Abstract
Liver dysfunction secondary to severe inflammation is associated with the release of enzymes normally sequestered within hepatocytes. The purpose of these studies was to test the hypothesis that these enzymes are released, at least in part, to modulate potentially deleterious inflammatory processes in distant tissues like the gut. Human Caco-2BBe enterocyte-like cells were exposed to cytomix (IFN-γ, TNF-α, and IL-1β) in the absence or presence of human liver cytosol (LC). Nitric oxide (NO•) and inducible nitric oxide synthase (iNOS) protein production were measured by the Griess assay and Western analysis, respectively. Cytomix induced the expression of iNOS and release of NO•. LC protein (400 μg/ml) added to the basal compartment but not apical compartment completely blocked the release of NO• but only slightly decreased the magnitude of iNOS protein induction. Ultrafiltration and ultracentrifugation studies demonstrated that microsome-associated arginase-1 activity was the iNOS-suppressing activity in LC. Liver arginase required activation by a <10-kDa factor that was present in supernatants of cytomix-stimulated cells. The selective iNOS inhibitor l-N6-(1-iminoethyl)-lysine·2HCl prevented production of this factor. The biotin switch assay detected increased S-nitrosylation of arginase-1 after incubation with supernatants from immunostimulated Caco-2 cells. Serum from endotoxemic mice contained significantly greater arginase activity compared with serum from control mice. Furthermore, the ratio of mucosal monomeric to dimeric iNOS increased in endotoxemic mice compared with controls. Thus reciprocal activation of arginase-1 and modulation of mucosal iNOS activity may be protective because it would be expected to decrease NO•-dependent intestinal barrier dysfunction on that basis.
Keywords: shock, systemic inflammatory response syndrome, liver damage
immunostimulated intestinal epithelial cells express inducible nitric oxide synthase (iNOS) and generate significant amounts of nitric oxide (NO•) (25, 32). Although NO• dilates arterioles in the gut increasing microcirculatory flow (35), excessive NO• production has been implicated in decreased mucosal barrier function (7). The latter process has been proposed to act as the “engine” that drives systemic inflammation during severe sepsis (9, 37). Thus altering iNOS activity during systemic inflammation may have both beneficial and exacerbating effects on pathological and physiological processes, respectively. Severe systemic inflammation is often associated with liver dysfunction that is monitored clinically by measuring the appearance of intracellular hepatic enzymes including γ-glutamyl transpeptidase, alkaline phosphatase, alanine aminotransferase, or aspartate aminotransferase in circulating plasma (16, 19, 30). Arginase type I (Arg-1), which is constitutively expressed predominantly in liver cytosol (LC), is also released to the circulatory system when liver function is compromised (27). For example, arginase activity in serum was shown to increase over 170-fold in a rat bile duct ligation model (15).
The energy expenditure associated with releasing significant quantities of normally cytosolic proteins from the liver to the circulatory compartment led us to hypothesize that these proteins may be actively secreted by inflamed hepatocytes to modulate metabolite-dependent processes in the host. We tested this hypothesis by determining whether enzymes present in normal LC can preserve intestinal epithelial barrier function during inflammation. We chose this readout because epithelial barrier dysfunction has been proposed as an important process underlying the progressive loss of organ function observed in severe sepsis (12).
NO• synthases (NOS) produce NO• and l-citrulline (Cit) using l-arginine (Arg) and O2 as substrates. Arg can also be metabolized to urea and l-ornithine (Orn) via Arg-1 and 2. This competition between NOS and arginase for Arg has been reported in diverse inflammatory states (4, 10, 21, 36). There have been several reports discussing the balance of iNOS and Arg-1 in the intestine under certain pathological conditions (14, 15). It has been proposed that bacteria and other microbes can deplete environmental Arg as a survival strategy by using their own arginase or host arginases to deplete Arg (6, 11, 38) and decrease NO• production on that basis. In light of these considerations, one can imagine conditions in which liver arginase will influence gut iNOS activity following a localized or systemic inflammatory response.
Herein, we report that LC when added to immunostimulated epithelial monolayers decreases the release of NO• and preserves barrier function on that basis. We further show that arginase activity is increased by incubation with immunostimulated but not resting cultures of enterocytes. Circulating Arg-1 protein increased early in endotoxemic mice, but the specific activity of the enzyme increased following a delay that appears to reflect time needed to increase iNOS activity in peripheral tissues. These findings may have important clinical implications for the use of Arg supplementation in the treatment of critically ill patients.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle medium (DMEM) and phosphate-buffered saline (PBS) were from Bio-Whittaker (Walkersville, MD). Heat-decomplemented fetal bovine serum (FBS) was from Hyclone (Logan, UT). Human liver S9 pool BD Gentest (cat. no. 45291, lot. no. 73024) was centrifuged at 12,000 g for 30 min and then filtered by using 0.22 μm Costar SPIN X centrifuge filters before use (Corning, NY). S-(2-boronoethyl)-l-cysteine (BEC) an inhibitor of Arg-1 and II (8) was from Calbiochem, EMD Chemical (San Diego, CA). l-N6-(1-iminoethyl)-lysine·2HCl (l-NIL) was from Axora (San Diego, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) unless otherwise noted.
Cells.
Caco-2BBe (referred to herein as Caco-2) cells (ATCC no. HTB37, human colon adenocarcinoma) were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in DMEM supplemented with 5% FBS, penicillin G (100 U/ml), streptomycin (100 g/ml), sodium pyruvate (2 mmol/l), 1% nonessential amino acids, and transferrin (0.1 mg/ml). Stock cultures were maintained in 75 cm2 BioCoat collagen-coated dishes at 37°C in a 5% CO2 atmosphere and were split weekly. For experiments, cells were harvested and suspended at a density of 5 × 105 cells/ml in culture media. Two milliliters of cells were added to each well of a six-well tissue culture plate or six-well Transwell bicameral chamber (0.4-μm pore size; Costar) depending on the assay. After 21 days, some wells were stimulated with cytomix (10 ng/ml TNF, 10 ng/ml IL-1β, and 1,000 U/ml IFN-γ) and incubated at 37°C in a 5% CO2 atmosphere for the times indicated.
Animals.
This research complied with regulations regarding animal care as published by the National Institutes of Health (NIH) and was approved by the Institutional Animal Use and Care Committee of the University of Pittsburgh. All animals were maintained in the University of Pittsburgh Animal Research Facility on a 12-h light-dark cycle with free access to standard laboratory chow and water. Animals were not fasted before experiments. Animals were anesthetized before surgical procedures with pentobarbital sodium (60–90 mg/kg ip). Heparin anticoagulated plasma was drawn from 25- to 35-g male 12-wk-old C57B1/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) at 0, 2, 6, 15, and 24 h after injection with PBS or lipopolysaccharide (LPS) (15 mg/kg) as published (42, 43). To induce a systemic inflammatory response, mice were injected intraperitoneally with Escherichia coli (strain 0127:B8) LPS (15 mg/kg) dissolved in 1.0 ml of PBS. Control animals were injected with a similar volume of PBS without LPS.
Separation of iNOS dimmers and monomers.
Caco-2 cells were incubated with or without cytomix, washed twice with ice-cold PBS, and then harvested in 1 ml of 25 mM Tris (pH 7.4) by use of a rubber policeman. Cells were sonicated at level 5 with a Fisher Scientific Sonic dismembrator using two 30-s pulses on ice. Insoluble material was collected by centrifugation at 15,000 g. The supernatant was filtered through 76-mm Advantec ultrafilters with a 200-kDa molecular weight cutoff (MWCO). The filtrate contained monomeric iNOS and the retentate contained dimeric active iNOS. The retentate represented a 15-fold concentration of the sample. This was subsequently adjusted to the starting volume with 20 mM Tris (pH 7.4). These proteins were then cleared of precipitated material with 0.22 μm Costar SPIN X centrifuge filters.
Griess assay.
Assays were performed as described (39). Briefly, 200 μl of sample was deproteinized with ZnSO4 (1.5% wt/vol). After centrifugation at 12,000 g for 5 min, the supernatants were shaken overnight with 0.6 g of activated Cd2+ filings to convert NO3− to NO2−. Cd2+ was removed and the samples were centrifuged at 12,000 g for 10 min, and 100 μl of supernatant was mixed with an equal volume of Griess reagent in a 96-well flat-bottom microtiter plate. Absorbance was measured at 550 nm with a BioTek Synergy HT microplate reader.
Measurement of iNOS and arginase enzymatic activity using [3H]l-Cit catabolism.
Cell-free medium was prepared from the supernatants of Caco-2 cells cultured for 18 h in fresh complete medium in the absence and presence of cytomix, 500 μl of each supernatant was harvested and centrifuged at 1,000 g for 10 min to remove cell debris. LC (2 μl; 20 mg/ml) was added to 25 μl of each supernatant. The entire volume of supernatant was adjusted to a final reaction volume of 40 μl and contained 50 mM Tris (pH 7.4), 1 mM NADPH, 20 mM tetrahydrobiopterin, 5 mM FAD, 5 mM flavin mononucleotide. The reaction was preincubated for 10 min at 37°C before addition of 10 μl of 1 μCi/μl [3H]Arg (35–70 Ci/mmol, GE Healthcare) and incubation for an additional 2 h. The reaction mixture was adjusted to 1.5 mM CaCl2 when iNOS activity was measured. The reaction was stopped by the addition of 0.4 ml ice-cold 5 mM HEPES stop buffer (pH 5.5) containing 5 mM EDTA. Reaction mixtures were applied to columns (0.5-cm diameter) containing 100 mg DOWEX 50W-X8 (Na+ form) cation exchange resin. The radioactivity of [3H]l-Cit in the eluates was measured on a liquid scintillation counter (RackBeta, LKB-Wallac, Turku, Finland). iNOS-specific arginase activity was calculated by performing the reactions in the absence or presence of l-NIL (40). The total conversion rate was subtracted by the conversion rate in the presence of l-NIL to obtain iNOS activity. In the same way, the activity of arginase in the extract was determined by use of BEC.
Arginase activity was measured as described previously with minor modifications (43). Briefly, a sample (150 μl) was added to 100 μl of 50 mM Tris (pH 7.5) containing 10 mM MnCl2. The hydrolysis reaction of Arg by arginase was performed by incubating the mixture containing activated arginase with 100 μl of Arg (0.5 M, pH 9.7) at 37°C for 1 h and was stopped by adding 900 μl of a mixture of concentrated H2SO4-H3PO4-H2O at a ratio of 1:3:7. The basal level of urea was measured in the same volume of sample that was kept on ice during the incubation time. For colorimetric determination of urea, α-isonitrosopropiophenone (25 μl, 9% in absolute ethanol) was added and the mixture was heated at 100°C for 15 min. After placing the sample in the dark for 10 min at room temperature, we determined the urea concentration spectrophotometrically with absorbance at 540 nm measured with a microplate reader. The amount of urea produced was calculated by subtracting the basal urea level detected in samples kept on ice from the level detected in samples incubated at 37°C and was used as an index for arginase activity in serum.
Microsomal-compartment isolation from LC.
LC (100 μl) was diluted to 10 ml with isotonic Tris buffer (25 mM Tris, pH 7.4, 130 mM NaCl), and the solution was centrifuged at 16,000 g at 4°C for 30 min to remove intracellular organelles and cell debris. The supernatants were filtered through 0.2-μm-pore filters and then subjected to ultracentrifugation at 100,000 g at 4°C for 1 h to pellet microsomes.
Biotin switch assay.
All steps were performed as described previously (17) with minor modifications. Five milliliters of cell supernatant were collected and centrifuged at 300 g for 10 min to remove cell debris followed by another centrifugation at 16,000 g for 15 min. The supernatants were filtered through 10-kDa MWCO ultrafilters, mixed with 200 μl of LC, and incubated at 37°C for 2 h. The samples were diluted to decrease the final protein concentration to 1.0 mg/ml followed by addition of freshly prepared S-methylmethanethiosulfonate (10% vol/vol in N,N-dimethylformamide; DMF) and SDS (25% vol/vol) to final concentrations of 30 mM and 2.5%, respectively. Following frequent vortexing at 50°C for 20 min, proteins were precipitated with two volumes of acetone at −20°C for 30 min. The proteins were recovered by centrifugation at 3,000 g for 10 min. The pellets were suspended in 40 μl of 25 mM HEPES, pH 7.7, 0.1 mM EDTA, 0.01 mM neocuproine (HEN) buffer containing 5% SDS and mixed with 230 μl of HEN buffer after pellets were completely dissolved. For labeling, the methylated samples were mixed with 90 μl of biotin-N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (HPDP) (4 mM in DMF; Pierce) and freshly prepared sodium ascorbate in pure water at a final concentration of 5 mM. Labeling reactions were performed in the dark at room temperature for 1 h.
The proteins were precipitated with two volumes of ice-cold acetone at −20°C for 30 min. The proteins were recovered by centrifugation at 3,000 g for 5 min, followed by gentle rinsing of the pellet with 1 ml of acetone. The washed pellet was then resuspended in 40 μl of HEN buffer containing 5% SDS, followed by addition of 200 μl of HEN buffer after pellets were completely dissolved. Subsequently, 750 μl of neutralization buffer (25 mM HEPES, 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, pH 7.5) were added. This material was incubated overnight at 4°C with 50 μl of streptavidin-agarose bead slurry. The beads were washed with 5 × 1.4 ml of wash buffer (neutralization buffer + 500 mM NaCl). The beads were eluted with 50 μl of elution buffer [20 mM HEPES, 100 mM NaCl, 1 mM EDTA and 100 mM 2-mercaptoethanol (2ME)] at 37°C for 15 min followed by incubation at 50°C for 3 min. The eluted mixture was then analyzed by SDS-PAGE, followed by immunoblotting with anti-Arg-1 antibodies (1:200; sc-18355, Santa Cruz Biotechnology).
Western blot analysis.
Equal volumes of cell-culture supernatant were mixed with 6× loading buffer (375 mM Tris, pH 6.8, 50% glycerol, 0.03% bromophenol blue, 10% SDS, and 4% 2ME). After being boiled, the samples were subjected to 10.5% SDS-PAGE electrophoresis. The resolved proteins were transferred to PVDF membrane (Amersham Pharamacia Biotech, Leicestershire, UK) and blocked with Blotto buffer (1× PBS, 1.0% nonfat milk, 0.2% Tween-20) for 1 h. The membrane was then incubated with a mouse anti-iNOS monoclonal antibody (BD Biosciences; Material no. 610432) diluted 1:1,000 in blocking buffer at 4°C overnight. The membrane was washed thrice with 1× PBS containing 0.3% Triton X-100 (PBST), and immunoblots were exposed for 1 h at room temperature to a 1:3,000 dilution of horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody. After three washes using 1× PBST, the membrane was impregnated with ECL reagent (Amersham Pharmacia Biotech) and used to expose X-ray film according to the manufacturer's instructions.
RESULTS
Basolateral application of human LC to immunostimulated Caco-2 monolayers inhibits iNOS activity in a dose- and time- dependent manner.
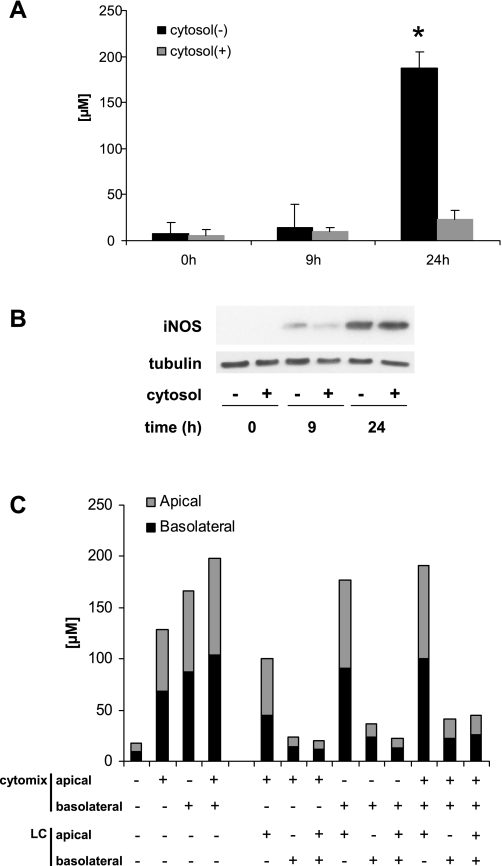
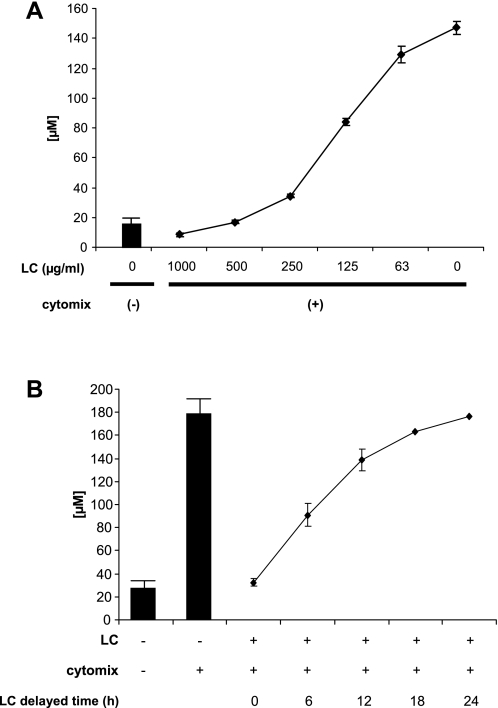
Our laboratory and others have shown that iNOS-dependent NO• production can decrease tight junction protein expression and decrease epithelial barrier function. We proposed that the multiple organ dysfunction syndrome is caused, at least in part, by decreased tight junction function, which results in loss of organ compartmentalization and function (12). We first examined the effect of human LC on NO• production by immunostimulated Caco-2 cells on collagen-coated dishes. LC suppressed iNOS activity completely when used at 400 μg/ml (Fig. 1A). However, the total amount of iNOS protein detected by Western blot analysis 24 h after stimulation was equivalent in cells cultured in both the absence and presence of LC (Fig. 1B). Although iNOS protein expression was slightly delayed in cells exposed to LC compared with control cells (9-h time point in Fig. 1B), this difference could not account for the huge decrease in the accumulation of NO2−/NO3− in these culture supernatants following exposure to LC. Monolayers cultured on semipermeable filters only responded to LC when added to the basolateral side regardless of the surface that was exposed to cytomix (Fig. 1C). Eckmann and colleagues (11) reported that the majority of Arg on the apical side of Caco-2 monolayers is rapidly transported to the basolateral surface. iNOS was reported to be located at the apical edge of Caco-2 cells (31), whereas its substrate is sequestered exclusively on the basolateral side. LC inhibition of iNOS activity was dose dependent ( Fig. 2A) and still exerted some effects even when it was added after cytomix exposure (Fig. 2B). This effect appears to be specific to liver cytosolic factors because Caco-2 cytosol had no effect on the production of NO• by Caco-2 monolayers exposed to cytomix (data not shown).
Fig. 1.
Liver cytosol (LC) decreases nitric oxide (NO•) production by suppressing inducible nitric oxide synthase (iNOS) activity only when applied to the basolateral side. Filtered human LC (400 μg/ml) and cytomix were added together to culture medium of Caco-2 cells growing on collagen-coated dishes which were then incubated for 24 h at 37°C. A: NO2−/NO3− concentration in medium was measured by the Griess assay. B: iNOS protein expression was measured by Western blot analysis. C: the polarity of the suppressive effect of LC on NO• was investigated with mature Caco-2 monolayers 21 days after seeding on the permeable membranes. Filtered LC (400 μg/ml) and cytomix were added to either or both the apical and basolateral sides of the monolayer as indicated in the graph. The concentration of NO2−/NO3− was measured after 24 h of stimulation. The data shown here are representative of the same experiment, all of which were equivalent. *P < 0.05 vs. 0 h control.
Fig. 2.
The suppressive effect of LC on NO• production is dose dependent and is apparent when LC is added after the inflammatory stimulus. A: a dose-response curve of LC-iNOS inhibition was generated by adding 0–1,000 μg of LC per milliliter to Caco-2 monolayers exposed to cytomix for 24 h. The y-axis represents the amount of NOx released from cells during the time period. B: the ability of LC (400 μg/ml) to inhibit NO• production was tested by adding LC to the basolateral surface of the cultures at times indicated following addition of cytomix.
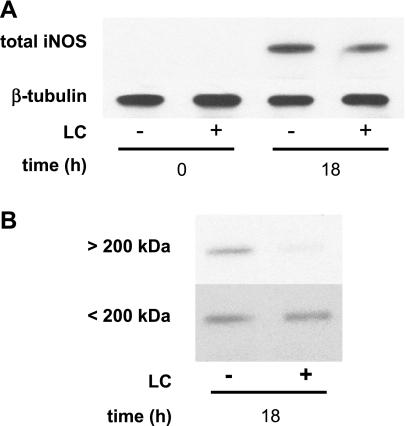
Monolayers exposed to LC express monomeric iNOS.
The data suggest that iNOS is inhibited posttranslationally. Monomeric and dimeric iNOS have molecular masses of 130 and 260 kDa, respectively, and its dimerization appears to be necessary for activation (1). Ultrafiltration of Caco-2 extracts through membranes with a MWCO of 200 kDa was used to monitor iNOS dimerization. Western blotting revealed that in the absence of LC Caco-2 cells expressed ∼25% dimeric and the remainder monomeric iNOS (Fig. 3B). Protein extracts from immunostimulated Caco-2 cells exposed to LC expressed similar amounts of monomeric iNOS but expressed almost undetectable levels of the active dimeric form.
Fig. 3.
LC inhibits iNOS dimerization. A: Caco-2 monolayers were exposed to cytomix for the times indicated in the figure, and total protein extracts were prepared and analyzed by Western blotting. B: after fractionation of the nonreduced whole Caco-2 cell lysates by filtration with 200-kDa molecular weight cutoff (MWCO) ultrafilters, each compartment was reconstituted to the same volume and proteins were reduced by boiling with loading buffer containing 2-mercaptoethanol. Dimeric and monomeric iNOS was detected by Western blot analysis of the >200-kDa and <200-kDa compartments, respectively.
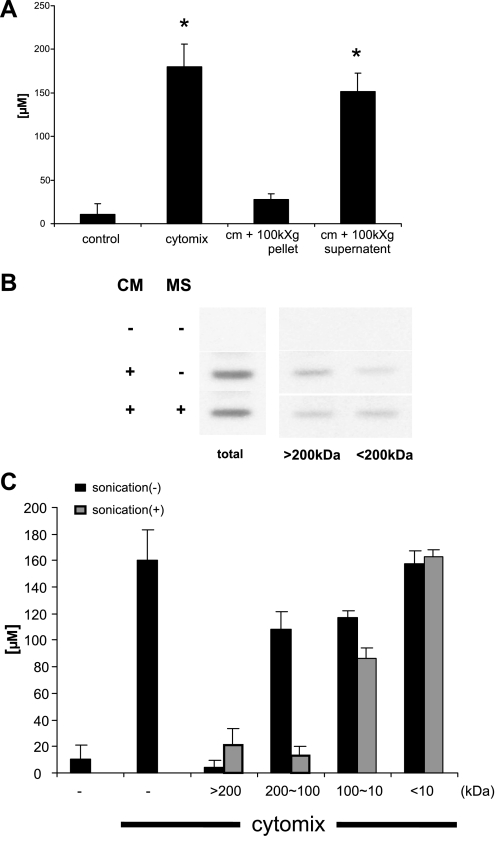
The iNOS-suppressing factor in LC is microsomal Arg-1.
To gain insight into the identity of the LC factor or factors that inhibit NO• release, we separated LC as follows. LC was subjected to filtration through 0.2-μm filters and then subjected to ultrafiltration to collect liver microsomes. The microsome pellet contained iNOS-suppressing activity (Fig. 4A) and inhibited iNOS dimerization (Fig. 4B) but not the supernatant. When LC was or was not sonicated before ultrafiltration sequentially through MWCO of 200-, 100-, and 10-kDa filters the only significant difference when comparing samples that were sonicated and those that weren't was observed with the 100- to 200-kDa fraction (Fig. 4C). These data indicate that this factor is a 100- to 200-kDa molecule present in the microsomal compartment of LC.
Fig. 4.
The iNOS-suppressing factor has an apparent molecular mass between 100 and 200 kDa and is present in the microsomal compartment of LC. The microsomal compartment was isolated from LC as described in materials and methods. Immunostimulated Caco-2 cells were exposed to either resuspended microsomes or the postmicrosomal supernatant for 24 h. A: NO2−/NO3− concentration was measured by Griess assay. B: inhibition of iNOS dimerization by the resuspended microsomes (MS) was performed as described in Fig. 3. C: 200 μl of LC was diluted to 1 ml with isotonic Tris buffer (25 mM Tris, pH 7.4, 130 mM NaCl) and was or was not sonicated for 30 s on ice and then spun at 15,000 g for 10 min. The supernatant was filtered sequentially through MWCO of 200-, 100-, and 10-kDa membranes. Each retentate was reconstituted to the original volume with Tris buffer and then added to immunostimulated Caco-2 cells, and NO2−/NO3− concentration was measured by Griess assay. *P < 0.05 vs. control.
The iNOS-suppressing factor in LC is Arg-1.
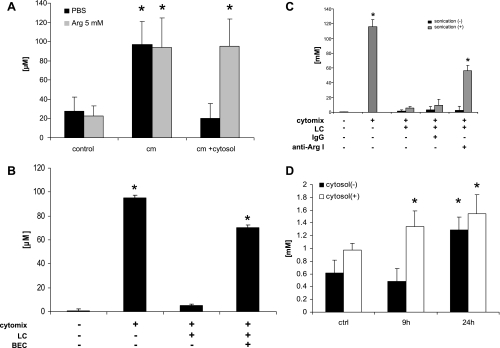
Taken together these data strongly suggest that Arg-1 is the factor in LC that decreases NO• production from Caco-2 cells exposed to cytomix. Arg-1 forms a 107-kDa homotrimer (5) that partitions into hepatocyte microsomal fractions (13). Arg depletion by Arg-1 inhibits iNOS dimerization in macrophages with a concomitant decrease in NOS activity (1). We tested this hypothesis by incubating Caco-2 cells in the presence of cytomix and LC in the absence or presence of 5 mM Arg, which completely blocked the ability of LC to inhibit NO• release (Fig. 5A). Similar results were obtained when measuring cytomix-induced Caco-2 epithelial barrier dysfunction in the presence and absence of Arg (data not shown). The Arg-1 and Arg-2 inhibitor BEC effectively blocked the iNOS-suppressing activity when it was included in the culture at 1 mM (Fig. 5B). Treating cells with anti-Arg-1 antibodies also blocked this affect of LC, but only after sonication of the extract (Fig. 5C). This effect was observed when supplying Arg even 18 h after cytomix/LC addition (data not shown). Finally, arginase activity was measured by evaluating urea production in the culture supernatant. Addition of LC resulted in near maximal accumulation of urea within 9 h of culture stimulation (Fig. 5D). These data lead us to conclude that the iNOS-suppressing factor in LC is Arg-1, which competes for available Arg with enterocyte iNOS.
Fig. 5.
The iNOS-suppressing factor in liver cytosol is arginase. Caco-2 monolayers were exposed to cytomix for 24 h, and supernatants were assayed for the release of NO2−/NO3− by the Griess assay. A: addition of 5 mM Arg completely blocked the ability of LC to prevent NO• production. B: 1 mM S-(2-boronoethyl)-l-cystein (BEC) completely blocked the ability of LC to prevent NO• production. C: LC (400 μg/ml) was or was not sonicated before addition to supernatants of immunostimulated Caco-2 cells in the presence or absence of either mouse control IgG (50 μg/ml) or anti-arginase type I (Arg-1) antibodies (50 μg/ml). Antibodies were added at the start of the cytomix stimulation. Anti-Arg-1 antibody significantly decreased the ability of sonicated but not nonsonicated LC to decrease NO• production from immunostimulated Caco-2 monolayers. D: urea concentration in the supernatants of immunostimulated Caco-2 cells was measured with or without LC (400 μg/ml). Addition of LC resulted in near maximal accumulation of urea within 9 h of culture stimulation. *P < 0.05 vs. control.
Liver arginase is activated only after contact with the supernatants from immunostimulated but not resting Caco-2 cells.
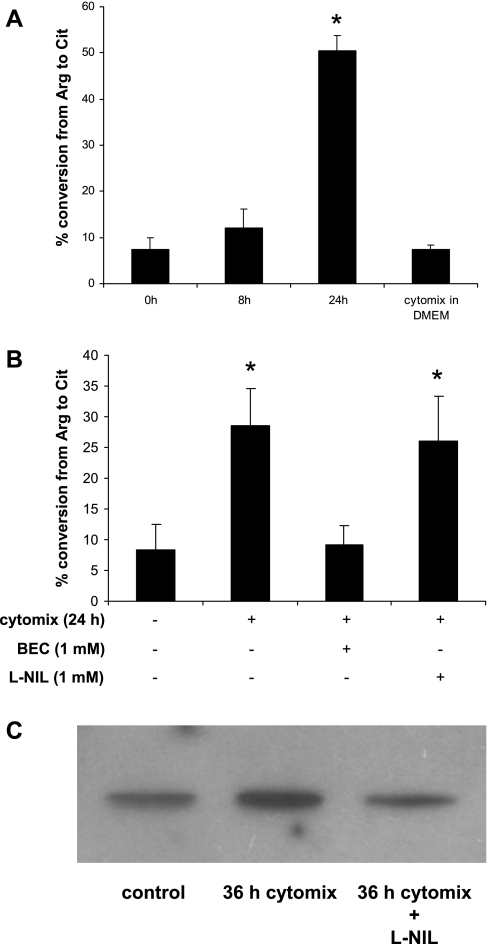
We next measured Arg-1 activity in LC by determining the conversion rate of Arg to Cit in vitro. LC arginase activity was low when it was measured in the presence of supernatant from nonstimulated Caco-2 cells or DMEM containing cytomix that had not been incubated with cells (Fig. 6A). However, LC acquired significantly higher arginase activity when mixed with supernatants from immunostimulated Caco-2 cells. As expected, BEC blocked in vitro activated Arg-1 activity, but when l-NIL was added to the culture supernatant before assembling the assay it had no effect on Arg-1 activation by immunostimulated Caco-2 culture supernatants (Fig. 6B). Similar results were observed with use of culture supernatants subjected to ultracentrifugation before mixing with LC (data not shown).
Fig. 6.
Arginase in liver cytosol is activated by supernatant of immunostimulated Caco-2 cells. A: Caco-2 cells were treated with vehicle alone or cytomix for 18 h, 500 μl of each supernatant was harvested and centrifuged at 1,000 g for 10 min to remove cell debris; 2 μl of LC (20 mg/ml) was added to 25 μl of each supernatant. After incubation for the times indicated in the figure the mixture was subjected to the arginase activity assay as described in materials and methods. As a negative control cytomix was added to 2 μl of LC in 25 μl of DMEM and subjected to the arginase activity assay. B: in the same system, BEC (1 mM) and l-N6-(1-iminoethyl)-lysine·2HCl (l-NIL; 700 μM) were added, respectively, to be subjected to the urea assay as described in the same way as in Fig. 5D. C: to investigate S-nitrosylation of the Arg-1 in LC, LC was subjected to biotin switch assay as described in materials and methods. *P < 0.05 vs. control.
One possible mechanism for this Arg-1 activation is S-nitrosylation of Arg-1 (42). The reported Km of NOS for Arg is in the 2–20 μM range with a Vmax of ∼1 μmol·min−1·mg−1, whereas Arg-1 has a Km of 2–20 mM with an estimated Vmax of about 1,400 μmol·min−1·mg−1 (26). It was recently shown that S-nitrosylation of Arg-1 decreases the Km sufficiently to allow Arg-1 to compete with iNOS for Arg over a wider concentration range (23, 33). Arginase activation was induced by the cell supernatants even after filtration through 10-kDa MWCO membranes (data not shown), suggesting that a small molecule present in the supernatant of immunostimulated Caco-2 cells was acting as an NO• donor. Consistent with this, we used the biotin switch assay to obtain data showing a 50% increase in S-nitrosylated Arg-1 in LC after incubation with supernatants of immunostimulated Caco-2 cells compared with resting cells, and this increase was blocked when l-NIL was included in the tissue culture media during cytomix stimulation (Fig. 6C). This led us to hypothesize that synthetic NO• donors should also activate Arg-1 present in LC. Culture supernatants from unstimulated Caco-2 cells was incubated with LC in the presence and absence of two distinct NO• donors, DETA-NONOate and S-nitrosoglutathione (GSNO). Interestingly, however, this treatment did not activate Arg-1 in LC even at a concentration as high as 5 mM (data not shown).
iNOS dimerization was inhibited in gut mucosa of endotoxemic mice.
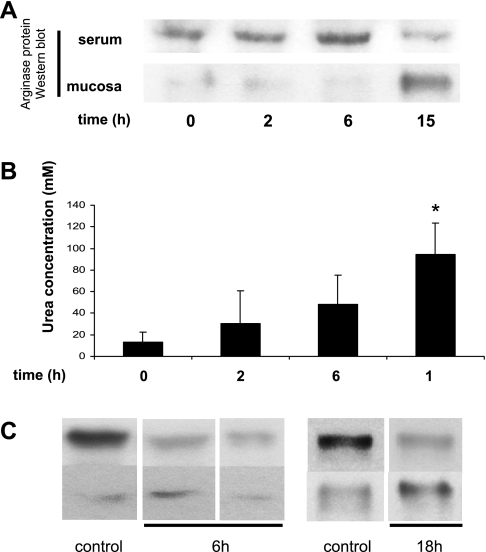
C57Bl/6J mice were injected with 15 mg LPS/kg ip. Arg-1 in plasma and in ileal mucosa was detected by Western blot, and serum arginase activity was measured at 0, 2, 6, and 15 h following injection. Serum Arg-1 protein levels increased during the first 6 h and had decreased by 15 h after injection (Fig. 7A). Mucosal levels of Arg-1 protein were very low during the first 6 h of endotoxemia and were sharply increased at the 15-h time point. Figure 7B shows the activity of arginase in serum by the production of urea. Interestingly, in contrast to decreased Arg-1 protein levels in serum after 15 h, the activity of circulating arginase activity peaked at that time point (Fig. 7B). This suggests activation of circulating arginase presumably from exposure to peripheral tissues producing NO•. Protein extracts were prepared from gut mucosa obtained from normal mice and mice 6 and 18 h after injection with endotoxin. Although the total amounts of iNOS varied with the individual animal, the relative amount of monomeric to dimeric iNOS was lower in mucosa at 18 h compared with 0 and 6 h (Fig. 7C).
Fig. 7.
Arg-1 activity is increased in the serum of endotoxemic mice model. A: arginase levels in serum and in ileal mucosa of endotoxemic mice challenged with 15 mg/kg of LPS was detected at 0, 2, 6, and 15 h after injection. The data shown are representative of 3 studies that gave equivalent results. B: arginase activity in serum was measured at each time point described in A (n = 3 mice per group). C: iNOS dimerization is blocked in mucosa of endotoxemic mice. Ileal mucosa was harvested from control (0 h), 6, and 18 h LPS-stimulated (15 mg/kg) mice, and proteins were extracted and fractionated by ultrafiltration with 200-kDa MWCO membranes as described in materials and methods (representative of 3 separate experiments).
DISCUSSION
We hypothesized that increased circulating levels of digestive enzymes derived presumably from the liver during systemic inflammation or resulting from localized hepatic damage caused by drug-induced liver injuries including antibiotics or acetaminophen overdose, alcoholic cirrhosis, and viral infection. Interestingly, Arg-1 is one of the earliest cytoplasmic components to be released from hepatocytes in response to an inflammatory stimulus (27). Given that Arg-1 does not need to be synthesized de novo, it would most likely be present in the circulation before iNOS protein is expressed in various tissues. Therefore, it would be available to modulate iNOS activity as it is activated in target organs and tissues. As a first test of this hypothesis we exposed immunostimulated Caco-2 cells to human LC to determine which if any liver cytosolic components would modulate cytokine-induced alterations in iNOS-dependent NO• production. Given the molecular complexity of LC, we were surprised that Arg-1 was the only factor needed to preserve epithelial barrier function (data not shown) and decrease iNOS activity in immunostimulated Caco-2 cells. Another enzyme to consider is Orn transcarbamylase, which converts Orn to Cit after arginase converts Arg to Orn and urea. This enzyme is known to exist in LC and may contribute to the depletion of Arg and inactivation of iNOS in addition to Arg-1. This enzyme exists predominantly in hepatocytes and small intestinal epithelial cells (24), and its potential contribution to our findings remains to be investigated.
Interplay between cellular NOS and arginase has been reported in vascular endothelium, smooth muscle cells, immune cells including macrophages and lymphocytes, and the respiratory system (4, 10, 11, 36). Most reports concerning induced circulating arginase levels during inflammation used Western blotting, reverse transcriptase PCR, and immunohistochemistry to detect changes in arginase expression. Values from conventional arginase assays were also reported. However, this assay was performed following heat activation of arginase in vitro. The usefulness of this information is limited as it represents total potential arginase activity in the sample and does not represent actual in vivo enzyme activity. Our studies are novel in that they show that immunostimulated cells in vitro are able to activate extracellular arginase in an iNOS-dependent manner. We also obtain in vivo evidence for the activation of circulating arginase activity by the local gut mucosa during a systemic inflammatory response.
It has been shown that iNOS requires Arg to induce its dimerization, which is required for NOS activation (11). We confirmed that arginase suppresses iNOS activity in Caco-2 cells predominantly by depleting Arg levels, thus inhibiting iNOS dimerization (Fig. 3B). Depletion of Arg also decreased the levels of iNOS protein to some extent (Figs. 1B and 3B). Astrocytes express significantly decreased levels of iNOS protein following Arg depletion by arginase, and this was associated with decreased phosphorylation of eukaryotic initiation factor elF2, which regulates translation of the iNOS message (20).
We observed an unexpected requirement for Caco-2 cell immunostimulation for maximal activation of arginase present in LC, since conditioned supernatants from Caco-2 monolayers failed to increase LC arginase activity in vitro (Fig. 6A). We found that this ability of activated Caco-2 cells to increase arginase activity could be inhibited by treating cells with l-NIL, supporting a role for NO• in this process (Fig. 6B). Addition of l-NIL to postculture supernatants from cytomix-treated cells failed to inhibit activation of LC Arg-1 in vitro. This “arginase-activating factor” released by immunostimulated Caco-2 cells was less than 10 kDa. Data obtained using the biotin switch assay is consistent with S-nitrosylation of LC Arg-1 in supernatants from activated cells (Fig. 6C). Nevertheless the NO• donors GSNO and DETA-NONOate failed to increase LC Arg-1 activity in the presence of culture medium conditioned by resting Caco-2 cells. Together these results support a model in which iNOS-derived NO• produced by immunostimulated enterocytes causes an unknown modification of key thiols in extracellular Arg-1, causing its activation. Our results suggest that activation of liver Arg-1 would still require some additional factor secreted by immunostimulated Caco-2 cells in addition to NO•. In this way, circulating Arg-1 that would be present during a systemic inflammatory response would be activated by NO• only at sites where NOS is active.
Finally we confirmed that arginase activity was significantly increased in endotoxemic murine serum between 6 and 18 h after injection with endotoxin (Fig. 7B). This was in contrast to Arg-1 protein levels in serum, which peaked during the first 6 h of endotoxemia then returned to near baseline levels by 15 h (Fig. 7A). Ileal mucosal Arg-1 protein staining was very low during the first 6 h of endotoxemia and greatest at 15 h. This also preceded the time point when iNOS existed primarily in its inactive monomeric form in mucosa (Fig. 7C).
An important question that is not addressed in these studies concerns the source of increased Arg-1 protein in ileal mucosa that was observed at 15 h of endotoxemia. Is this increased Arg-1 synthesized de novo in immunostimulated mucosal tissue or does it reflect captured liver Arg-1 that has been activated by iNOS-derived NO• generated in villous enterocytes? The finding that increased mucosal protein levels of Arg-1 were maximal at a time point when serum Arg-1 protein levels were lowest supports the latter hypothesis. Our results using Caco-2 cells would suggest that circulating Arg-1 is activated in an NO•-dependent manner when it is in the vicinity of the inflamed mucosa. Additional studies will be required to actually identify the source of ileal mucosal Arg-1 in this murine model of systemic inflammation.
The importance of circulating Arg levels was reviewed in a paper by Barbul in 1986 (2) in which he argued that Arg supplementation could be helpful in treating various diseases. Clinical studies of Arg supplementation to reverse the Arg-deficient state observed in critically ill patients have met with mixed reviews (3, 4, 18, 22). Our results suggest that Arg-1 is only activated systemically in patients that also experience iNOS activation. It can be argued that these patients should not receive Arg supplementation since it may exacerbate the effects of iNOS on epithelial dysfunction and increase morbidity and/or mortality associated with the multiple organ dysfunction syndrome (12). Because liver arginase was reported to improve the survival rate in an LPS shock model following intravenous injection (41), we believe that further studies of the interplay between parenchymal iNOS and circulating Arg-1 are supported.
GRANTS
This research was supported by NIH grants NIH R01 GM-37631 (R. L. Delude) and P50 GM-53789 (Dr. Timothy R. Billiar, Department of Surgery, University of Pittsburgh School of Medicine).
REFERENCES
- 1.Baek KJ, Thiel BA, Lucas S, Stuehr DJ. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem 268: 21120–21129, 1993 [PubMed] [Google Scholar]
- 2.Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr 10: 227–238, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Barbul A, Uliyargoli A. Use of exogenous arginine in multiple organ dysfunction syndrome and sepsis. Crit Care Med 35: S564–S567, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Beruter J, Colombo JP, Bachmann C. Purification and properties of arginase from human liver and erythrocytes. Biochem J 175: 449–454, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol 5: 641–654, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chavez AM, Menconi MJ, Hodin RA, Fink MP. Cytokine-induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med 27: 2246–2251, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Colleluori DM, Ash DE. Classical and slow-binding inhibitors of human type II arginase. Biochemistry 40: 9356–9362, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg 125: 403–404, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, Gillin FD. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J Immunol 164: 1478–1487, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin 21: 177–196, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Galeva N, Altermann M. Comparison of one-dimensional and two-dimensional gel electrophoresis as a separation tool for proteomic analysis of rat liver microsomes: cytochromes P450 and other membrane proteins. Proteomics 2: 713–722, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Gobert AP, Cheng Y, Akhtar M, Mersey BD, Blumberg DR, Cross RK, Chaturvedi R, Drachenberg CB, Boucher JL, Hacker A, Casero RA, Jr, Wilson KT. Protective role of arginase in a mouse model of colitis. J Immunol 173: 2109–2117, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Houdijk AP, Teerlink T, Visser JJ, van Lambalgen AA, van Leeuwen PA. Arginine deficiency in bile duct-ligated rats after surgery: the role of plasma arginase and gut endotoxin restriction. Gastroenterology 113: 1375–1383, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Huber-Lang M, Sarma VJ, Lu KT, McGuire SR, Padgaonkar VA, Guo RF, Younkin EM, Kunkel RG, Ding J, Erickson R, Curnutte JT, Ward PA. Role of C5a in multiorgan failure during sepsis. J Immunol 166: 1193–1199, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3: 193–197, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Kalil AC, Danner RL. l-Arginine supplementation in sepsis: beneficial or harmful? Curr Opin Crit Care 12: 303–308, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kanai S, Honda T, Uehara T, Matsumoto T. Liver function tests in patients with bacteremia. J Clin Lab Anal 22: 66–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA 100: 4843–4848, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis CA, Reichner JS, Henry WL, Jr, Mastrofrancesco B, Gotoh T, Mori M, Albina JE. Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. Am J Physiol Regul Integr Comp Physiol 274: R775–R782, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE. Sepsis: an arginine deficiency state? Crit Care Med 32: 2135–2145, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J Nutr 137: 1616S–1620S, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Mori M, Gotoh T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem Biophys Res Commun 275: 715–719, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Morin MJ, Unno N, Hodin RA, Fink MP. Differential expression of inducible nitric oxide synthase messenger RNA along the longitudinal and crypt-villus axes of the intestine in endotoxemic rats. Crit Care Med 26: 1258–1264, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Morris S. Regulation of arginine availability and its impact on NO synthesis. In: Nitric Oxide Biology and Pathobiology, edited by Ignarro LJ. Los Angeles, CA: Academic, 2005, p. 187–197 [Google Scholar]
- 27.Murayama H, Ikemoto M, Fukuda Y, Nagata A. Superiority of serum type-I arginase and ornithine carbamyltransferase in the detection of toxicant-induced acute hepatic injury in rats. Clin Chim Acta 391: 31–35, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Nijveldt RJ, Siroen MP, van der Hoven B, Teerlink T, Prins HA, Girbes AR, van Leeuwen PA. High plasma arginine concentrations in critically ill patients suffering from hepatic failure. Eur J Clin Nutr 58: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ochoa JB, Bernard AC, O'Brien WE, Griffen MM, Maley ME, Rockich AK, Tsuei BJ, Boulanger BR, Kearney PA, Morris SM., Jr Arginase I expression and activity in human mononuclear cells after injury. Ann Surg 233: 393–399, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oismuller C, Mayer N, Micksche M, Steltzer H, Hammerle AF. In vivo modulation of human neutrophil function by pentoxifylline in patients with septic syndrome. Shock 4: 161–165, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Rumbo M, Courjault-Gautier F, Sierro F, Sirard JC, Felley-Bosco E. Polarized distribution of inducible nitric oxide synthase regulates activity in intestinal epithelial cells. FEBS J 272: 444–453, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salzman AL, Eaves-Pyles T, Linn SC, Denenberg AG, Szabo C. Bacterial induction of inducible nitric oxide synthase in cultured human intestinal epithelial cells. Gastroenterology 114: 93–102, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Santhanam L, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res 101: 692–702, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Sonoki T, Nagasaki A, Gotoh T, Takiguchi M, Takeya M, Matsuzaki H, Mori M. Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J Biol Chem 272: 3689–3693, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Spain DA, Wilson MA, Bar-Natan MF, Garrison RN. Role of nitric oxide in the small intestinal microcirculation during bacteremia. Shock 2: 41–46, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Tadie JM, Henno P, Leroy I, Danel C, Naline E, Faisy C, Riquet M, Levy M, Israel-Biet D, Delclaux C. Role of nitric oxide synthase/arginase balance in bronchial reactivity in patients with chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 294: L489–L497, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology 113: 1246–1257, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Vincendeau P, Gobert AP, Daulouede S, Moynet D, Mossalayi MD. Arginases in parasitic diseases. Trends Parasitol 19: 9–12, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Vodovotz Y. Modified microassay for serum nitrite and nitrate. Biotechniques 20: 390–392, 394, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med 178: 605–613, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang SR. Method for protecting against endotoxin-induced shock using ornithine, edited by USPTO. US Patent 5,686,493. Nov. 11,1997 [Google Scholar]
- 42.Witthoft T, Eckmann L, Kim JM, Kagnoff MF. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am J Physiol Gastrointest Liver Physiol 275: G564–G571, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension 44: 935–943, 2004 [DOI] [PubMed] [Google Scholar]