Abstract
Angiotensin II promotes liver fibrogenesis by stimulating nonphagocytic NADPH oxidase (NOX)-induced oxidative stress. Angiotensin II type 1 (AT1) receptor blockers attenuate experimental liver fibrosis, yet their effects in human liver fibrosis are unknown. We investigated the effects of losartan on hepatic expression of fibrogenic, inflammatory, and NOX genes in patients with chronic hepatitis C (CHC). Fourteen patients with CHC and liver fibrosis received oral losartan (50 mg/day) for 18 mo. Liver biopsies were performed at baseline and after treatment. The degree of inflammation and fibrosis was evaluated by histological analysis (METAVIR). Collagen content was measured by morphometric quantification of Sirius red staining. Overall collagen content and fibrosis stage remained stable in the whole series, yet the fibrosis stage decreased in seven patients. Inflammatory activity improved in seven patients. The effect of losartan on hepatic expression of 31 profibrogenic and inflammatory genes and components of the NOX complex was assessed by quantitative PCR. Losartan treatment was associated with a significant decrease in the expression of several profibrogenic and NOX genes including procollagen α1(I) and α1(IV), urokinase-type plasminogen activator, metalloproteinase type 2, NOX activator 1 (NOXA-1) and organizer 1 (NOXO-1), and Rac-1. Losartan was well tolerated in all patients and was effective in attenuating the activity of the systemic renin-angiotensin system. No effects on serum liver tests or viral load were observed. We conclude that prolonged administration of losartan, an oral AT1 receptor blocker, is associated with downregulation of NOX components and fibrogenic genes in patients with CHC. Controlled studies are warranted to assess the effect of AT1 receptor blockers in chronic liver injury.
Keywords: angiotensin, liver fibrosis, antifibrogenic
accumulating evidence indicates that the renin-angiotensin system (RAS) is a major mediator in liver fibrogenesis. Key components of the RAS are locally expressed in chronically injured livers and activated hepatic stellate cells de novo generate angiotensin II (5), the main effector peptide of this system. Angiotensin II induces an array of fibrogenic actions in hepatic stellate cells (HSC) including cell proliferation, migration, secretion of proinflammatory cytokines, and collagen synthesis (34). The fibrogenic and inflammatory actions of angiotensin II in the liver are largely mediated by angiotensin type 1 (AT1) receptors (5). The production of intracellular reactive oxygen species generated by the nonphagocytic NADPH oxidase (NOX) complex is a key event in the angiotensin II-mediated fibrogenesis (6). The nonphagocytic NOX complex is directly expressed and functionally active in HSC (6), whereas the phagocytic form of NOX expressed in Kupffer cells only indirectly activate HSC through extracellular reactive oxygen species (39). Most importantly, pharmacological and/or genetic ablation of the RAS attenuates experimental liver fibrosis and oxidative stress (21, 23, 40).
On the basis of these studies, AT1 receptor blockers have been proposed to treat liver fibrosis in patients with chronic liver diseases. However, only four small studies have explored the effects of AT1 receptors blockers in human liver fibrosis: 1) two retrospective studies indicate that angiotensin II inhibitors may attenuate liver fibrosis progression in liver transplanted patients with hepatitis C recurrence (33) and hypertensive patients with chronic hepatitis C (CHC) (13); and 2) two small pilot studies in patients with nonalcoholic steatohepatitis (42) and CHC (38) suggest that oral losartan is associated with improvement in liver fibrosis. Noteworthy, none of these studies assessed whether oral AT1 receptor blockers were effective in inhibiting liver fibrogenesis by evaluating the hepatic expression of procollagen and other putative fibrosis-related genes. The assessment of changes on gene expression in human tissues after a therapeutic intervention is a novel approach that provides a more “dynamic” view of the wound healing response to chronic tissue injury (17, 29).
The present study investigates whether prolonged blockade of AT1 receptors attenuates the hepatic expression of key fibrogenic genes in patients with CHC and active viral infection who were not candidates for antiviral therapy. We evaluated the hepatic expression of profibrogenic and NOX genes involved in the hepatic response to chronic hepatitis C virus (HCV) infection before and after 18-mo treatment with losartan. We provide evidence that oral treatment with an AT1 receptor blocker to patients with CHC and active viral infection slows down fibrosis progression, as indicated by stabilization in collagen deposition and decreased expression of fibrosis-related genes and components of the NOX complex.
PATIENTS AND METHODS
Study design.
Fourteen patients with CHC with liver fibrosis and active viral infection were included in the Hospital Clínic of Barcelona in an uncontrolled open-label study. Inclusion criteria were as follows: 1) age between 35 and 65 yr; 2) chronic elevation of serum aminotransferases for more than 6 mo and positive RNA-HCV; 3) significant liver fibrosis (≥F2 in METAVIR score); 4) no previous response and/or contraindications to antiviral therapy. Exclusion criteria were 1) existence of other cause of chronic liver disease; 2) past history of hepatic decompensations or hepatocellular carcinoma; 3) alcohol consumption (>20 g/day); 4) arterial hypertension; 5) serum creatinine >1.5 mg/dl; 6) treatment with AT1 receptor blockers, angiotensin converting enzyme inhibitors, or interferon in the preceding 12 mo; and 7) contraindications to oral losartan. Patients were treated for 18 mo with oral losartan 50 mg/day (Cozaar, MSD, Wilmington, DE). In all patients, two liver biopsies were performed: the first one within a 24-h period before the initiation of treatment and the second one on the day following the last dose of losartan. Liver biopsies were performed in the right lobe of the liver with a Tru-Cut 14-gauge needle (20 mm length). The protocol was approved by the Ethics Committee of the Hospital Clínic of Barcelona and the Agencia Española del Medicamento as a phase IV trial (ARAHEPC 02-0491), and registered into the protocol registration system (NCT00298714). An untreated control group undergoing paired liver biopsies was not included because of ethical concerns. All patients gave written, informed consent.
Measurements.
Patients' visits were every week the first month, every month the following 5 mo, and every 3 mo thereafter. At these time points, arterial pressure and standard serum liver tests were measured. At baseline and at the end of treatment quantitative HCV-RNA determinations were performed by use of Amplicor HCV-RNA (Roche Diagnostic Systems, Basel, Switzerland). The activity of the systemic RAS was assessed by measuring plasma renin activity (PRA) and serum angiotensin II levels at baseline, day 7, day 30, day 120, day 270, and the end of treatment (1). Treatment compliance was evaluated monthly by pill count and it was defined as an intake of more than 90% of monthly dose.
Liver histological analysis.
Liver specimens were divided into two fragments: 2/3 were formalin fixed and paraffin embedded for standard histological analysis and 1/3 was immediately frozen in liquid nitrogen for RNA isolation. Paraffin-embedded samples were stained with hematoxylin-eosin and Masson's trichrome. The degree of fibrosis and inflammation in liver specimens was blindly evaluated by the same pathologist (M. Bruguera) using the METAVIR scoring system (7). Briefly, this semiquantitative score evaluates the degree of fibrosis (F0: no fibrosis; F1: portal; F2: rare septa; F3: numerous septa; F4: cirrhosis) and inflammatory activity (A0: none; A1: mild; A2: moderate; A3: severe) by combining piecemeal necrosis and lobular inflammation (0: none or mild; 1: moderate; 2: severe). Steatosis was scored as follows depending on the percentage of lobular hepatic parenchyma involved: 0: <10%; 1: 10–33%; 2: 33–66%; 3: >66%. Histological improvement in necroinflammatory activity and fibrosis stage was defined as a decrease of at least of 1 degree in the METAVIR scoring system in the liver biopsy performed at the end of treatment compared with pretreatment biopsy (10). All samples for histological study were at least 10 mm in length and included at least 10 portal tracts (35). There were no differences both in length (14.1 ± 2.3 mm vs. 13.8 ± 1.7 mm) and number of portal tracts (12.7 ± 2.7 vs. 12.5 ± 2.3) between liver biopsies obtained before and after treatment with losartan.
Assessment of collagen and ut-PA hepatic protein expression.
The amount of collagen content was estimated by measuring the percentage of the whole biopsy area stained with Sirius red staining (Sirius red F3B; Gurr-BDH Lab Supplies, Poole, UK). Morphometric analysis of the area with positive staining was blindly performed by the same operator as described in detail elsewhere (12). Protein detection of urokinase-type plasminogen activator (ut-PA) was performed by using mouse monoclonal antibodies against ut-PA (cat. no. 3689, American Diagnostica, Greenwich, CT). We blindly quantified ut-PA before and after treatment with losartan in a semiquantitatively manner (degrees: none, mild, moderate, severe).
Hepatic gene expression analysis.
We investigated hepatic gene expression in patients with chronic hepatitis C (n = 14) before and after treatment with losartan and in normal livers (n = 6). Gene selection was performed according to previously reported genes involved in human liver fibrogenesis (2, 6, 11, 12, 16, 44). We selected procollagen α1(I) and α1(IV) as end products of liver fibrosis; TGF-β1, TIMP-1, MMP-2, and ut-PA as markers of liver fibrogenesis; TNF-α, IL-6, Gro-α (CXCL-1), and MCP-1 as inflammatory mediators. We also explored the expression of components of the nonphagocytic NOX system: 1) the catalytic subunit gp91phox, NOX type 2 (NOX-2 or CYBB) and its isoforms: NOX-1 (enterocytes isoform), NOX-3 (inner ear cells isoform), NOX4 (renal isoform), NOX-5 (lymphocytes/spermatozoa isoform), and dual oxidase (Duox) 1 and 2; 2) the regulatory subunit p22phox (CYBA); 3) the p47phox isoform, NOX organizer 1 (NOXO-1); 4) the p67phox isoform, NOX activator 1 (NOXA-1); and 5) Rac 1 and 2. We additionally evaluated the expression of other prooxidant molecules: cytochrome P-450 monooxygenase (CYP2E1), heme oxidase 1 (HO-1), catalase, and the antioxidant superoxide dismutase type 2 (SOD-2). Finally, we also evaluated the expression of components of the RAS [angiotensin converting enzyme (ACE) 1 and 2, and AT1 receptor type I] and two apoptosis mediators (Bcl-2 and Fas ligand). Total RNA was extracted with Trizol (Life Technologies, Rockville, MD). RNA purity was assessed with a spectrophotometer Ultrospec 3300 pro (GE Healthcare). All RNAs had a 260/280 absorption ratio from 1.80 to 2. RNAs were further analyzed with a microfluidic glass chip platform (Bioanalizer 2100, Agilent, Palo Alto, CA). RNAs were considered suitable for RT-PCR if they showed lack of degradation, preserved 18S rRNA, an area under both bands >30%, and absence of contamination. One microgram of total RNA was reverse transcribed with a high-capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Thirty-two predesigned TaqMan assays for target genes were selected (Table 1; for information on primers used for RT-PCR assays see Supplementary Table S1) and distributed into a 384 wells TaqMan Low Density Array card (Applied Biosystems). Samples were analyzed in duplicate on an ABI PRISM 7900 (Applied Biosystems). Gene expression values were calculated on the basis of the cycle threshold (ΔCt) method (28) and normalized to expression of 18S rRNA. Results are expressed as 2−ΔΔCt. Normal livers were obtained from optimal cadaveric liver donors (n = 3) or resection of liver metastases (n = 3). Criteria to obtain normal livers have been described in detail elsewhere (12).
Table 1.
List of genes included in the study
| Group | Gene Symbol | Name | Gene Name |
|---|---|---|---|
| Apoptosis | BCL2 | Bcl-2 | B-cell CLL/lymphoma 2 |
| Apoptosis | TNFSF6 | Fas ligand | tumor necrosis factor (ligand) superfamily, member 6 |
| Cytokine | CXCL1 | GRO-α | chemokine (C-X-C motif) ligand 1 (growth-related oncogene α) |
| Cytokine | IL6 | IL-6 | interleukin 6 (interferon, β2) |
| Cytokine | TNF-α | TNF-α | tumor necrosis factor (TNF superfamily, member 2) |
| Endogenous control | 18S rRNA | 18S rRNA | 18S ribosomal RNA |
| Extracellular matrix | COL1A1 | Procollagen α1(I) | collagen, type I, α1 |
| Extracellular matrix | COL4A1 | Procollagen α1(IV) | collagen, type IV, α1 |
| Growth factor | CCL2 | MCP-1 | chemokine (C-C motif) ligand 2; monocyte chemotactive protein 1 |
| Growth factor | TGFB1 | TGF-β1 | transforming growth factor, β1 |
| Inhibitor matrix proteases | TIMP1 | TIMP-1 | tissue inhibitor of metalloproteinase 1 (erythroid potentiating activity, collagenase inhibitor) |
| Inhibitor matrix proteases | PLAU | ut-PA | plasminogen activator, urokinase-type |
| Matrix proteases | MMP2 | MMP-2 | matrix metalloproteinase 2 (gelatinase A, 72 kDa gelatinase, 72 kDa type IV collagenase) |
| Metabolism/oxidative stress | CAT | Catalase | catalase |
| Metabolism/oxidative stress | CYP2E1 | Cytochrome P-450 2E1 | cytochrome P-450, family 2, subfamily E, polypeptide 1 |
| Metabolism/oxidative stress | SOD2 | SOD-2 | superoxide dismutase 2, mitochondrial |
| Metabolism/oxidative stress | HMOX | HO-1 | heme oxygenase (decycling) 1 |
| NAPDH oxidase | RAC1 | Rac-1 | ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1) |
| NAPDH oxidase | RAC2 | Rac-2 | ras-related C3 botulinum toxin substrate 2 (rho family, small GTP binding protein Rac2) |
| NAPDH oxidase | NOXO1 | p67phox isoform: NOXO-1 | NADPH oxidase activator 1 |
| NAPDH oxidase | NOXA1 | p67phox isoform: NOXA-1 | NADPH oxidase activator 1 |
| NAPDH oxidase | NOX4 | gp91phox isoform: NOX-4 | (renal) NADPH oxidase 4 |
| NAPDH oxidase | CYBA | p22phox | cytochrome b-245, α polypeptide |
| NAPDH oxidase | CYBB | gp91phox or NOX-2 | cytochrome b-245, β polypeptide (chronic granulomatous disease) |
| NAPDH oxidase | DUOX1 | gp91phox isoform: Duox-1 | dual oxidase 1 |
| NAPDH oxidase | DUOX2 | gp91phox isoform: Duox-2 | dual oxidase 2 |
| NAPDH oxidase | NOX1 | gp91phox isoform: NOX-1 | NADPH oxidase 1 |
| NAPDH oxidase | NOX3 | gp91phox isoform: NOX-3 | NADPH oxidase 3 |
| NAPDH oxidase | NOX5 | gp91phox isoform: NOX-5 | NADPH oxidase 5 |
| Renin angiotensin system | ACE | ACE-1 | angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 |
| Renin angiotensin system | ACE2 | ACE-2 | angiotensin I converting enzyme (peptidyl-dipeptidase A) 2 |
| Renin angiotensin system | AGTR1 | AT1 receptor | angiotensin II receptor, type 1 |
Data analysis.
Quantitative variables were expressed as median (95% confidence interval) unless otherwise specified. Statistical methods included Mann-Whitney U-test and Wilcoxon's paired test for continuous variables and Fisher's exact test for categorical variables. Correlations were performed by the Pearson's linear correlation. A correction for controlling the false positive rate in multiple comparisons was performed by the Benjamini-Hochberg procedure (20). Unsupervised hierarchical clustering of gene expression of the selected genes in normal livers and patients with CHC before treatment using a specific software (dChip MFC application version 1.1) (27). The P value threshold for calling significant clusters was <0.001 for gene clustering and <0.05 for sample clustering. Statistical analysis was performed with SPSS version 14.0 for Windows (SPSS, Chicago, IL).
RESULTS
Baseline characteristics of the patients.
Patients were predominantly male (71%) with a median age of 55 yr [95% confidence interval (CI): 47–57] and a median body mass index of 26 (95% CI: 24–27). All patients were infected by HCV genotype 1 (64% type 1b). The estimated duration of infection, which was available in 12 patients, was 20 yr (95% CI: 15–30). Twelve patients were previous nonresponders to combined antiviral therapy (either because of lack of response or development of complications related to interferon) and two patients declined to give their consent to antiviral therapy. All patients had a preserved synthetic hepatic function with moderate elevation of serum liver aminotransferases (Table 2) and normal values of arterial pressure, PRA, and serum angiotensin II levels (Table 3).
Table 2.
Clinical and biochemical characteristics of the patients before and after losartan treatment
| Before Treatment |
After Treatment |
P | |||
|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | ||
| Serum AST, IU/l | 65 | 52–94 | 61 | 52–92 | ns |
| Serum ALT, IU/l | 81 | 70–133 | 96 | 68–131 | ns |
| Serum γ-GT, IU/l | 60 | 38–187 | 63 | 48–122 | ns |
| Serum bilirubin, mg/dl | 0.85 | 0.67–0.94 | 0.7 | 0.57–1.07 | ns |
| Serum cholesterol, mg/dl | 192 | 169–221 | 167 | 160–201 | ns |
| Serum albumin, g/l | 44 | 42–45 | 43 | 41–45 | ns |
| Prothrombin time, % | 88 | 84–92 | 90 | 85–94 | ns |
| Hemoglobin, g/dl | 14.7 | 12.0–15.4 | 14.4 | 12.3–15.4 | ns |
| Platelet count, ×109/l | 155 | 137–208 | 165 | 146–206 | ns |
| Serum HCV-RNA, IU/ml*106 | 2.48 | 1.67–5.15 | 2.81 | 1.95–4.01 | ns |
95% CI, 95% confidence interval; ns, not significant; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GT, glutamyl transferase; HCV, hepatitis C virus.
Table 3.
Effects of losartan on arterial pressure, renal function, and the systemic renin-angiotensin system
| Baseline | Day 7 | Day 30 | Day 120 | Day 270 | End of Treatment | |
|---|---|---|---|---|---|---|
| Systolic arterial pressure, mmHg | 120 (116–128) | 113 (110–122) | 120 (112–124) | 120 (113–125) | 120 (113–125) | 120 (112–121) |
| Diastolic arterial pressure, mmHg | 70 (69–78) | 70 (65–75) | 70 (67–76) | 70 (66–76) | 70 (66–77) | 70 (68–76) |
| Mean arterial pressure, mmHg | 90 (85–94) | 83 (80–90)* | 87 (83–92) | 88 (80–90) | 88 (80–90) | 87 (80–90) |
| Plasma renin activity, ng·ml−1·h−1† | 0.16 (0.08–0.25) | 1.13 (0.28–1.99) | 0.96 (0.22–1.69) | 1.01 (0.31–1.91) | 1.06 (0.32–1.8) | 0.89 (0.33–1.46) |
| Serum angiotensin II, pg/ml* | 10.04 (7.4–12.7) | 18.65 (10.7–26.6) | 21.87 (9.4–34.4) | 22.8 (8.9–37.8) | 23.9 (8.5–39.3) | 20.6 (11.8–29.4) |
| Serum creatinine, mg/dl | 1.0 (0.9–1.1) | 1.0 (0.9–1.0) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
Results are expressed as median (95% CI).
P < 0.001 vs. baseline;
P < 0.0001 vs. baseline.
Baseline biopsies showed that the degree of fibrosis was F2 in seven patients (50%), F3 in four patients (29%), and F4 in three patients (21%) and the degree of inflammation was A1 in seven patients (50%), A2 in four patients (29%), and A3 in three patients (21%). Baseline piecemeal necrosis was severe in two patients (14%), moderate in five patients (36%), and mild or absent in seven patients (50%). Baseline lobular activity was severe in one patient (7%), moderate in six patients (43%), and mild or absent in seven patients (50%). Baseline steatosis was <10% in three patients, 10–33% in 10 patients, and 33–66% in one patient.
Effect of losartan on clinical and biochemical parameters.
Treatment with losartan for 18 mo was well tolerated in all patients. Treatment compliance assessed by pill count showed an excellent average adherence (over 90% pills per month in all patients). No patient showed any liver-related complications during the treatment period. Losartan was not associated with changes in arterial pressure or renal function (Table 3). Importantly, losartan treatment induced an increase in PRA and serum angiotensin II levels (P < 0.001). Changes in the systemic RAS were found throughout the treatment period in all patients. This finding is indicative of a sustained AT1 receptor blockade with compensatory increase of PRA and serum angiotensin II levels. Losartan treatment did not affect serum liver tests or viral load (Table 2).
Effect of oral losartan on hepatic fibrosis and inflammation.
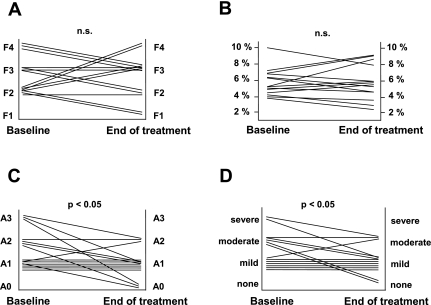
Following treatment with oral losartan the degree of fibrosis decreased in seven patients (50%, Fig. 1A), remained unchanged in three patients (21%), and progressed in four patients (29%), resulting in stabilization of the mean stage of fibrosis (Table 4). We next assessed the amount of collagen content in liver specimens by quantification of the positive area stained with Sirius red. AT1 receptor blockade was associated with stabilization of total collagen content in liver biopsies (Table 4, Fig. 1B).
Fig. 1.
Changes in the degree of inflammation and fibrosis before and after losartan treatment assessed by histological analysis using the METAVIR score (A, C, D: hematoxylin and eosin and Masson's trichromic staining) and morphometry (B: Sirius red staining). A: changes in the stage of liver fibrosis [F0: no fibrosis; F1: portal; F2: rare septa; F3: numerous septa; F4: cirrhosis; not significant (n.s.)]. B: changes in collagen content (not significant). C: changes in the inflammatory activity (A0: none; A1: mild; A2: moderate; A3: severe; P < 0.05). D: changes in the degree of piecemeal necrosis (P < 0.05).
Table 4.
Changes in the degree of liver fibrosis, inflammation, and collagen content before and after AT1 receptor blockade with losartan
| Before Treatment | After Treatment | P | |
|---|---|---|---|
| METAVIR | |||
| Fibrosis | 2.71±0.83 | 2.57±0.94 | ns |
| Activity score | 2.29±1.14 | 1.57±0.94 | <0.05 |
| Piecemeal | 1.64±0.75 | 1.07±0.61 | <0.05 |
| Lobular | 0.64±0.63 | 0.50±0.52 | ns |
| Morphometric analysis | |||
| Sirius red positive area, % | 5.86±1.65 | 5.90±2.17 | ns |
Values are means ± SD.
At the end of treatment, inflammatory activity improved in seven patients (50%, P < 0.05; Fig. 1C), remained unchanged in six patients (43%), and worsened in one patient (7%). Interestingly, the degree of piecemeal necroinflammation decreased in six patients (43%, P < 0.05; Fig. 1D), worsened in one patient (7%), and remained stable in seven patients (40%). The improvement in inflammation and piecemeal activity after treatment with losartan was significant (P < 0.05 for both; Table 4). In contrast, lobular inflammation did not change in nine patients (65%, not significant; Table 4), improved in three patients (21%), and worsened in two patients (14%).
Patients with improvement in liver fibrosis or inflammation were not different regarding age, gender, body mass index, duration of infection, baseline serum viral load, baseline liver tests, degree of activation of the systemic RAS at baseline, or treatment compliance. Moreover, the degree of activation of the systemic RAS following treatment with losartan was not different between responder and nonresponder patients.
Changes in hepatic gene expression of fibrosis-related genes after treatment with oral losartan.
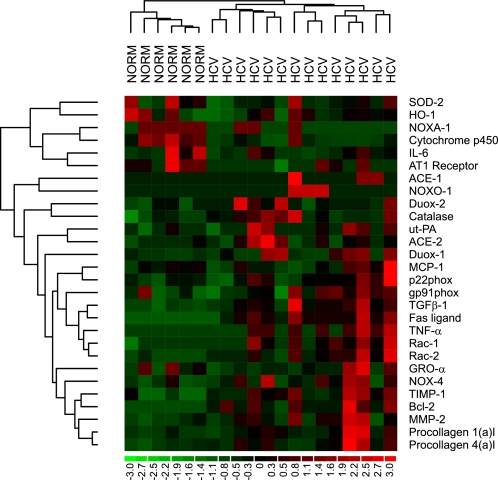
We first analyzed hepatic gene expression in patients with CHC compared with normal livers (n = 6). Most candidate genes were differentially expressed in livers with CHC compared with control livers. Gene expression of extracellular proteins (α1 chains of procollagen type I and type IV), major fibrogenic (TGF-β1, ACE-1 and 2) and proinflammatory mediators (TNF-α) as well as regulators of matrix degradation (MMP-2, TIMP-1, and ut-PA) were upregulated in livers of patients with chronic HCV infection. In contrast, genes mainly expressed by hepatocytes (i.e., cytochrome P-450 2E1) and AT1 receptors were downregulated in CHC livers compared with normal livers. These findings are in agreement with previous reports on hepatic gene expression in patients with CHC (2, 36). Components of the NOX complex (NOX-4, Rac-1, and Rac-2) were upregulated in livers with chronic HCV infection. We performed an unsupervised hierarchical clustering of normal livers and patients with chronic HCV infection according to the expression profile of the selected genes. On the basis of similarity in gene expression profiles, this procedure adequately clustered livers from patients with CHC and normal livers in two different groups (Fig. 2).
Fig. 2.
Unsupervised hierarchical clustering of normal (NORM) livers (n = 6) and patients with chronic hepatitis C (HCV) before treatment with oral losartan (n = 14) according to the expression profile of the selected genes.
Following treatment with losartan for 18 mo, there was a significant reduction in the expression of nine genes (Table 5). Importantly, four of these genes are known to be involved in liver fibrogenesis and were upregulated at baseline compared with normal livers. Expression of procollagen α1(I) and α1(IV), two major extracellular proteins in the fibrotic liver, decreased in the majority of patients after AT1 receptor blockade. Procollagen α1(I) decreased in 11 patients with a mean reduction of −31% (P < 0.05 vs. baseline). Procollagen α1(IV) decreased in 12 patients with a mean decrease of 23% (P < 0.05 vs. baseline). Losartan also reduced the expression of two important mediators of liver fibrogenesis such as ut-PA (−40%, P < 0.05 vs. baseline) and MMP-2 (−27%, P < 0.05 vs. baseline).
Table 5.
Hepatic expression of genes involved in liver fibrogenesis in normal livers compared with patients with CHC before and after treatment with losartan
| Gene Name | CHC Before Treatment (n = 14) | CHC After Treatment (n = 14) | CHC After Treatment vs. Before Treatment |
||
|---|---|---|---|---|---|
| Fold | P | Corrected P value† | |||
| Fibrogenic | |||||
| Procollagen α1(I)* | 21.6±4.6 | 15.1±2.3 | 0.69 | 0.0464 | 0.047 |
| Procollagen α1(IV)* | 19.0±3.7 | 14.7±2.7 | 0.77 | 0.0277 | 0.039 |
| MMP-2* | 7.0±1.5 | 5.1±1.3 | 0.73 | 0.0392 | 0.046 |
| ut-PA* | 0.96±0.13 | 0.56±0.10 | 0.60 | 0.0277 | 0.036 |
| TIMP-1* | 78.1±15.5 | 67.2±11.8 | 0.0747 | ||
| TGF-β1* | 19.0±4.2 | 17.90±3.9 | 0.6496 | ||
| ACE-1* | 1.06±0.32 | 1.02±0.28 | 0.3821 | ||
| ACE-2* | 3.2±0.78 | 4.5±1.48 | 0.9721 | ||
| AT1 receptor | 2.00±0.24 | 1.63±0.29 | 0.1961 | ||
| Prooxidant | |||||
| NOX-1 | NE | NE | |||
| NOX-2 (gp91phox) | 36.9±5.8 | 32.3±4.3 | 0.2487 | ||
| NOX-3 | NE | NE | |||
| NOX-4 (Renox)* | 0.10±0.02 | 0.10±0.02 | 1.0000 | ||
| NOX-5 | NE | NE | |||
| p22phox* | 30.4±3.9 | 29.3±3.5 | 0.8613 | ||
| Duox-1 | 0.05±0.01 | 0.05±0.01 | 0.9372 | ||
| Duox-2 | 0.05±0.01 | 0.07±0.03 | 0.6947 | ||
| NOXA-1 (p67phox)* | 0.73±0.07† | 0.57±0.05 | 0.74 | 0.0107 | 0.031 |
| NOXO-1 (p47phox)* | 0.74±0.06† | 0.53±0.04 | 0.71 | 0.0119 | 0.031 |
| Rac-1* | 2.90±0.43 | 2.39±0.39 | 0.81 | 0.0159 | 0.031 |
| Rac-2* | 4.48±0.77 | 4.31±0.52 | 0.8613 | ||
| Catalase* | 308±32 | 252±24 | 0.82 | 0.0046 | 0.031 |
| Cytochrome P-450 2E1* | 2817±289 | 2204±192 | 0.78 | 0.0131 | 0.031 |
| SOD-2 | 150±27 | 146±14 | 0.4454 | ||
| HO-1 | 21.3±2.7 | 18.9±3.0 | 0.1961 | ||
| Inflammatory | |||||
| TNF-α* | 1.23±0.23 | 1.06±0.20 | 0.1961 | ||
| GRO-α | 3.9±1.0 | 4.00±1.1 | 0.9721 | ||
| IL-6 | 0.07±0.02 | 0.08±0.02 | 0.8139 | ||
| MCP-1 | 6.0±1.4 | 5.19±1.0 | 0.2787 | ||
| Apoptotic | |||||
| Fas ligand | 0.97±0.20* | 0.82±0.13 | 0.5067 | ||
| Bcl-2 | 2.81±0.54* | 2.64±0.48 | 0.5767 | ||
Results are expressed as 2−ΔΔCt. Values are means ± SE. CHC, chronic hepatitis C; NE, not expressed.
P < 0.05 vs. normal liver;
Corrected P values after correction for multiple comparisons.
Changes in hepatic gene expression nonphagocytic NOX components after treatment with oral losartan.
We previously demonstrated that nonphagocytic NOX plays a key role in liver fibrosis and is stimulated by angiotensin II (6, 12). Therefore, we examined whether AT1 receptor blockade downregulates the expression of key components of this system in patients with CHC. As shown in Table 5, losartan treatment was associated with a significant decrease in the expression of key components of nonphagocytic NOX complex such as NOXO-1 (−29%), NOXA-1 (−26%), and Rac-1 (−19%). NOXO-1, the homologue of p47phox, and Rac-1 are crucial components of the NOX complex in HSC (15). Interestingly, the decrease of Rac-1 strongly correlated with the decrease of procollagen α1(I) (r = 0.684, P = 0.001), procollagen α1(IV) (r = 0.797, P = 0.001), and ut-PA (r = 0.689, P = 0.009). Since NOX mediates angiotensin II fibrogenic activity in the liver, the decrease in expression of key components of NOX and procollagen supports an antifibrogenic effect of losartan. The expression of CYP2E1 (−22%) and catalase (−18%) decreased after treatment with losartan while expression of SOD-2 and HO-1 remained unchanged. We did not detect expression of NOX-1, NOX-3, and NOX-5 in the liver before or after losartan.
Changes in hepatic gene expression in patients with decreased liver fibrosis.
We observed that patients with improvement in liver fibrosis had higher expression of AT1 receptor at baseline compared with patients without improvement in liver fibrosis (mean 2−ΔΔCt AT1 receptor expression 2.39 ± 0.69 vs. 1.38 ± 0.44 in responders vs. nonresponders, respectively; P = 0.004). We next analyzed changes in gene expression vs. baseline in patients with improved liver fibrosis after treatment compared with patients without improvement in liver fibrosis. As shown in Table 6, patients with improvement in liver fibrosis showed a pronounced downregulation of genes encoding extracellular matrix proteins, profibrogenic genes, and NOX genes. In contrast, no changes in hepatic gene expression were found in those patients without fibrosis improvement. These results suggest that changes in hepatic gene expression could decrease collagen accumulation in patients with CHC.
Table 6.
Changes in hepatic expression of fibrogenic genes vs. baseline in patients with improved liver fibrosis compared with patients without improvement in liver fibrosis
| Gene Name | Improvement in Liver Fibrosis (n = 7) |
No Improvement in Liver Fibrosis (n = 7) |
||
|---|---|---|---|---|
| Fold vs. baseline | P | Fold vs. baseline | P | |
| Fibrogenic | ||||
| Procollagen α1(I) | 0.53 | 0.028 | 0.96 | 0.499 |
| Procollagen α1(IV) | 0.67 | 0.018 | 1.06 | 0.310 |
| MMP-2 | 0.66 | 0.018 | 1.03 | 0.176 |
| ut-PA | 0.53 | 0.028 | 0.83 | 0.237 |
| TIMP-1 | 0.68 | 0.048 | 0.92 | 0.310 |
| NOX-2 (gp91phox) | 0.82 | 0.046 | 1.06 | 0.735 |
| NOXA-1 (p67phox) | 0.73 | 0.046 | 0.82 | 0.128 |
| Rac-1 | 0.71 | 0.046 | 0.88 | 0.176 |
Finally, inflammatory cytokines as MCP-1 and GRO-α were reduced in patients with histological improvement of inflammation by 27 and 43%, respectively (P < 0.05 vs. baseline). These results suggest that reduced expression of chemokines may mediate the anti-inflammatory effects of AT1 receptor blockade in CHC patients. The decreased expression of inflammatory genes was not found in patients without improvement in the degree of inflammation.
Effects of losartan treatment on ut-PA protein expression.
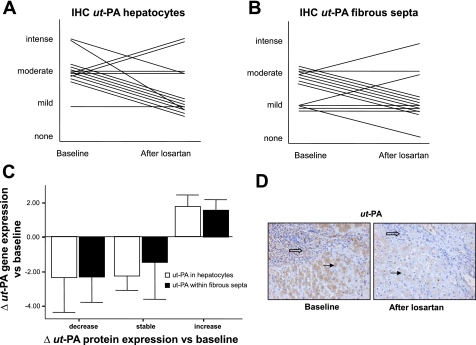
Gene expression analysis revealed that losartan decreased ut-PA hepatic expression. Since ut-PA plays a role in experimental hepatic fibrosis and mediates the profibrogenic effects of angiotensin II in several tissues (25, 44), we quantified ut-PA protein expression in liver samples using immunohistochemistry. ut-PA protein expression was detected in hepatocytes and within the fibrous septa. Following treatment with losartan, we observed a decrease in protein levels of ut-PA in 64% of patients (9/14, P < 0.05 vs. baseline). Moreover, a positive correlation between changes in ut-PA mRNA levels and changes in the degree of ut-PA protein expression was noted (Fig. 3). These data support the results obtained at the gene expression level.
Fig. 3.
A: changes in the degree of urokinase-type plasminogen activator (ut-PA) expression in liver samples assessed by immunohistochemistry (IHC) (P < 0.05 vs. baseline). B: representative picture of ut-PA immunohistochemistry in 1 patient at baseline and after prolonged treatment with losartan (magnification ×200). D: ut-PA expression was noted in hepatocytes (solid arrows) and within the fibrous stroma (open arrows). C: correlation between changes in gene expression of ut-PA (fold vs. baseline) and changes in protein expression of ut-PA (vs. baseline) following treatment with losartan.
DISCUSSION
The identification of drugs that attenuate fibrosis progression in patients with chronic liver diseases in whom the causative agent cannot be removed is an important goal in hepatology. We conducted a pilot study to explore whether AT1 receptor blockers attenuate the hepatic expression of genes related to liver fibrosis progression in patients with CHC. The primary aim of this study was to investigate the effects of AT1 receptor blockers on liver fibrogenesis in patients with CHC by measuring the hepatic expression of candidate genes encoding extracellular matrix proteins and fibrogenic mediators. An accurate detection of changes in collagen deposition requires large long-term controlled trials and has limitations in the interpretation of liver biopsies (sampling error, observer variability, etc.). In contrast, the assessment of hepatic expression of collagen and other fibrogenic genes correlates with the degree of liver fibrosis and provides a more “dynamic” approach to the fibrogenic activity (2).
At the gene expression level, the most important finding of this study was the demonstration that AT1 receptor blockade is associated with a decrease in procollagen α1(I) mRNA, a key component of the fibrous scar in CHC (2). This result suggests that losartan inhibits collagen synthesis in these patients and warrants long-term controlled studies to evaluate the antifibrotic efficacy of AT1 receptor blockers in CHC. Losartan treatment was associated to a reduced expression of important genes involved in angiotensin II-mediated oxidative stress in the fibrotic liver. These genes include NOXO-1 and Rac-1, key components of the NOX activity in HSC (15). This result supports previous observations indicating that AT1 receptor blockers inhibit oxidative stress in rodent models of chronic liver injury (21). We also observed a reduction in the expression of MMP-2 and ut-PA, two regulators of extracellular matrix turnover. ut-PA regulates matrix degradation (45) and is involved in inflammation and angiogenesis (14). Plasmin generated by ut-PA is required for the release of active TGF-β1 from its latent form bound to the latency-associated peptide (9). Finally, downregulation of TIMP-1 in the subgroup of patients with improvement in liver fibrosis is in agreement with previous reports on the effects of AT1 receptor blockers in experimental models of liver injury (21, 22). Further studies should specifically address the effects of AT1 receptor blockers on oxidative stress and collagen degradation in patients with chronic liver disease.
We also evaluated the effect of losartan on the degree of liver fibrosis in liver specimens. Our study cohort includes CHC patients who are at a high risk for developing progressive liver fibrosis (19, 43) (i.e., age older than 40 yr and significant liver fibrosis). Losartan treatment was associated with a decrease of at least one degree of fibrosis in half of the patients. Previous reports indicate that only 5–24% of patients with CHC show a spontaneous decrease in liver fibrosis (18, 37). On the contrary, a reduction in liver fibrosis in half of the patients has been reported in patients with CHC after viral clearance (10). When collagen accumulation is evaluated by morphometry in untreated patients with CHC, the mean increase in collagen content in 12 mo ranges from 43 to 94% (19). In contrast, in our cohort we observed only 1% increase after 18-mo treatment with oral losartan. Taken together, these results suggest that AT1 receptor blockade slows down the progressive collagen accumulation in patients with CHC.
Another relevant finding of this study is the reduction in the extent of piecemeal necrosis. The degree of necroinflammatory injury in patients with CHC correlates with fibrosis and predicts worsening fibrosis in CHC (18, 26, 41). The anti-inflammatory effect of losartan could be anticipated from previous experimental studies. Angiotensin II exerts powerful proinflammatory effects in the liver both in culture and in vivo and promotes myofibroblast survival (3, 4, 8, 24, 31, 32). In particular, angiotensin II activates intracellular signaling pathways such as NF-κB and JNK, leading to increased expression of inflammatory cytokines such as RANTES and cell adhesion molecules such as ICAM-1 (30). These effects are markedly blunted by AT1 receptor blockers in experimental models of liver fibrosis (40). The blockade of AT1 receptors in patients with CHC could result in decreased expression of inflammatory mediators, as indicated by downregulation of MCP-1 and GRO-α in the subgroup with improved inflammation. Interestingly, patients with improvement in fibrosis had higher expression of AT1 receptor at baseline compared with patients without fibrosis improvement. This finding suggests that activation of the intrahepatic RAS and subsequent modulation of Ang II-induced signaling pathways [e.g., P-Ser536-RelA (32)] varies among CHC patients and could influence the response to AT1 receptor blockers.
The present study also assessed whether AT1 receptor blockers could be safely administered to patients with CHC. In our series, losartan effectively induced a compensatory activation of the systemic RAS without inducing renal impairment. This finding confirms that prolonged administration of AT1 blockers to patients with CHC without activation of the systemic RAS is safe.
This study has two main limitations. First, it is an uncontrolled study including a small number of patients. Second, the mean size of liver tissue available for histological study is smaller than 1.5 cm in length. Nonetheless, the aim of this study was to evaluate the effects of AT1 receptor blockade on liver fibrogenesis at the gene expression level, rather than evaluating the efficacy of losartan as an antifibrotic therapy. An untreated control group undergoing paired liver biopsies was not used, nor were two needle passes performed to obtain enough liver tissue for both histology and gene expression studies, because of ethical considerations. Moreover, the presence of at least 10 portal tracts is also accepted as a requirement for liver biopsy adequacy and the quality of RNA samples from controls and patients was adequate in all cases.
In conclusion, we provide evidence that prolonged blockade of AT1 receptor for 18 mo in patients with chronic HCV infection is associated with downregulation of hepatic profibrogenic and NOX genes and with stabilization of collagen deposition and is well tolerated. Further controlled studies are needed to evaluate the effect of long-term administration AT1 receptor blockers in patients with CHC and other types of chronic liver diseases in whom the causative agent of liver injury cannot be removed.
GRANTS
This work is supported by grants from the Ministerio de Ciencia y Tecnología, Dirección General de Investigación (SAF 2005-06245), the Instituto de Salud Carlos III (CO3/02), FIS 2005-06245-O, FIS 2005-050567-O, FIS 2008-PI040048, the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK072237-01), and the Instituto Reina Sofía de Investigación Nefrológica. P. Sancho-Bru and M. Moreno had a grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS). M. Domínguez had a grant from the Fundación Banco Bilbao Vizcaya Argentaria (FBBVA). The study was not supported by any pharmaceutical company.
ACKNOWLEDGMENTS
We thank Elena Juez and Cristina Millán for excellent technical support and Jose María Sánchez-Tapias for kind contribution to patient recruitment.
REFERENCES
- 1.Asbert M, Jiménez W, Gaya J, Ginès P, Arroyo V, Rivera F, Rodés J. Assessment of the renin-angiotensin system in cirrhotic patients. Comparison between plasma renin activity and direct measurement of immunoreactive renin. J Hepatol 15: 179–183, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Asselah T, Bièche I, Laurendeau I, Paradis V, Vidaud D, Degott C, Martinot M, Bedossa P, Valla D, Vidaud M, Marcellin P. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology 129: 2064–2075, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bataller R, Gabele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, Brenner DA, Rippe RA. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology 41: 1046–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bataller R, Gabele E, Schoonhoven R, Morris T, Lehnert M, Yang L, Brenner DA, Rippe RA. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol 285: G642–G651, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bataller R, Sancho-Bru P, Ginès P, Lora JM, Al-Garawi A, Sole M, Colmenero J, Nicolás JM, Jiménez W, Weich N, Gutiérrez-Ramos JC, Arroyo V, Rodés J. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 125: 117–125, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 112: 1383–1394, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24: 289–293, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem 212: 155–169, 2000 [PubMed] [Google Scholar]
- 9.Breitkopf K, Lahme B, Tag CG, Gressner AM. Expression and matrix deposition of latent transforming growth factor beta binding proteins in normal and fibrotic rat liver and transdifferentiating hepatic stellate cells in culture. Hepatology 33: 387–396, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cammà C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, Balart L, Alberti A, Craxi A. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 39: 333–342, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, Li YX, Goldschmidt-Clermont PJ, Diehl AM. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology 44: 1267–1277, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, Jares P, Bosch J, Arroyo V, Caballería J, Ginès P. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology 132: 687–697, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Corey KE, Shah N, Misdraji J, Abu Dayyeh B, Zheng H, Bhan AK, Chung RT. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int 29: 748–753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alessio S, Blasi F. The urokinase receptor as an entertainer of signal transduction. Front Biosci 14: 4575–4587, 2009 [DOI] [PubMed] [Google Scholar]
- 15.De MS, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology 131: 272–275, 2006 [DOI] [PubMed] [Google Scholar]
- 16.De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys 462: 266–272, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feld JJ, Nanda S, Huang Y, Chen W, Cam M, Pusek SN, Schweigler LM, Theodore D, Zacks SL, Liang TJ, Fried MW. Hepatic gene expression during treatment with peginterferon and ribavirin: identifying molecular pathways for treatment response. Hepatology 46: 1548–1563, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, Park Y, Liang TJ, Hoofnagle JH. Progression of fibrosis in chronic hepatitis C. Gastroenterology 124: 97–104, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Goodman ZD, Becker RL, Jr, Pockros PJ, Afdhal NH. Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology 45: 886–894, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Green GH, Diggle PJ. On the operational characteristics of the Benjamini and Hochberg False Discovery Rate procedure. Stat Appl Genet Mol Biol 6: Article27, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hirose A, Ono M, Saibara T, Nozaki Y, Masuda K, Yoshioka A, Takahashi M, Akisawa N, Iwasaki S, Oben JA, Onishi S. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology 45: 1375–1381, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Yamamoto N, Uchida K, Terai S, Sakaida I. Telmisartan prevents hepatic fibrosis and enzyme-altered lesions in liver cirrhosis rat induced by a choline-deficient l-amino acid-defined diet. Biochem Biophys Res Commun 364: 801–807, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology 121: 148–155, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kanno K, Tazuma S, Nishioka T, Hyogo H, Chayama K. Angiotensin II participates in hepatic inflammation and fibrosis through MCP-1 expression. Dig Dis Sci 50: 942–948, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kenichi M, Masanobu M, Takehiko K, Shoko T, Akira F, Katsushige A, Takashi H, Yoshiyuki O, Shigeru K. Renal synthesis of urokinase type-plasminogen activator, its receptor, and plasminogen activator inhibitor-1 in diabetic nephropathy in rats: modulation by angiotensin-converting-enzyme inhibitor. J Lab Clin Med 144: 69–77, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Lagging LM, Westin J, Svensson E, Aires N, Dhillon AP, Lindh M, Wejstal R, Norkrans G. Progression of fibrosis in untreated patients with hepatitis C virus infection. Liver 22: 136–144, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98: 31–36, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA, Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med 346: 1357–1365, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Moreno M, Bataller R. Cytokines and renin-angiotensin system signaling in hepatic fibrosis. Clin Liver Dis 12: 825–52, ix, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Nabah YN, Mateo T, Estelles R, Mata M, Zagorski J, Sarau H, Cortijo J, Morcillo EJ, Jose PJ, Sanz MJ. Angiotensin II induces neutrophil accumulation in vivo through generation and release of CXC chemokines. Circulation 110: 3581–3586, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Oakley F, Teoh V, Ching AS, Bataller R, Colmenero J, Jonsson JR, Eliopoulos AG, Watson MR, Manas D, Mann DA. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 136: 2334–2344, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Rimola A, Londoño MC, Guevara G, Bruguera M, Navasa M, Forns X, Garcia-Retortillo M, Garcia-Valdecasas JC, Rodés J. Beneficial effect of angiotensin-blocking agents on graft fibrosis in hepatitis C recurrence after liver transplantation. Transplantation 78: 686–691, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Sancho-Bru P, Bataller R, Gasull X, Colmenero J, Khurdayan V, Gual A, Nicolas JM, Arroyo V, Ginès P. Genomic and functional characterization of stellate cells isolated from human cirrhotic livers. J Hepatol 43: 272–282, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Schiano TD, Azeem S, Bodian CA, Bodenheimer HC, Jr, Merati S, Thung SN, Hytiroglou P. Importance of specimen size in accurate needle liver biopsy evaluation of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 3: 930–935, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Schulte S, Oidtmann A, Kociok N, Demir M, Odenthal M, Drebber U, Dienes HP, Nierhoff D, Goeser T, Toex U, Steffen HM. Hepatocyte expression of angiotensin II type 1 receptor is downregulated in advanced human liver fibrosis. Liver Int 29: 384–391, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, Fujiyama S, Yoshida H, Omata M. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 132: 517–524, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Sookoian S, Fernández MA, Castaño G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World J Gastroenterol 11: 7560–7563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, González FJ, Samulski RJ, Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med 31: 1544–1549, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Bataller R, Dulyx J, Coffman TM, Ginès P, Rippe RA, Brenner DA. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J Hepatol 43: 317–323, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, Lefkowitch JH, Ludwig J, Okuda K. The long-term pathological evolution of chronic hepatitis C. Hepatology 23: 1334–1340, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 40: 1222–1225, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Zarski JP, Mc Hutchison J, Bronowicki JP, Sturm N, Garcia-Kennedy R, Hodaj E, Truta B, Wright T, Gish R. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol 38: 307–314, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Zhang LP, Takahara T, Yata Y, Furui K, Jin B, Kawada N, Watanabe A. Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells. J Hepatol 31: 703–711, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Zhang YJ, Yang XS, Wu PS, Liao GQ, Yang GP, Zhang XF, Chen XQ. Expression of AT(1a) mRNA in rat hepatic stellate cells and its effects on cell growth collagen production. Chin Med J (Engl) 117: 772–774, 2004 [PubMed] [Google Scholar]