Abstract
Glucagon-like peptide-2 (GLP-2) is an important neuroendocrine peptide in intestinal physiology. It influences digestion, absorption, epithelial growth, motility, and blood flow. We studied involvement of GLP-2 in intestinal mucosal secretory behavior. Submucosal-mucosal preparations from guinea pig ileum were mounted in Ussing chambers for measurement of short-circuit current (Isc) as a surrogate for chloride secretion. GLP-2 action on neuronal release of acetylcholine was determined with ELISA. Enteric neuronal expression of the GLP-2 receptor (GLP-2R) was studied with immunohistochemical methods. Application of GLP-2 (0.1–100 nM) to the serosal or mucosal side of the preparations evoked no change in baseline Isc and did not alter transepithelial ionic conductance. Transmural electrical field stimulation (EFS) evoked characteristic biphasic increases in Isc, with an initially rapid rising phase followed by a sustained phase. Application of GLP-2 reduced the EFS-evoked biphasic responses in a concentration-dependent manner. The GLP-2R antagonist GLP-2-(3-33) significantly reversed suppression of the EFS-evoked responses by GLP-2. Tetrodotoxin, scopolamine, and hexamethonium, but not vasoactive intestinal peptide type 1 receptor (VPAC1) antagonist abolished or reduced to near zero the EFS-evoked responses. GLP-2 suppressed EFS-evoked acetylcholine release as measured by ELISA. Pretreatment with GLP-2-(3-33) offset this action of GLP-2. In the submucosal plexus, GLP-2R immunoreactivity (-IR) was expressed in choline acetyltransferase-IR neurons, somatostatin-IR neurons, neuropeptide Y-IR neurons, and vasoactive intestinal peptide-IR neurons. We conclude that submucosal neurons in the guinea pig ileum express GLP-2R. Activation of GLP-2R decreases neuronally evoked epithelial chloride secretion by suppressing acetylcholine release from secretomotor neurons.
Keywords: enteric nervous system, gastrointestinal hormones, intestine, mucosal secretion
the digestive tract uses neuroendocrine peptides in multiple signaling functions. They may function as neurotransmitters, hormones, or mediators released in paracrine fashion (7). One such peptide is glucagon-like peptide-2 (GLP-2), which is a 33-amino acid peptide secreted by intestinal endocrine L cells in response to nutrient intake (37). It acts as a hormone that has multifaceted signaling functions, which include 1) action as a mucosal trophic factor that increases bowel weight and nutrient absorption; 2) enhanced capacity for carbohydrate, amino acid, and lipid absorption due to augmented activity of epithelial brush-border enzymes and nutrient transporters; 3) enhanced enterocyte proliferation and suppression of crypt cell death; 4) restoration of epithelial growth following periods of fasting; 5) maintenance of mucosal integrity by enhancement of intestinal barrier function and decrease in transcellular and paracellular epithelial permeability; 6) suppression of gastric acid secretion and motility; and 7) action as an anti-inflammatory agent, which reduces mucosal inflammatory cytokine production, crypt cell proliferation, and apoptosis (4, 5, 10–14, 23, 28, 30, 32, 33, 37).
Results from Phase II and III trials suggest that GLP-2 action, as a growth factor, has therapeutic potential for short bowel syndrome and gastrointestinal disorders associated with mucosal damage (e.g., inflammatory bowel disease) (38). Improvement of bowel adaptation after resection may account in part for GLP-2 efficacy to enhance intestinal absorption and thereby reduce patient dependence on parenteral nutrition. The action of GLP-2 is initiated by binding to a specific receptor. Receptor activity is terminated by enzymatic action of dipeptidyl peptidase IV, which starts at the NH2-terminal and generates the inactive truncated fragment, GLP-2-(3-33) (12). Therapeutic strategies include treatment with enzyme-resistance analogs, one of which is GLP-2 (h[Gly 2] GLP-2), known as teduglutide or GATTEX. Efficacy of teduglutide was found in placebo-controlled Phase III trials (21, 25, 34). A second strategy, which has shown promise in preclinical models of intestinal inflammatory damage, is to prolong the action of GLP-2 at its receptor by inhibiting dipeptidyl peptidase IV (38).
GLP-2 receptor.
The GLP-2 receptor (GLP-2R) is a G protein-coupled receptor expressed mainly in the gut and brain. Nevertheless, the exact cellular localization, particularly in the intestine, is unresolved. Others reported expression of GLP-2R in human enteroendocrine cells (39) and in human and rodent subepithelial myofibroblasts (28). Mice express messenger RNA for GLP-2R in enteric neurons, but not the intestinal epithelium (3). In human and porcine intestine, GLP-2R localizes to vasoactive intestinal peptide (VIP)-immunoreactive neurons and to enteroendocrine cells (20).
Epithelial chloride secretion.
Data for effects of GLP-2 on mucosal chloride secretion are generally unavailable. Chloride secretion receives attention because it is a major determinant for movement of NaCl and H2O across the mucosal epithelium into the intestinal lumen. Opening of chloride channels elevates the intraluminal concentration of chloride followed by movement of Na+ down its electrochemical gradient. As NaCl accumulates in the lumen, water moves across the mucosa down the osmotic gradient and this increases the liquidity of the intestinal contents. The enteric nervous system regulates chloride secretion (7). We aimed to test the hypothesis that GLP-2 action on neurons in the submucosal division of the enteric nervous system is a factor in regulation of chloride secretion.
MATERIALS AND METHODS
Tissue preparation.
Male albino Hartley guinea pigs weighing 300–400 g were killed by stunning and exsanguination from the cervical vessels according to procedures reviewed and approved by the Ohio State University Laboratory Animal Care and Use Committee and the United State Department of Agriculture inspectors.
Segments of the ileum were removed and placed in ice-cold Krebs solution containing (in mM) 120.9 NaCl, 5.9 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 14.4 NaHCO3, 2.5 CaCl2, and 11.5 glucose. The segments were opened along the mesenteric border, the intraluminal contents were removed, and the segments were stretched tautly and pinned flat in a Sylgard-lined Petri dish. For immunohistochemical studies, the tissues were immersed in Zamboni's fixative (4% formaldehyde plus 0.2% picric acid in 0.1 M sodium phosphate buffer, pH 7.0) for 3 h at room temperature. After fixation, the tissues were washed three times for 10 min each time in phosphate-buffered saline (PBS; 0.9% NaCl in 0.1 M sodium phosphate buffer, pH 7.0). Whole mounts of the submucosal plexus were obtained from these segments. To enhance immunostaining for vasoactive intestinal peptide (VIP) in ganglion cell somas, some preparations were incubated in Dulbecco's modified Eagle's medium, containing 40 μM colchicine at 37°C for 4 h prior to fixation. After incubation, the tissues were fixed and processed as described above.
Ussing chamber methods.
The muscularis externa, together with the myenteric plexus, was removed by microdissection to obtain flat-sheet preparations for the Ussing chamber studies. The submucosal plexus remained intact with the mucosa. The flat-sheet preparations of the submucosa/mucosa were mounted between halves of Ussing flux chambers, which had a total cross-sectional area of 0.64 cm2. The tissues were bathed on both sides, with 10 ml of oxygenated (95% O2 and 5% CO2) Krebs solution, maintained at 37°C by circulation from a temperature controlled water bath. Each Ussing chamber was equipped with a pair of Ag-AgCl electrodes connected via Krebs-agar bridges to calomel half-cells for measurement of transmural potential difference (PD). A second pair of electrodes was connected to an automated voltage-clamp amplifier to inject the required short-circuit current (Isc) to maintain a zero PD between the PD-sensing bridges. The current necessary to change the transepithelial PD by 2.5 mV was used to monitor tissue conductance, calculated according to Ohm's law, as a determinant of tissue viability. Isc, measured in microamperes per square centimeter, was monitored by a voltage-clamp apparatus (DVC-1000; World Precision Instruments, Sarasota, FL) and displayed on a chart recorder (Dash IV; Astro-Med, West Warwick, RI). Electrical current passed between pairs of aluminum foil electrodes placed on the submucosal surface of the tissue at the intersection between the two halves of the Ussing chamber activated the submucosal neurons in the preparations (8). Grass SD 88 stimulators (Grass Instruments, Quincy, MA) supplied electrical current to the electrodes. The stimulus parameters were stimulus strength 1–5 mA, frequency 10 Hz, pulse duration 0.5 ms, total stimulus duration 90 s. Measurements of the change in Isc during electrical stimulation were calculated as the difference between the peak response and baseline Isc before stimulation.
Experimental protocol.
We allowed the preparations to equilibrate for 20 min at 37°C before application of electrical field stimulation (EFS), after which stimulation at 5-min intervals evoked reproducible responses, and then repeated the stimulation in the presence of increasing cumulative concentrations of GLP-2 (0.1–100 nM). The incubation time for each concentration was 7 min. In a different series of experiments, GLP-2 was tested 30 min after placement of GLP-2-(3-33) (10 nM) in the Ussing chambers. GLP-2-(3-33) is a murine GLP-2R antagonist (31). Following discovery that GLP-2 suppressed EFS-evoked secretory responses, we tested GLP-2 in the presence of the muscarinic receptor antagonist scopolamine (1 μM), the nicotinic receptor antagonist hexamethonium (100 μM), and a selective antagonist of VIP type 1 (VPAC1) receptor (1 μM). These agents were added 20 min before addition of GLP-2.
ACh release.
We studied acetylcholine (ACh) release from flat-sheet preparations of submucosa/mucosa mounted between the halves of Ussing chambers. Each half-chamber was filled with agar to reduce the volume to 300 μl. Electrode and fluid ports were blocked with agar. The preparations were incubated with GLP-2 alone, GLP-2 and GLP-2-(3-33) applied in combination, or GLP-2-(3-33) alone for 30 min followed by EFS, as described above. The contents of the chambers were collected immediately at the end of stimulation and stored at −20°C for subsequent analysis of ACh release.
Immunofluorescence.
Table 1 lists sources for all primary and secondary antibodies and the optimal dilutions used in the immunohistochemical study. Whole-mount preparations of submucosal plexus were placed in PBS containing 10% normal donkey serum and 0.3% Triton X-100 for 30 min at room temperature to minimize nonspecific binding and increase tissue permeability. The tissues were incubated overnight at 4°C in primary antibodies for GLP-2R or a mixture of primary antibodies (Table 1) for double labeling. Competition with the control peptide (cat. no. LS-P1312, Lifespan Biosciences) established antibody specificity for GLP-2R. The GLP-2R antibody was preabsorbed with the control peptide for 2 h and then used for immunohistochemical localization of the receptor. After being washed with PBS, the tissues were incubated with a single secondary antibody or a mixture of appropriate secondary antibodies conjugated with fluorescein isothiocyanate (FITC) or indocarbocyanin (Cy3), at room temperature, for 1 h followed by washing in PBS and mounting with VECTASHIELD (Vector, Burlingame, CA). Fluorescence labeling was examined with a Nikon Eclipse 90i fluorescence microscope (Nikon Instruments, Melville, NY). Photomicrographs were acquired with a Cool Snap HQ2 monochrome digital camera and MetaMorph software (Molecular Devices, Sunnyvale, CA), stored on disk, and analyzed with MetaMorph. Images were minimally adjusted for brightness and contrast by using MetaMorph. Immunoreactivity (IR) for the GLP-2R and enteric neurochemical codes and numbers of double-labeled cells were assessed in randomly chosen ganglia situated throughout the preparations.
Table 1.
Codes and sources of primary and secondary antibodies
| Antigen | Host | Code | Dilution | Source |
|---|---|---|---|---|
| Anti-Hu | Mouse | A212-71 | 1:50 | Molecular Probes |
| GLP-2R | Rabbit | LS-A1312 | 1:200 | Lifespan Biosciences |
| GLP-2 peptide | Rabbit | LS-P1312 | 1:100 | Lifespan Biosciences |
| ChAT | Goat | AB144P | 1:100 | Chemicon |
| NPY | Sheep | AB1583 | 1:1500 | Chemicon |
| Somatostatin | Rat | MAB354 | 1:200 | Chemicon |
| VIP | Sheep | AB1581 | 1:1000 | Chemicon |
| Calretinin | Goat | AB1550 | 1:2500 | Chemicon |
| Rabbit IgG | Donkey Cy3 | 711-165-152 | 1:500 | Jackson |
| Mouse IgG | Donkey FITC | 715-095-150 | 1:100 | Jackson |
| Goat IgG | Donkey FITC | 713-095-003 | 1:100 | Jackson |
| Sheep IgG | Donkey FITC | 713-095-147 | 1:100 | Jackson |
| Rat IgG | Donkey FITC | AP189F | 1:100 | Chemicon |
Anti-Hu: anti-human neuronal protein; GLP-2R, glucagon-like peptide (GLP-2) receptor; ChAT, choline acetyltransferase; NPY, neuropeptide Y; VIP, vasoactive intestinal peptide.
Chemicals.
The following drugs were used and stock solutions were prepared in distilled H2O or dimethyl sulfoxide. Scopolamine and hexamethonium bromide were purchased from Sigma (St. Louis, MO), tetrodotoxin from Alomone Labs (Jerusalem, Israel), GLP-2 from Tocris (Elisville, MO), GLP-2-(3-33) from California Peptide Research (Napa, CA), and VPAC1 receptor antagonist [Ac-His1, d-Phe2, Lys15, Arg16, Leu27]-VIP(3-7)-GRF(8–27) from Phoenix Pharmaceuticals. GLP-2 and GLP-2-(3-33) were dissolved in dimethyl sulfoxide (DMSO, 0.1% final concentration). Application of DMSO alone did not alter basal Isc or responses to EFS as reported earlier (15).
Data analysis and statistics.
Preparations from the same animal were studied simultaneously in four Ussing chamber setups. Data are expressed as means ± SE; n values refer to the number of animals. Statistical significance was determined with paired Student's t-test between control and experimental populations or ANOVA followed by Bonferroni post hoc test, when appropriate. Differences were considered significant at P < 0.05.
Labeled neurons in 30 submucosal ganglia were counted in the immunohistochemical studies. Total number of neurons labeled with a specific neuronal marker and the percent overlap of those markers with GLP-2R were determined.
RESULTS
Baseline Isc was 145.4 ± 2.5 μA/cm2 (n = 15) and the corresponding conductance was 36.8 ± 1 mS/cm2. Application of GLP-2 (0.1–100 nM) to the serosal or mucosal side of the preparations evoked no change in the baseline Isc (143.8 ± 1.9 μA/cm2, n = 8, P > 0.05) and did not alter the total tissue conductance (35.6 ± 1.2 mS/cm2, n = 8, P > 0.05).
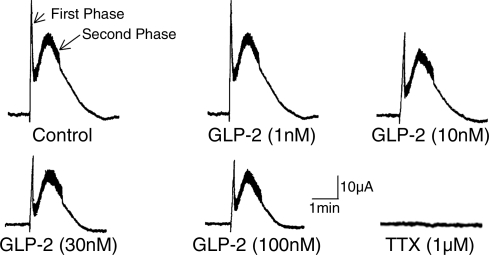
EFS evoked a biphasic increase in Isc, which consisted of a spikelike rapid rise followed by a second sustained response (Fig. 1). Tetrodotoxin (100 nM, n = 3) abolished both phases of the EFS-evoked responses (Fig. 1). Blockade of the EFS-evoked responses by tetrodotoxin was evidence that the responses were neurally mediated.
Fig. 1.
Electrical field stimulation (EFS) evoked increases in short-circuit current (Isc), which consisted of a spikelike first phase and a delayed second phase. The first and second phases represent cholinergically evoked and putative peptidergic/cholinergic-evoked chloride secretion, respectively. glucagon-like peptide-2 (GLP-2), applied in the serosal compartment of the Ussing chamber, suppressed the first and second phases in a concentration-dependent manner. Tetrodotoxin (TTX) abolished both the first phase and the delayed second phase.
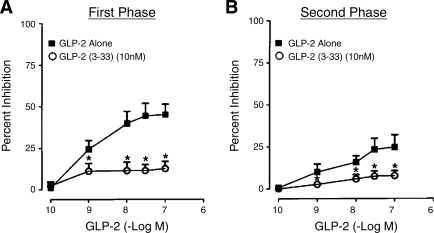
GLP-2 (0.1–100 nM), added to the serosal side of the chamber, produced a concentration-dependent reduction in the first and second phases of the EFS-evoked response (Figs. 1 and 2). This action of GLP-2 (0.1–100 nM) was suppressed by the GLP-2R antagonist GLP-2-(3-33) (Fig. 2).
Fig. 2.
Cumulative concentration-response curves for the inhibitory action of GLP-2 on the first and second phases of Isc evoked by EFS. A: inhibition of the first phase of EFS-evoked Isc by GLP-2 alone (▪) or in the presence of the antagonist GLP-2-(3-33) (○). B: inhibition of the second phase of EFS-evoked Isc by GLP-2 alone (▪) or in the presence of the antagonist GLP-2-(3-33) (○). Data are expressed as percent inhibition of EFS-evoked Isc by GLP-2. Data points are means ± SE. Each mean is data for preparations from 6 animals. *P < 0.05 for GLP-2-(3-33) relative to GLP-2 alone.
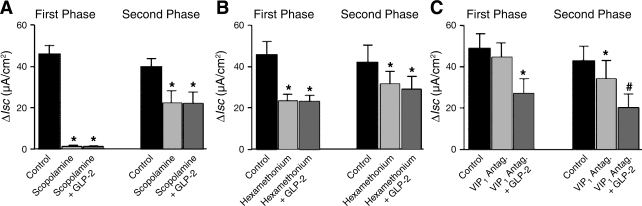
Application of the muscarinic receptor antagonist scopolamine (1 μM) alone abolished the first phase and significantly reduced the second phase of the EFS-evoked responses. In the presence of scopolamine, GLP-2 in a concentration range of 0.1–100 nM failed to suppress further the first and second phases of the EFS-evoked secretory responses (Fig. 3A).
Fig. 3.
Pharmacology for action of GLP-2 on first and second phases of neurally mediated Isc responses to transmural EFS. A: GLP-2 (10 nM) applied in the presence of the muscarinic receptor antagonist scopolamine (1 μM). B: GLP-2 (10 nM) applied in the presence of the nicotinic receptor antagonist hexamethonium (100 μM). C: GLP-2 (10 nM) applied in the presence of the vasoactive intestinal peptide (VIP) receptor antagonist VPAC1 (1 μM). ΔIsc is the difference between baseline current and evoked current. Data points are means ± SE. Each mean represents data for ileal preparations from 5 guinea pigs. *P < 0.05 for GLP-2 relative to control; #P < 0.05 for GLP-2 relative to VPAC1.
Exposure to 100 μM hexamethonium, which is a nicotinic receptor antagonist, reduced both the first and the second phase of the EFS-evoked responses. In the presence of hexamethonium, GLP-2 (0.1–100 nM) failed to suppress further the first and second phases of the EFS-evoked secretory responses (Fig. 3B).
The presence of the VIP receptor antagonist VPAC1 (1 μM) in the bathing medium on the serosal side of the preparation did not modify the first phase but reduced significantly the second phase of the EFS-evoked responses. In the presence of VIPAC1, GLP-2 (0.1–100 nM) continued to suppress both the first and second phases of the EFS-evoked secretory responses (Fig. 3C).
ACh release.
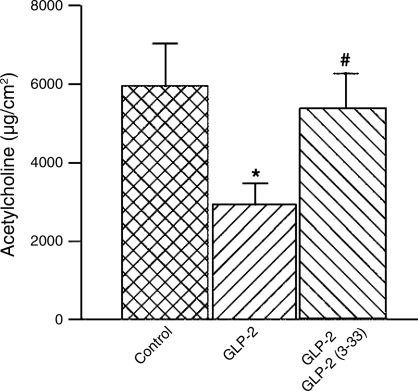
The results with tetrodotoxin and cholinergic receptor antagonists suggested that GLP-2 acted to suppress neuronal release of ACh from secretomotor neurons. We tested this by measuring the amount of ACh released from the submucosal-mucosal preparations by EFS in the absence or presence of GLP-2. The presence of GLP-2 (10 nM) significantly reduced the released ACh (Fig. 4). Pretreatment with 10 nM GLP-2-(3-33) reduced suppression of ACh release by GLP-2 to near zero (Fig. 4).
Fig. 4.
Release of acetylcholine evoked by transmural EFS. GLP-2 (10 nM), when applied alone in the serosal compartment of the Ussing chamber, suppressed neural release of acetylcholine evoked by transmural EFS. Coapplication of the GLP-2 receptor antagonist GLP-2-(3-33) (10 nM) with GLP-2 (10 nM) reversed suppression by GLP-2 of acetylcholine release during transmural EFS. Data points are means ± SE.
Immunofluorescence.
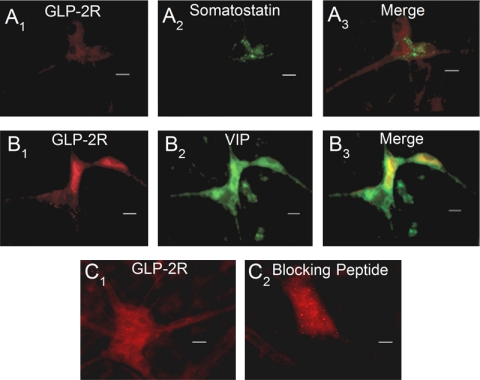
We used immunohistochemical staining to localize GLP-2R in whole-mount preparations of the submucosal plexus and found that ganglion cell bodies expressed IR for GLP-2R at the cell surface (Fig. 5A). Preabsorption of the anti-GLP-2R antibody with the immunogen (i.e., synthetic peptide-keyhole limpet hemocyanin conjugated) quenched all immunofluorescence (Fig. 6C).
Fig. 5.
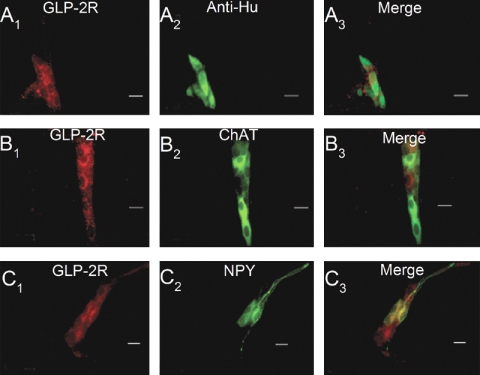
Expression of GLP-2R immunoreactivity (IR) in whole mounts of guinea pig small intestinal submucosal plexus. A1–3: coexpression of GLP-2R-IR with anti-Hu-IR, which marks all enteric neurons, reveals expression GLP-2R-IR restricted to neuronal cell bodies. B1–3: coexpression of GLP-2R-IR with choline acetyltransferase (ChAT-IR), which is a neurochemical marker for secretomotor neurons. C1–3: coexpression of GLP-2R-IR with neuropeptide Y (NPY-IR), which is a neurochemical marker for secretomotor/nonvasodilator motoneurons when coexpressed with ChAT-IR. Scale bars = 20 μm.
Fig. 6.
Expression of GLP-2R IR in whole mounts of guinea pig small intestinal submucosal plexus. A1–3: coexpression of GLP-2R-IR with somatostatin-IR, which is a neurochemical marker for interneurons that provide inhibitory input to secretomotor neurons. B1–3: coexpression of GLP-2R-IR with vasoactive intestinal peptide (VIP-IR), which is a neurochemical marker for noncholinergic secretomotor/vasodilator motoneurons. C1: GLP-2R-IR. C2: GLP-2R-IR after preabsorbing the antibodies with the corresponding blocking peptide provided by the manufacturer.
IR for HuC/D is a convenient panneuronal marker for all enteric neurons (24). Double labeling with anti-Hu showed that GLP-2R-IR nerve cell bodies accounted for about one-half of the total population of anti-Hu-IR neurons in the submucosal plexus (Fig. 5, A–C; Table 2). Double-label immunohistochemistry for enteric neurochemical codes identified the major classes of ganglion cells that expressed GLP-2R-IR. GLP-2R-IR was expressed by neurons that expressed IR for choline acetyltransferase, which is a neurochemical code for cholinergic secretomotor/vasodilator motoneurons (Fig. 5, D–F; Table 2) (17, 18). About three-fourths of the neurons with IR for neuropeptide Y coexpressed GLP-2R-IR (Fig. 5, G–I; Table 2). Neuropeptide Y is a code for cholinergic secretomotor/nonvasodilator motoneurons in the guinea pig submucosal plexus (17, 18). More than half of the neurons with IR for somatostatin coexpressed GLP-2R-IR (Fig. 5, G–I; Table 2). Somatostatinergic neurons in the guinea pig submucosal plexus provide inhibitory synaptic input to secretomotor neurons in the guinea pig submucosal plexus (30). Fewer than half of the neurons with IR for VIP expressed GLP-2R-IR (Fig. 5, O–Q; Table 2). Guinea pig submucosal neurons, which express VIP-IR, belong to the noncholinergic secretomotor/vasodilator class of submucosal neurons (17, 18). A much smaller proportion (1%) of the submucosal neurons that expressed calretinin-IR also expressed GLP-2-IR (Table 2).
Table 2.
GLP-2R-IR in relation to chemical codes
| Chemical Code | GLP-2R/Chemical Code |
|---|---|
| Anti-Hu | 51.7±6.1% |
| ChAT | 61.5±7.9% |
| NPY | 71.4±0% |
| Somatostatin | 61.8±3.3% |
| VIP | 40.9±7.0% |
| Calretinin | 1.0±1.2% |
Data are means ± SE from 4 preparations. IR, immunoreactivity.
DISCUSSION
We studied GLP-2 because it is an important factor in the regulation of the morphology, function, and integrity of the intestinal mucosa and it is emerging as a novel therapeutic modality in a variety of conditions associated with intestinal injury (33, 34, 38). We aimed to investigate, in vitro, a putative role for GLP-2 in enteric nervous regulation of intestinal secretion.
Ussing chamber methods revealed no changes in baseline Isc or the corresponding conductance during application of GLP-2, which suggests that it does not change ionic channel conductance or mucosal barrier function when applied in vitro. This disagrees with earlier reports of others that GLP-2 reduced passive permeability to ions and restricted transmucosal movement of macromolecules, in vivo, in chronic animal models for food allergy, stress, or diabetes (2, 5, 6, 20). Our study in vitro differs from the in vivo studies reported by others and might account for the discrepancy. Chronic administration of GLP-2 in vivo reportedly tightens the mucosal barrier to movement of ions and macromolecules, whereas acute application of GLP-2 in our Ussing chamber studies in vitro did not change Isc or ionic conductance.
Our finding that functional classes of neurons in the submucosal plexus expressed GLP-2R and that application of GLP-2 suppressed EFS-evoked increases in Isc suggest that GLP-2 suppression of chloride secretion reflects action at enteric ganglion cells in the submucosal plexus. The inhibitory action of GLP-2 on EFS-evoked Isc appears to be specific because it was concentration-dependent and suppressed by GLP-2-(3-33), which is an antagonist at GLP-2R (29). GLP-2-(3-33) alone did not affect the EFS-evoked biphasic responses, which suggests that it behaved like an antagonist at receptors on neurons in our preparations and that it had no direct action on the enterocytes or on paracellular epithelial conduction pathways.
The first and second phases of EFS-evoked Isc responses reflect muscarinic cholinergic and VIPergic-cholinergic transmission at neuroepithelial junctions, respectively (9, 23). Our pharmacological data suggest that the inhibitory action of GLP-2 is mainly in pathways with cholinergic neurotransmission and minimal in VIPergic pathways. The finding that the muscarinic receptor antagonist scopolamine suppressed the inhibitory action of GLP-2 on neurally evoked Isc responses was consistent with an action of GLP-2 on cholinergic secretomotor pathways. Scopolamine suppression of the inhibitory action of GLP-2 was probably due to blockade of muscarinic transmission at the neuroenterocyte junctions, which would prevent EFS-evoked responses and in this manner mask the inhibitory action of GLP-2 on the responses.
EFS directly activates secretomotor neurons to release their neurotransmitters at the neuroepithelial junctions and simultaneously activates interneurons in the enteric microcircuitry to release their neurotransmitters at synapses with other interneurons. Enteric interneurons, which are connected into networks by nicotinic synapses, provide excitatory nicotinic synaptic input to the secretomotor neurons (35). Suppression of the inhibitory action of GLP-2 by the nicotinic receptor antagonist hexamethonium is evidence for an action of GLP-2 to suppress neuronal release of ACh at synapses in the interneuronal microcircuitry and for action to suppress ACh release at excitatory nicotinic synapses on secretomotor neurons. Failure of the VIP receptor antagonist VPAC1 to alter the inhibitory action of GLP-2 suggests that GLP-2's enteric neuronal action is restricted to cholinergic signal pathways to the mucosa. Our finding that GLP-2 reduced the neuronal release of ACh into the bathing medium and that the GLP-2R antagonist offset this action of GLP-2 supports this conclusion.
Absence of suppression of EFS-evoked Isc responses by a VIP receptor antagonist is puzzling in view of our finding of expression of GLP-2R-IR by the class of ganglion cells with VIP as their chemical code. This might reflect differences in parallel secretomotor pathways for chloride and bicarbonate secretion. VIP is a neurotransmitter at neuroepithelial junctions in a neural pathway that activates chloride secretion and a separate pathway that stimulates bicarbonate secretion (16). Expression of the GLP-2R by VIPergic secretomotor neurons in the bicarbonate stimulatory pathway and not by VIPergic secretomotor neurons in the chloride pathway might explain the discrepancy. GLP-2 relaxes the mouse stomach by stimulating release of VIP from inhibitory motoneurons in the gastric enteric nervous system (1). An excitatory action of GLP-2 on VIPergic secretomotor neurons in a bicarbonate secretory pathway might be the case. Nevertheless, this has not been confirmed.
Our evidence for GLP-2 inhibitory influence on intestinal chloride secretion is reminiscent of reports for gastric secretion in which GLP-2 suppresses the stimulation of acid secretion evoked by pentagastrin or sham feeding in humans (26, 27). These effects of GLP-2 on acid secretion in the stomach, like the effects on neurally evoked chloride secretion in the intestine in vitro, might reflect suppression of ACh release at neuroeffector junctions. Whether the actions of GLP-2 in vitro are indicative of physiologically significant action in vivo is undetermined because physiological concentrations of GLP-2 in the plasma of the guinea pig model are unknown.
Conclusion.
Our results are new evidence that functional subclasses of neurons in the submucosal plexus of guinea pig small intestine express the GLP-2R. GLP-2 acts at the receptor to suppress ACh release from secretomotor neurons at muscarinic junctions with the epithelium and from interneurons in the enteric neural networks, which have nicotinic synaptic connections that control mucosal chloride secretion. This action of GLP-2 translates to suppression of secretion of NaCl and H2O and a decrease in the liquidity in the intestinal lumen at the organ system level of organization.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1 DK 37238 and RO1 DK 57075 to J. D. Wood, Pharmaceutical Manufacturers of America Foundation Research Starter Award to S. Liu, and a PRIN 2007 (Italy) study grant to S. Baldassano.
ACKNOWLEDGMENTS
Present address for S. Baldassano: Dipartimento di Biologia cellulare e dello Sviluppo and Dipartimento di Medicina, Pneumologia, Fisiologia e Nutrizione Umana, Università di Palermo, 90128 Palermo, Italy.
Present address for F. Mule: Dipartimento di Biologia cellulare e dello Sviluppo, Università di Palermo, 90128 Palermo, Italy.
REFERENCES
- 1.Amato A, Baldassano S, Serio R, Mulè F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol 296: G678–G684, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut 47: 112–119, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci USA 98: 12497–12502, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol Endocrinol Metab 272: E1050–E1058, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Cameron HL, Perdue MH. Stress impairs murine intestinal barrier function: improvement by glucagon-like peptide-2. J Pharmacol Exp Ther 314: 214–220, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cameron HL, Yang PC, Perdue MH. Glucagon-like peptide-2-enhanced barrier function reduces pathophysiology in a model of food allergy. Am J Physiol Gastrointest Liver Physiol 284: G905–G912, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cooke HJ, Christofi F. Enteric neural regulation of mucosal secretion. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR.San Diego: Elsevier Academic, 2006, p. 737–762 [Google Scholar]
- 8.Cooke HJ, Shonnard K, Wood JD. Effects of neuronal stimulation on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 245: G290–G296, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Cooke HJ, Zafirova M, Carey HV, Walsh JH, Grider J. Vasoactive intestinal polypeptide actions on the guinea pig intestinal mucosa during neural stimulation. Gastroenterology 92: 361–370, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Deniz M, Bozkurt A, Kurtel H. Mediators of glucagon like peptides 2-induces blood flow: responses in different vascular sites. Regul Pept 142: 7–15, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 93: 7911–7916, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drucker DJ, DeForest L, Brubaker PL. Intestinal response to growth factors administered alone or in combination with human [Gly2]glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 273: G1252–G1262, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Dube PE, Brubaker PL. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab 293: E460–E465, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Estall JL, Drucker DJ. Glucagon and glucagon-like peptide receptors as drug targets. Curr Pharm Des 12: 1731–1750, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Fei G, Wang YZ, Liu S, Hu HZ, Wang GD, Qu MH, Wang XY, Xia Y, Sun X, Bohn LM, Cooke HJ, Wood JD. Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol 296: G823–G832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fie GJ, Fang X, Wang XY, Wang GD, Liu S, Gao N, Hu HZ, Xia Y, Wood JD. Neurogenic mucosal bicarbonate secretion mediated by the purinergic P2Y1 receptor in guinea-pig duodenum (Abstract). Gastroenterology 130: A–380, 2006 [Google Scholar]
- 17.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst 3: 87–96, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Furness JB. The Enteric Nervous System. Oxford, UK: Blackwell, 2006, p. 29–102 [Google Scholar]
- 19.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150–164, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hadjiyanni I, Li KK, Drucker DJ. Glucagon-like peptide-2 reduces intestinal permeability but does not modify the onset of type 1 diabetes in the nonobese diabetic mouse. Endocrinology 150: 592–599, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Jeppesen PB. Glucagon-like peptide-2: update of the recent clinical trials. Gastroenterology 130: S127–S131, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB. Glucacon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806–815, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kuwahara A, Tien XY, Wallace LJ, Cooke HJ. Cholinergic receptors mediating secretion in guinea pig colon. J Pharmacol Exp Ther 242: 600–606, 1987 [PubMed] [Google Scholar]
- 24.Lin Z, Gao N, Hu Hz Liu S, Gao C, Kim G, Ren J, Xia y Peck OC, Wood JD. Immunoreactivity of Hu proteins facilitates identification of myenteric neurones in guinea pig small intestine. Neurogastroenterol Motil 14: 197–204, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology 130: 44–54, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Nagell CF, Wettergren A, Pedersen JF, Mortensen D, Holst JJ. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand J Gastroenterol 39: 353–358, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Orskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept 124: 105–112, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology 128: 1340–1353, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Shen KZ, Surprenant A. Somatostatin-mediated inhibitory postsynaptic potential in sympathetically denervated guinea-pig submucosal neurones. J Physiol 470: 619–65, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 293: G211–G221, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Tsai CH, Hill M, Asa SL, Brubaker PL, Drucker DJ. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol Endocrinol Metab 273: E77–E84, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Wallis K, Walters JRF, Forbes A. Glucagon-like peptide 2—current applications and future directions. Aliment Pharmacol Ther 25: 365–372, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Wood JD. Cellular neurophysiology of enteric neurons. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR.San Diego: Elsevier Academic, 2006, p. 629–664 [Google Scholar]
- 34.Wøjdemann M, Wettergren A, Hartmann B, Hilsted L, Holst JJ. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab 84: 2513–2517, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Xiao Q, Boushey RP, Drucker DJ, Brubaker PL. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 117: 99–105, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119: 744–755, 2000 [DOI] [PubMed] [Google Scholar]