, which appears to be mediated (at least in part) via a decrease in transcriptional activity of SLC19A2 and SLC19A3 promoters. Further studies, however, are needed to confirm the latter possibility.
Following delineation and characterization of effect of EPEC on thiamin uptake by Caco-2 cells, we examined the bacterial structural and secretory requirements that are needed to induce the observed inhibition in thiamin uptake. As mentioned earlier, EPEC possess functional TTSS, which is comprised of a number of structural proteins that forms molecular syringes for the translocation of the effector proteins into the host cells and is dependent on translocating intact ATPase (EscN) (14). Thus we examined the requirement for an intact EPEC TTSS in exerting the inhibition in thiamin uptake. We used an ATPase defective EPEC mutant (ΔescN) for this purpose. The results showed lack of inhibition in thiamin uptake by Caco-2 cells with this mutant, indicating a requirement for an intact TTSS in causing the observed inhibition. We also examined the role of several TTSS structural components in forming translocation apparatus (EspA and EspB) and in forming pore (EspD) on host cell for injecting effector proteins in causing the inhibition in thiamin uptake by Caco-2 cells. Again we used mutants that lack such structural proteins and obtained results that show lack of inhibition in thiamin uptake, suggesting importance of these structural components in mediating EPEC inhibitory effect on thiamin uptake. In other studies, we tested the effect of the EPEC-secreted effector molecules EspF (known to disrupt tight junctions; Ref. 25), EspG (disrupts microtubules and produce subtle alterations in barrier functions; Ref. 37), EspH (known to alter pedestal morphology and filopodia formation; Ref. 38), and Map (reported to alter mitochondrial membrane potential; Ref. 20) in causing the observed inhibition in thiamin uptake. We used EPEC mutants that lack the ability to produce these effector molecules. The results showed that although these mutants continue to cause marked inhibition in thiamin uptake compared with untreated Caco-2 cells, the degree of inhibition was significantly lower than that observed with wild-type EPEC. These findings suggest that these secreted effecter molecules contribute, yet to different degree, in causing the inhibition in thiamin uptake.
In summary, our studies demonstrate for the first time that EPEC infection of human intestinal epithelial cells leads to a significant inhibition in thiamin uptake. Furthermore, the studies show that whereas the initial inhibitory effect is due to a decrease in the level of expression (and possibly activity) of hTHTR-1 and hTHTR-2 protein at the apical membrane, the inhibition could then be prolonged (and compounded) by the inhibitory effect of EPEC on hTHTR-1 and hTHTR-2 mRNA levels that is mediated in part via a decrease in the activity of the SLC19A2 and SLC19A3 promoters. Moreover, the inhibition in intestinal thiamin uptake caused by EPEC is dependent on existence of an intact TTSS system.
GRANTS
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK58057 and DK56061 to H. M. Said; DK050694 and DK067887 to G. A. Hecht) and the Department of Veterans Affairs.
REFERENCES
- 1.Babei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 52: 2110–2120, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Berdanier CD. Micronutrients. In: Advanced Nutrition Boca Raton, FL: CRC, 1998 [Google Scholar]
- 3.Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol 290: G30–G35, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol 58: 946–958, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Collington GK, Booth IW, Knutton S. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut 42: 200–207, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean P, Maresca M, Schuller S, Phillips AD, Kenny B. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc Natl Acad Sci USA 103: 1876–1881, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz GA, Banikazemi M, Oishi K, Desnick RJ, Gelb BD. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anaemia syndrome. Nat Genet 22: 309–312, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg MS, Kaper JB. Enteropathogenic E coli. Infect Immun 60: 3953–3961, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudeja PK, Tyagi S, Kavilaveettil RJ, Gill R, Said HM. Mechanism of thiamin uptake by human jejunal brush-border membrane vesicles. Am J Physiol Cell Physiol 281: C786–C792, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Fagundes U, Andrade J. Acute diarrhea and malnutrition: lethality risk in hospitalized infants. J Am Coll Nutr 18: 303–308, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Fagundes U, Scaletsky IC. The gut at war: the consequences of enteropathogenic Escherichia coli infection as a factor of diarrhea and malnutrition. Sao Paulo Med J 118: 21–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming JC, Tartaglini E, Steinkamp MP, Schorderet DF, Cohen N, Neufeld EJ. The gene mutated in thiamine-responsive anemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet 22: 305–308, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Frederikse PH, Farnsworth P, Zigler JS., Jr Thiamin deficiency in vivo produces fiber cell degeneration in mouse lenses. Biochem Biophys Res Commun 258: 703–707, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Gauthier A, Puente JL, Finlay BB. Secretion of the enteropathogenic E. coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect Immun 71: 3310–3319, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerrant RL, Oriá RB, Moore SR, Oriá MOB, Lima AAM. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 66: 487–505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemia damage and prevents experimental diabetic retinopathy. Nat Med 9: 294–299, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, Ramaswamy K, Dudeja PK. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 287: G370–G378, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hecht G, Marrero JA, Danilkovich A, Matkowskyj KA, Savkovic SD, Koutsouris A, Benya RV. Pathogenic Echerichia coli increase Cl− secretion from intestinal epithelia by upregulation galanin-1 receptor expression. J Clin Invest 104: 253–262, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenny B, Jepson M. Targeting of an EPEC effector protein to host mitochondria. Cell Microbiol 2: 579–590, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Laforenza U, Patrini C, Alvisi C, Faelli A, Licandro A, Rindi G. Thiamine uptake in human intestinal biopsy specimens including observations from a patient with acute thiamine deficiency. Am J Clin Nutr 66: 320–326, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Levine MM, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev 6: 31–51, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Matsushima K, MacManus JP, Hakim AM. Apoptosis is restricted to the thalamus in thiamin-deficient rats. Neuroreport 8: 867–870, 1997 [PubMed] [Google Scholar]
- 24.McDaniel TK, Kaper JB. A cloned pathogenicity island from enteropathogenic E. coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol 23: 399–407, 1997 [DOI] [PubMed] [Google Scholar]
- 25.McNamara BP, Koutsouris A, O'Connell CB, Nougayrede JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic E. coli disrupts host intestinal barrier function. J Clin Invest 107: 621–629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen HTT, Dalmasso G, Yan Y, Powell K, Bhatt S, Kalman D, Gewirtz AT, Sitaraman SV, Merlin D. Pathogenic bacteria transcriptionally induce colonic PepT1 expression—an implication in modulating bacterial-epithelial interaction (Abstract). Gastroenterology 136: A–87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reidling JC, Subramanian VS, Dudeja PK, Said HM. Expression and promoter analysis of SLC19A2 in the human intestine. Biochim Biophys Acta 1561: 180–187, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Rindi G, Laforenza U. Thiamin intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med 224: 246–255, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Said HM, Balamurugan K, Subramanian VS, Marchant JS. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am J Physiol Gastrointest Liver Physiol 286: G491–G498, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Said HM, Ortiz A, Kumar C, Chatterjee N, Dudeja P, Rubin S. Transport of thiamine in the human intestine: mechanism and regulation in an intestinal epithelial cell model Caco-2. Am J Physiol Cell Physiol 277: C645–C651, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Spitz J, Yuhan R, Koutsouris C, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol Gastrointest Liver Physiol 268: G374–G379, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Stagg AR, Fleming JC, Baker MA, Sakamoto M, Cohen N, Neufeld EJ. Defective high-affinity thiamin transporters lead to cell death in thiamin-responsive megaloblastic anemia syndrome fibroblasts. J Clin Invest 103: 723–729, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian VS, Marchant JS, Parker I, Said HM. Cell biology of the human thiamin transporter-1 (hTHTR1): intracellular trafficking and membrane targeting. J Biol Chem 278: 3976–3984, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamin transporter-2 (hTHTR2) in epithelial cells. J Biol Chem 281: 5233–5245, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Todd K, Butterworth RF. Mechanisms of selective neuronal cell death due to thiamine deficiency. Ann NY Acad Sci 893: 404–411, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Tomson FL, Viswanathan VK, Kanack K, Kanteti RP, Straub KV, Menet M, Kaper JB, Hecht G. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol Microbiol 56: 447–464, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Tu X, Nisan I, Yona C, Hanski E, Rosenshine I. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic E. coli. Mol Microbiol 47: 595–606, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition Philadelphia, PA: Davis, 1989 [Google Scholar]
- 40.Zimnicka AM, Maryon EB, Kaplan JH. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem 282: 26471–26480, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 Zo-2 and PKC redistribution in tight junction and epithelial barrier repair. Cell Microbiol 9: 804–816, 2007 [DOI] [PubMed] [Google Scholar]