Abstract
P2Y5 is a G protein-coupled receptor that binds and is activated by lysophosphatidic acid (LPA). We determined that P2Y5 transcript is expressed along the intestinal mucosa and investigated the intracellular pathways induced by P2Y5 activation, which could contribute to LPA effects on intestinal cell adhesion. P2Y5 heterologously expressed in CHO and small intestinal hBRIE 380i cells was activated by LPA resulting in an increase in intracellular calcium ([Ca2+]i) when the cells concurrently expressed GαΔ6qi5myr. P2Y5 activation also increased the phosphorylation of ERK1/2 that was sensitive to pertussis toxin. Together these indicate that P2Y5 activation by LPA induces an increase in [Ca2+]i and ERK1/2 phosphorylation through Gαi. We discovered that P2Y5 was activated by farnesyl pyrophosphate (FPP) without a detectable change in [Ca2+]i. The activation of P2Y5 by LPA or FPP induced the activity of a serum response element (SRE)-linked luciferase reporter that was inhibited by the RGS domain of p115RhoGEF, C3 exotoxin, and Y-27632, suggesting the involvement of Gα12/13, Rho GTPase, and ROCK, respectively. However, only LPA-mediated induction of SRE reporter activity was sensitive to inhibitors targeting p38 MAPK, PI3K, PLC, and PKC. In addition, only LPA transactivated the epidermal growth factor receptor, leading to an induction of ERK1/2 phosphorylation. These observations correlate with our subsequent finding that P2Y5 activation by LPA, and not FPP, reduced intestinal cell adhesion. This study elucidates a mechanism whereby LPA can act as a luminal and/or serosal cue to alter mucosal integrity.
Keywords: farnesyl pyrophosphate, GPCR, serum response element, Rho, mucosa
lysophosphatidic acid (LPA) is a phospholipid derivative that can act as a potent extracellular signaling molecule through the activation of G protein-coupled receptors (GPCRs). The better known LPA receptors belong to the endothelial differentiation gene (EDG) family, designated as LPA1/EDG2, LPA2/EDG4, and LPA3/EDG7 (41). Cellular processes initiated by LPA receptor activation include neurite retraction, cytoskeletal reorganization, smooth muscle cell contraction, and cell proliferation (53, 59).
The intestinal mucosal integrity essential for the functions of healthy intestine requires the coordination of renewal and migration of epithelial cells from the crypt to villus tip where anoikis [a programmed cell death caused by the loss of integrin-mediated cell adhesion (15)] occurs (62). LPA contributes to the maintenance of the intestinal epithelial integrity (60) by regulating cellular events such as epithelia restitution and inflammation responses (6, 60, 73). The intestine is a unique tissue that is exposed to both exogenous LPA from the diet (45) and endogenous LPA from the circulation and cells in the mucosa (3, 13, 42). The EDG family receptors such as LPA1 and LPA2 are expressed in the intestine; however, specific functions of non-EDG family LPA-activated GPCRs have not been elucidated.
Recently, the activation of a family of GPCRs, with sequence similarities to the purinergic receptors (P2Y family), by LPA was reported (7, 31, 33, 44, 47–49). Some of these receptors are also activated by other agonists in addition to LPA (7, 44, 48). The multiple effects of LPA on the intestine might be explained by the presence of these non-EDG family LPA responsive GPCRs, which are capable of having their activation by LPA enhanced by the presence of other ligands to this group of receptors. Of these newly identified LPA-responsive receptors, we have observed that P2Y5 mRNA appears to be at similar levels along the proximal to distal intestine. The distribution of P2Y5 transcript along the intestinal mucosa would suggest that the activation of this receptor might be important for maintaining events involved in mucosal homeostasis.
P2Y5, originally designated as 6H1, was later renamed as P2Y5 because of its similarities to nucleotide receptors (P2Y family) and its ability to bind [35S]dATPαS (67). Nevertheless, the identity of the agonist of P2Y5 remained elusive since nucleotides did not activate second messenger systems in P2Y5 transfected cells regardless of their high binding affinities (35). The prior establishment of LPA as an agonist of the P2Y5-related GPCRs [i.e., P2Y9 and GPR93 (also named GPR92) (7, 31, 33, 47, 48)] led to the discovery that P2Y5 is activated by LPA as well (49). However, the mechanism(s) of the biological effects resulting from the activation of P2Y5 remains to be clarified. Critical to the understanding of the role(s) that P2Y5 could play in the intestine is the determination of its downstream effector molecules upon activation.
In the present study, we demonstrate P2Y5 activation by LPA and farnesyl pyrophosphate (FPP). This activation is coupled to Gαi and Gα12/13, resulting in a reduced forskolin-stimulated cAMP level, an increase in the phosphorylation of ERK1/2, an induction of serum response element (SRE)-linked reporter activity, and a reduction in small intestinal hBRIE 380i cell attachment to extracellular matrix substratum. The increase in ERK1/2 phosphorylation is through a Gαi pathway and mediated by MEK1/2. The activation of SRE reporter activity is through a Gα12/13 pathway and mediated by Rho kinase (ROCK), possibly through the activation of Rho family small GTPase. This study demonstrates that P2Y5 may play an important role in intestinal cell adhesion in response to molecules that can be presented to the cell at both basolateral and apical surfaces such as LPA and FPP.
MATERIALS AND METHODS
Materials.
1-Oleoyl [oleoyl-9, 10-3H(N)] lysophosphatidic acid (47.0 Ci/mmol) and [1-3H(N)]-farnesyl pyrophosphate (26.2 Ci/mmol) were obtained from PerkinElmer Life Sciences. Oleoyl LPA (18:1-LPA), oleoyl thiophosphate, and tetradecyl phosphonate were purchased from Cayman Chemical. Stearoyl LPA (18:0-LPA), palmitoyl LPA (16:0-LPA), myristoyl LPA (14:0-LPA), dioleoyl phosphatidic acid (18:1-PA), and oleoyl phosphatidylcholine (18:1-PC) were purchased from Avanti Polar Lipids. Pertussis toxin (PTX), Y-27632, AG 1478, LY294002, genistein, PD 98059, edelfosine, Gö 6976, and U-73122 were purchased from Calbiochem. All other reagents used in this study, including meat protein hydrolysate (peptone; a mixture of enzymatically derived peptide fragments, mostly between 120 and 1,200 Da, and free amino acids) type I, were purchased from Sigma-Aldrich unless indicated differently. The LPA used in this study was dissolved in PBS (pH. 7.4) with 0.1% fatty acid-free BSA (ffBSA) as a carrier.
Plasmid construction.
The mitochondria-targeted aequorin (mtAEQ), Gα15, GαΔ6qi5myr, β2-adrenergic receptor (β2AR), P2Y5, P2Y5 tagged at the COOH terminus with the enhanced green fluorescent protein (EGFP) (P2Y5-EGFP fusion), and P2Y9 expression vectors were constructed as previously described (7). All primers used in the construction of the plasmids are listed in Table 1.
Table 1.
Oligonucleotide primers used for RT-PCR analyses and cDNA cloning
| Gene | Forward Primer | Reverse Primer | ||
|---|---|---|---|---|
| Plasmid Construction | ||||
| P2Y5-EGFP | CGGGATCCATTATTGCAGATTCATTATCAAATATC | |||
| RGS-p115 | CCCACCATGTACCCATACGATGTTCCAGATTACGCCATGGGAGAA | GCGCTAGCTTATCAATTCCCCATCACCTTTTTCCGGAAGAAG | ||
| GTCGC | TTCCTTC | |||
| RGS-GRK2 | GTCCACCATGGGAAAGTATCTAGAGGATCGAGGAGAAG | CATTACCCGGGTCACTTCCACTGGCAGAACCGTGTGAACTTG | ||
| RT-PCR Analysis | ||||
| P2Y5 | GGGTGCATGTTCAGCATGGT | GGGTAGACAATTGCCAGAAATCG | ||
The primer sequences are listed in 5′ to 3′ direction.
Plasmids encoding the RGS domains of rat p115RhoGEF and GRK2 overexpressing plasmids were constructed following the method described by Huang et al. (24) and Hains et al. (16).
The vector base for the reporter constructs, pBV-luc, was a generous gift from Dr. Bert Vogelstein (The John Hopkins Kimmel Cancer Center). The SRE (GGATGTCCATATTAGGACATC)- and the cAMP response element (CRE; TGACGTCA)-linked luciferase reporter constructs containing eight tandem repeats of the respective response elements were constructed as previously described (7).
The C3 exotoxin expression vector was constructed by excising the open reading frame of C3 exotoxin from a bacterial expression vector (a generous gift from Dr. Steve Martin; University of California, Berkeley) and subcloning it into a mammalian expression vector, pCI-neo (Promega).
All constructs were verified by DNA sequencing (DNA Sequencing Facility, University of California, Berkeley).
Cell culture conditions and transfection.
The hybrid Berkeley Rat Intestinal Epithelial 380i (hBRIE 380i) cell line is nontumorigenic and expresses enterocyte phenotypes and intestine-specific protein markers (4, 17, 19). The hBRIE 380i cells used in these experiments were from passages 11 to 18. The cell culture and electroporation conditions for hBRIE 380i and Chinese hamster ovary (CHO) cells were as previously described (7). HeLa cells were kindly provided by Dr. John Forte (University of California, Berkeley). The culture conditions for HeLa cells were as described by Forte and coworkers (74), and the Superfect transfection reagent (Qiagen) was used to transfect following the manufacturer's protocol.
The P2Y5 or empty vector stable transfectants were developed as follows: 36 h after the electroporation (3 μg plasmid DNA/106 cells), resistant clones were selected and subsequently maintained in the presence of 800 μg/ml G418 (Invitrogen). The overexpression of P2Y5 was verified by PCR and the [Ca2+]i mobilization assay.
Tissue preparation and RNA isolation for P2Y5 expression profile.
Total RNA from hBRIE 380i cells and rat tissues including intestinal cells isolated by laser microscopy dissection (LMD) were prepared as previously described (7). In brief, 12-h fasted male Sprague-Dawley rats (14 wk old) were used as tissue sources (n = 3). After animals were euthanized, brain, heart, lung, kidney, pancreas, liver, stomach, and small and large intestine were isolated for mRNA analysis. Intestinal epithelial samples were prepared as follows: intestines were extracted, cleaned, and cut into segments. The mucosal layer of the intestine was obtained by gentle scraping of the exposed luminal surface, and the purity of the epithelial preparations were verified by determining the relative expression of villin and intestinal fatty acid binding proteins (I-FABP) by use of RT-PCR. Duodenal samples used for LMD were prepared by cutting duodenum into 2-mm sections after a 70% ethanol fixation. The tissue sections were washed with ice-cold PBS and immersed in ice-cold 30% (wt/vol) sucrose in PBS overnight at 4°C. The sucrose-equilibrated sections were cryosectioned at 10-μm thickness and then stored at −80°C. LMD and analysis of mRNA were performed as previously described (9) by using a Leica AS LMD system followed by semiquantitative RT-PCR. Animals used in these studies received humane care according to National Institutes of Health (NIH) guidelines; studies were performed after approval by the Animal Care and Use Committee of the University of California at Berkeley.
Semiquantitative RT-PCR.
Reverse transcription was performed as we previously described (34). The PCR primers for P2Y5 (sequence listed in Table 1) were designed on the basis of the rat P2Y5 sequence (Ensembl Gene ID: ENSRNOG00000015577). Taq DNA polymerase (New England Biolabs) was used to PCR amplify a 302-bp fragment of P2Y5 cDNA. The PCR primers for the ribosomal 18S RNA, villin, and I-FABP were as described previously (34). The PCR parameters were: 20 s at 94°C, 15 s at 55°C, and 30 s at 72°C; for 19–35 cycles.
AEQ-based [Ca2+]i mobilization assay.
CHO or hBRIE 380i cells were electroporated with the mtAEQ expression plasmid (2 μg/106 cells) and either P2Y5 alone (4 μg/106 cells) or P2Y5 plus Gα protein cDNA (2 μg/106 cells). The amount of electroporated DNA was equalized by using the empty vector. Cells were allowed to recover for 20 h in Iscove's modified Dulbecco's medium (IMDM; Invitrogen)/10% bovine calf serum (BCS; Hyclone Laboratories), and then a [Ca2+]i mobilization assay was performed as previously described (7). Luminescence [as relative light units (RLU)] was recorded continuously. Fractional RLU is defined as the increased RLU due to a stimulus normalized to the total RLU. Total RLU is the integrated RLU value for 30 s after the injection of the stimulus plus the 20 s after the addition of the lysis buffer.
Localization of P2Y5 in hBRIE 380i cells.
The hBRIE 380i cells were transfected with the P2Y5-EGFP fusion construct by electroporation (4 μg plasmid DNA/106 cells). After a recovery incubation in IMDM-10% BCS under normal culture conditions for 24 h, cells were trypsinized, resuspended in phenol red-free IMDM-10% BCS media, and plated on six-well slides coated with collagen type I at a density of 104/well for 16 h. The images of EGFP-tagged P2Y5 were acquired by using a Zeiss 510 Meta confocal microscope and a ×63 water-dipping lens. The samples were excited by using a 488-nm argon laser line. A 505-to 550-nm barrier filter was used to filter the emission light.
Measurement of intracellular cAMP.
CHO cells were electroporated with the P2Y5 expression plasmid or empty vector (6 μg of DNA/106 cells) and then plated in 12-well plates (5 × 105 cells/well) in IMDM-10% BCS. After 24 h, cells were washed three times with PBS and preincubated in HBSS/0.1% ffBSA for 30 min, followed by an additional 30 min incubation in the presence of 1 mM of 3-isobutyl-1-methylxanthine (IBMX). Cells were then treated with stimuli for 7 min. The treatments were terminated by placing the cells on ice and rinsing three times with ice-cold PBS. Cells were scraped on ice with 200 μl of 0.1 M HCl. The cytosolic fraction of each sample was obtained by centrifugation (10,000 g, for 10 min at 4°C), and the cAMP concentration was determined by an enzyme immunoassay method (Cayman Chemical). Under this condition, 2.5 μM forskolin increased intracellular cAMP by 7.4 ± 1.2-fold, and 100 μM isoproterenol increased intracellular cAMP by 8.8 ± 1.3-fold in β2AR transfected cells. The interassay coefficient of variance was less than 20%, and the lowest detectable level of cAMP was 3 nM.
Ligand binding assay.
hBRIE 380i cells stably transfected with P2Y5 or the empty vector were laid down in T150 flasks (4 × 106 cells/flask). After 24 h in IMDM-10% BCS, cells were placed on ice and scraped with 5 ml of binding buffer [20 mM Tris·Cl, pH 7.5; 1 mM EDTA; and protease inhibitor cocktail (Roche)] per T150 flask. Cell lysates were incubated on ice for 20 min and then homogenized on ice for 40 strokes/sample. The crude membrane from hBRIE 380i cells was prepared by centrifugation at 16,000 g, for 3 min at 4°C after homogenization. The pellets obtained from the centrifugation, which contained cell membranes, were resuspended in ice-cold binding buffer and the protein concentration was determined by the Bio-Rad protein assay. The crude cell membrane (30 μg) was incubated with various doses of [3H]18:1-LPA or 100 nM [3H]FPP in the presence of 0.1% ffBSA for 30 min at 25°C with gentle shaking. The binding reaction was stopped by placing the samples on ice, followed by a 1:10 dilution with ice-cold binding buffer containing 0.1% ffBSA. The membrane bound [3H]18:1-LPA was immediately vacuum collected on a GF/C glass microfiber filter disk (Whatman). The filter was then rinsed three times with ice-cold binding buffer containing 1% BSA and dried overnight at room temperature. The radioactivity of each sample was measured in a liquid scintillation counter (Beckman). Nonspecific binding was determined in the presence of 10 μM unlabeled 18:1-LPA or FPP. The value of Kd and Bmax and the saturation plot were generated by using the GraphPad Prism version 4 with one-site binding nonlinear regression. The competition binding experiment of [3H]18:1-LPA with different phospholipids was performed to investigate the specific binding of 10 nM [3H]18:1-LPA in the presence or absence of different competitors. The specific binding of 10 nM [3H]18:1-LPA was arbitrarily set to 100%. The values of Ki were generated by using the GraphPad Prism version 4 with one-site competition nonlinear regression algorithm.
Western immunoblotting.
The hBRIE 380i cells stably transfected with the P2Y5 cDNA or empty vector were grown on six-well plates (seeded at an initial density of 5 × 105 cells/well) in IMDM-10% BCS for 24 h. The cells were then serum starved for 12 h in IMDM/0.1% ffBSA prior to the treatment with various doses of 18:1-LPA for the time indicated in the figures. Sample preparation and the Western blot procedure were as previously described (7). The amount of protein per lane was 40 μg for phospho-ERK1/2 detection and 100 μg for epidermal growth factor receptor (EGFR). For loading control, the membrane was stripped after probing with the phospho-ERK antibody and reprobed with the total ERK antibody. The primary antibody against ERK1 (Santa Cruz Biotechnology) was used at a dilution of 1:6,000; phospho-ERK1/2 (Cell Signaling) at 1:1,000; EGFR (Cell Signaling) at 1:2,000; and the horseradish peroxidase-conjugated secondary antibody at 1:10,000.
Luciferase reporter assay.
The hBRIE 380i cells were electroporated with the reporter plasmid DNA (2 μg/106 cells) and the P2Y5 expression construct or empty vector (6 μg/106 cells). The transfectants were allowed to recover on 24-well plates (2.5 × 105 cells/well) for 24 h in IMDM-10% BCS, followed by serum starvation for 2 h in IMDM/0.1% ffBSA. The treatments with LPA or FPP were for 6 h. Luciferase activity from the samples was determined as previously described (7).
In the experiments that require cotransfection with C3 exotoxin, hBRIE 380i cells were electroporated with the reporter plasmid DNA (2 μg/106 cells), the P2Y5 expression construct or empty vector (2 μg/106 cells), and the C3 exotoxin (2 μg/106 cells).
In the experiments that require cotransfection with the RGS domain in HeLa cells, cells were transfected by using Superfect with the reporter plasmid DNA (2 μg/106 cells), P2Y5 expression construct or empty vector (2 μg/106 cells), and the RGS domain (4 μg/106 cells). After 24 h in Dulbecco's modified Eagle's medium (DMEM; Invitrogen)/10% fetal bovine serum (FBS; Hyclone Laboratories), the cells were serum starved for 2 h in DMEM/0.1% ffBSA, followed by 6-h treatments with LPA or FPP.
Adhesion assay.
hBRIE 380i cells stably transfected with P2Y5 cDNA or the empty vector control were seeded in T75 flasks (1 × 106 cells/flask) 24 h prior to the experiments. On the day of the experiments, cells were trypsinized, resuspended in 1 ml IMDM/0.1% ffBSA, and gently rolled for 2 h for recovery as well as acclimation to the serum free condition. Cells were collected by centrifugation at 100 g for 5 min, resuspended in fresh IMDM/0.1% ffBSA, and then plated on 24-well plates (1.2 × 105 cells/well) coated with type I collagen. The cells were allowed to adhere for 2 h in 37°C in the presence of either 18:1-LPA or FPP. The assay was terminated by washing the cells four times with ice-cold PBS (pH 7.4). The effect of each treatment on cell adhesion was determined by crystal violet cell staining as previously described (34).
Inhibitors treatment.
The inhibitors used in this study and their respective targets are listed in Table 2.
Table 2.
The inhibitors used in this study and their respective targets
| Inhibitor Name | Target Signal Molecules | |
|---|---|---|
| Chemical Inhibitors | ||
| Pertussis toxin | Gαi family and GαΔ6qi5myr | |
| PD 98059 | ERK1/2 | |
| Genistein | Tyrosine kinase | |
| AG 1478 | EGFR | |
| RGS-p115RhoGEF | Gα12/13 family | |
| RGS-GRK2 | Gαq family | |
| C3-exoenzyme | Rho family GTPase | |
| Y-27632 | Rho kinase | |
| SB202190 | p38 MAPK | |
| LY294002 | Phosphoinositide-3 kinase | |
| Edelfosine | Phosphatidylinositol-specific PLC | |
| Gö 6976 | PKCα, PKCβI, PKCγ, PKD1 (PKCμ) | |
| PKD2, and PKD3 (PKCν) | ||
| U-73122 | PLCβ | |
| FTI-277 | Farnesyltransferase | |
| Gene Inhibitors | ||
| RGS-p115RhoGEF | Gα12/13 family | |
| RGS-GRK2 | Gαq family (except Gα15/16) | |
The inhibitors were used as described in materials and methods.
Cells were preincubated with 80 ng/ml of PTX for 24 h prior to [Ca2+]i mobilization assay, Western blotting analysis, or luciferase reporter assay. AG 1478, genistein, or PD98059 was added with LPA to the cells used for Western blotting analysis. Cells for the luciferase reporter assay were pretreated with Y-27632 for 24 h or with an inhibitor (FTI-277, LY294002, SB 202190, edelfosine, or Gö 6976) for 2 h prior to the addition of LPA/FPP. For the cell adhesion assay, the treatment with AG 1478, edelfosine, genistein, Gö 6976, or U-73122 was for 2 h prior to the addition of LPA.
The doses of the inhibitors used for these studies were chosen by generating a dose-response curve for each inhibitor and using the minimum effective dose in each experiment. The concentrations of each inhibitor used were lower or equal to those reported by others when performing experiments under similar conditions.
Statistical analysis.
Where applicable, data were expressed as means ± SD. Statistical difference between multiple groups was determined by one-way ANOVA with Duncan's post hoc test performed with SPSS version 11. Significance was accepted at P < 0.05. Dose-response curves were generated by using the curve fitting software GraphPad Prism version 4 (GraphPad Software).
RESULTS
P2Y5 transcript is expressed along the proximal-to-distal intestinal mucosa.
The mRNA expression of P2Y5 in various rat tissues was quantified by semiquantitative RT-PCR, and the abundance of the P2Y5 transcripts was similar in all tested tissues (i.e., brain, heart, lung, kidney, pancreas, liver, stomach, small and large intestine) (data not shown). In the intestine, P2Y5 mRNA is expressed in mucosa from the duodenum to the ileum at similar levels (Fig. 1A), with a twofold higher expression in the villus tip than in the crypt region (Fig. 1B). This is consistent with the higher P2Y5 expression in differentiated hBRIE 380i cells (Fig. 1C). Both LPA and FPP are found in the circulation (41, 55), and LPA is also found in food (45). The villus tip expression of P2Y5 could provide a convenient location for the receptor to have access to agonists that are present in both the apical and basolateral plasma membranes. Although the mRNA levels of GPCRs can represent the protein levels in certain cell types [e.g., the sphingosine-1-phosphate receptor's mRNA levels reflect the protein levels in T cell (65)], the possibility that P2Y5 mRNA levels might not reflect the protein levels in the intestine in vivo remains to be determined.
Fig. 1.
Expression of P2Y5 mRNA is highest in villus and differentiated hBRIE 380i cells. A: the mucosal layers of rat duodenum, jejunum, ileum, and colon were removed as described in materials and methods. Total RNA was extracted and semiquantitative RT-PCR was performed with primers specific for rat P2Y5 and 18S RNA (resulting in 302 and 542 bp of amplified cDNA fragments, respectively). A representative image of ethidium bromide-stained agarose gel of amplified fragments is depicted. Duo, duodenum mucosa; Jej, jejunum mucosa; Ile, ileum mucosa; Col, colon mucosa. B: cells from the villus and crypt areas were removed by laser microscopy dissection as described in materials and methods. Total RNA was extracted and semiquantitative RT-PCR was performed with primers specific for rat P2Y5, intestinal fatty acid binding protein (I-FABP; resulting in 564 bp of cDNA fragment), and 18S RNA. I-FABP is a differentiation-dependent marker for enterocytes. A representative image of ethidium bromide-stained agarose gel of amplified fragments is depicted. C: P2Y5 transcript levels at different stages of hBRIE 380i cell maturity (from preconfluency to 3 days postconfluency) were determined by semiquantitative RT-PCR using primers specific for rat P2Y5, villin (resulting in 516 bp of cDNA fragment), and 18S RNA. Villin is another differentiation-dependent marker for enterocytes. Depicted is a representative image of ethidium bromide-stained agarose gel. P, proliferating hBRIE 380i cells; C, hBRIE 380i cells at confluency; 1D, hBRIE 380i cells at 1 day after confluency; 3D, hBRIE 380i cells at 3 days after confluency.
The hBRIE 380i cells express EGFP-tagged P2Y5 on the plasma membrane.
LPA could exert its effects both intra- and extracellularly (39). The cellular localization of the LPA responsive receptors would help in determining the initial site of regulation of the signaling pathways responsive to LPA. Therefore, the localization of heterologously overexpressed P2Y5 tagged with EGFP at its COOH terminus was determined in hBRIE 380i cells. Fluorescence confocal microscopy revealed expression of the receptor on the surface plasma membrane (Fig. 2A). These data suggest that the observed P2Y5 activation in our subsequent studies occurred at the cell surface.
Fig. 2.
Lysophosphatidic acid (LPA)-activated P2Y5 changes intracellular Ca2+ and cAMP levels. A: the localization of P2Y5 tagged with EGFP on the cell surface plasmalemma was visualized by laser scanning confocal microscopy. The scale bar represents 10 μm. B: Chinese hamster ovary (CHO) cells were transfected with the mitochondria-targeted aequorin (mtAEQ) plus P2Y5 or P2Y9 cDNA and the empty vector (●), GαΔ6qi5myr (▵), or Gα15 (▾). After a 20-h recovery, cells were loaded with coelenterazine-H for 2 h during a gentle rolling. The intracellular Ca2+ concentration ([Ca2+]i) in response to various doses of LPA, as indicated in the figure, was assayed as described in materials and methods. Integrated RLU represents the integrated fractional relative light units (RLU) value for a 30-s period after the introduction of LPA. Data points are means ± SD (n = 4–8). C: hBRIE 380i cells were transfected with mtAEQ, GαΔ6qi5myr, and either P2Y5 (■) or the empty vector (□). The changes in [Ca2+]i were assayed as described in B. Data points are means ± SD (n = 4–8). D: CHO cells transfected with mtAEQ, P2Y5, and GαΔ6qi5myr cDNA were loaded with coelenterazine-H. [Ca2+]i was assayed with various doses of 18:1-LPA (■), 18:1-PA (●), 18:0-LPA (▴), 16:0-LPA (▾), and 14:0-LPA (▵). Normalized integrated RLU represents the integrated RLU value obtained from P2Y5 transfected cells subtracted from that of empty vector-transfected cells. Data points are means ± SD (n = 3). E: CHO cells were transfected with P2Y5 cDNA or the empty vector. After a 48-h recovery, cells were treated with 2.5 μM of forskolin/1 mM IBMX in the presence or absence of 1 μM LPA for 7 min. Intracellular cAMP level was measured by enzyme immunoassay as described in materials and methods. Percent cAMP is the ratio of cAMP concentration in LPA- and forskolin-treated cells to that in forskolin-treated cells, which is arbitrarily set to 100. Bars are means ± SD (n = 3). *P < 0.05 vs. corresponding color in bar 1.
Activation of P2Y5 by LPA in CHO and hBRIE 380i cells leads to Gαi-mediated signaling pathways.
It had been observed that LPA mediated the induction of CRE reporter activity in P2Y5-overexpressing CHO cells (49). This induction occurred in a dose-responsive manner that correlated with increased LPA binding in P2Y5-overexpressing HEK cells. It was, therefore, proposed that P2Y5 was an LPA-responsive GPCR (49). Since CRE reporter activity is induced by more than one family of Gα (40), the Gα protein coupling preferences of P2Y5 remained unclear. All known LPA responsive GPCRs can couple with multiple families of Gα (41); therefore, the Gα coupling preferences of P2Y5 in response to LPA were first examined by using an aequorin-based calcium assay to characterize the downstream signal cascades that are activated by P2Y5. Although both the 1-acyl and 2-acyl LPA isomers exist in the circulation in vivo (51, 63), 2-acyl LPA is not stable under physiological conditions (51). Therefore, we used the 1-acyl isoform in these studies.
We found that the [Ca2+]i in P2Y5-overexpressing CHO cells was not different from the empty vector transfected CHO cells upon stimulation by oleoyl LPA (18:1-LPA), which is similar to what was reported by others (47), suggesting that P2Y5 is not a Gαq-coupled GPCR. The activation of Gαs and Gαi-coupled GPCRs are not usually associated with detectable changes in [Ca2+]i. By cotransfecting a promiscuous Gα, in this case Gα15 or GαΔ6qi5myr (30), the activation of Gαs- or Gαi-coupled GPCRs, respectively, can be quantified in a calcium assay, thus allowing changes in [Ca2+]i to be used as an indicator for the activation of Gαs-, Gαi-, and Gαq-coupled GPCRs. In the presence of GαΔ6qi5myr, a dose-dependent rise in [Ca2+]i in response to 18:1-LPA (EC50 = 195.3 nM) was higher in P2Y5-overexpressing CHO cells than in the cells that were transfected with the vector and GαΔ6qi5myr (Fig. 2B). The enhanced induction of [Ca2+]i in response to LPA in P2Y5 and GαΔ6qi5myr transfected cells was abolished in cells that were pretreated with 80 ng/ml PTX (a specific inhibitor of Gαi family and GαΔ6qi5myr) (data not shown). This confirmed that the cotransfected GαΔ6qi5myr was the mediator for enhancing the induction of [Ca2+]i. Cotransfecting Gα15 only enhanced the [Ca2+]i increase in P2Y5 transfected cells at micromolar levels of 18:1-LPA (Fig. 2B). Experiments repeated in hBRIE 380i cells also demonstrated that GαΔ6qi5myr was necessary for the detection of the P2Y5-specific [Ca2+]i response (EC50 = 1.59 μM; Fig. 2C), suggesting that P2Y5 might be an LPA-responsive Gαi- (and not Gαq)-coupled GPCR.
To investigate whether the activation of P2Y5 was only specific to the 18:1-LPA, the potencies of other related phospholipids on P2Y5 activation were tested in CHO cells cotransfected with GαΔ6qi5myr (Fig. 2D). Listed in Table 3 are the EC50 values of the tested phospholipids. The rank of potency was 18:1-LPA > 16:0-LPA > 18:0-LPA > 18:1-PA > 14:0- LPA. In all subsequent experiments, unless indicated otherwise, the 18:1-LPA was used as the agonist for P2Y5. Some phospholipid responsive receptors are also activated by changes in proton concentration ([H+]), such as OGR1, GPR4, G2A, and TDAG8 (64). Therefore, the effects of varying [H+] on the activation of P2Y5 in CHO cells cotransfected with GαΔ6qi5myr were assayed by the calcium mobilization assay. Different [H+] (pH 6.8, pH 7.2, pH 7.8, or pH 8.2) had no effect on the [Ca2+]i in the presence and absence of 10 μM LPA. Listed in Table 4 are the tested compounds that did not activate P2Y5 in CHO cells cotransfected with GαΔ6qi5myr.
Table 3.
Phospholipids' and other related compounds' potencies are determined by monitoring [Ca2+]i induction in CHO cells
| Name | EC50 (μM) | Emax |
|---|---|---|
| 18:1 LPA | 0.16 | 0.45 |
| 18:0 LPA | 4.56 | 0.38 |
| 16:0 LPA | 0.23 | 0.34 |
| 14:0 LPA | 61.46 | 0.42 |
| 18:1 PA | 9.62 | 0.45 |
| Oleoyl thiophosphate | 9.32 | 0.50 |
Chinese hamster ovary (CHO) cells were transfected with mtAEQ, P2Y5 cDNA (or the empty vector), and GαΔ6qi5myr. P2Y5-specific intracellular Ca2+ concentration ([Ca2+]i) induction was determined by subtracting the change in the [Ca2+]i level in vector-transfected cells from that in P2Y5-transfected cells.
Table 4.
Tested compounds that do not elevate [Ca2+]i in P2Y5- and GαΔ6qi5myr-cotransfected CHO cells
| Name | Concentration |
|---|---|
| 18:1-PC | NE ≤100 μM |
| Tetradecyl phosphonate* | NE ≤10 μM |
| Oleic acid | NE ≤20 μM |
| Platelet activating factor* | NE ≤80 μM |
| ATP | NE ≤100 μM |
| dATP | NE ≤100 μM |
| Protein hydrolysate | NE ≤50 mg/ml |
| Farnesyl diphosphates* | NE ≤10 μM |
| Geranylgeranyl diphosphates | NE ≤10 μM |
| Geranyl diphosphates | NE ≤10 μM |
| Farnesol | NE ≤10 μM |
CHO cells were transfected with mtAEQ, P2Y5 cDNA (or the empty vector), and GαΔ6qi5myr. P2Y5-specific [Ca2+]i induction was determined as described in Table 3. PC, phosphatidylcholine. NE, not effective.
Tested for antagonism against lysophosphatidic acid (LPA).
For comparison, we also explored the effects of 18:1-LPA on the activation of P2Y9, an LPA-responsive “P2Y5-like” GPCR that couples to both Gαi and Gαs families (25, 32), using the aequorin-based calcium assay in the presence or absence of the promiscuous Gα proteins. Changes in [Ca2+]i in response to 18:1-LPA in P2Y9-overexpressing CHO cells were enhanced with cotransfection of either Gα15 (EC50 = 153.3 nM) or GαΔ6qi5myr (EC50 = 166 nM) (Fig. 2B). The EC50 values of the [Ca2+]i induction of P2Y5 and P2Y9 cells were comparable, verifying the LPA responsiveness of P2Y5. In addition, the EC50 value of P2Y5 was similar to the LPA receptors of the EDG family, such as LPA3 (EC50 = 214 nM) (11). Because GPR93 is also activated by protein hydrolysate, we tested for a similar activation of P2Y5. As also observed for P2Y9 (9), protein hydrolysate (up to 50 mg/ml) did not enhance [Ca2+]i in CHO cells transfected with P2Y5 and GαΔ6qi5myr.
LPA-activated P2Y5 reduces forskolin-stimulated [cAMP]i in CHO cells.
The preference of P2Y5 to couple to GαΔ6qi5myr over Gα15 suggests that P2Y5 prefers coupling with the Gαi over the Gαs family. In CHO cells, LPA (up to 10 μM) did not significantly increase intracellular cAMP level ([cAMP]i) in either vector or P2Y5 transfected CHO cells (data not shown), suggesting that P2Y5 activation was not coupled to Gαs. A 7-min treatment of LPA (1 μM) significantly reduced the forskolin (2.5 μM)-elevated [cAMP]i in P2Y5-transfected cells by 44% (Fig. 2E). These results indicated that P2Y5 activation was coupled to the Gαi, not to the Gαs family, which confirmed the results of the calcium mobilization assay. Consistent with this finding, LPA (up to 10 μM) did not induce CRE-linked luciferase reporter activity in hBRIE 380i cells (data not shown).
P2Y5 activation by LPA, in hBRIE 380i cells, induces PTX-sensitive ERK1/2 phosphorylation.
LPA treatment of cell lines, such as hBRIE 380i, can induce an increase in ERK1/2 phosphorylation through Gαi-mediated signal cascades (7, 10, 41). Therefore, we explored the effects of P2Y5 activation on ERK1/2 phosphorylation in hBRIE 380i cells. LPA at 10 μM induced a rapid phosphorylation of ERK1/2 that reached a maximum at 4 min followed by a gradual decrease (Fig. 3A). P2Y5 overexpression in hBRIE 380i cells enhanced the phosphorylation of ERK1/2 in response to LPA at all time points. Because the 4-min time point produced the highest phosphorylation of ERK1/2 in response to LPA, the effects of various LPA concentrations on ERK1/2 phosphorylation were examined at 4 min. P2Y5 specific enhancement of ERK1/2 phosphorylation was dose dependent and peaked at 5 μM (Fig. 3B), which was in a range similar to the EC50 value obtained by the aequorin-based calcium assay in hBRIE 380i cells. The phosphorylation of ERK1/2 in response to LPA activated P2Y5 was blocked by 50 μM PD98059, an inhibitor of MEK1/2 (Fig. 3C). PTX at 80 ng/ml almost eliminated the P2Y5 mediated increase in ERK1/2 phosphorylation, suggesting that the activation of the Gαi family was required for the P2Y5 induction of ERK1/2 phosphorylation (Fig. 3C).
Fig. 3.
LPA induces a pertussis toxin (PTX)- and AG 1478-sensitive increase in ERK1/2 phosphorylation in P2Y5-overexpressing hBRIE 380i cells. A: hBRIE 380i cells stably transfected with either P2Y5 cDNA (hBRIE 380i-P2Y5) or empty vector (hBRIE 380i-vector) were treated with 10 μM LPA over various time periods as indicated in the figure. Time-dependent change in ERK1/2 phosphorylation is depicted, and the levels of ERK1/2 phosphorylation were determined as described in materials and methods. B: hBRIE 380i-P2Y5 or hBRIE 380i-vector cells were treated with various doses of LPA as indicated in the figure for 4 min. Depicted is a representative image. C: effects of pertussis toxin (80 ng/ml) and PD98059 (50 μM) on the ERK1/2 phosphorylation induced by 5 μM LPA for 4 min in hBRIE 380i-P2Y5 or hBRIE 380i-vector cells were determined by Western blot and densitometry analysis as described in materials and methods. The relative quantitative values are shown in the histogram. Fold induction represents the ratio of phospho-ERK1/2 band intensity to the band intensity of total ERK1 from each treatment normalized to that of the control (bar 1 of the corresponding color; no treatment), which was arbitrarily designated as 1. Bars are means ± SD (n = 3). aP < 0.05 vs. corresponding color in bar 1. bP < 0.05 vs. corresponding color in bar 2. D: effects of genistein (GE; 1 or 10 μM) and AG 1478 (AG; 1, 5, or 50 nM) on 5 μM LPA-induced ERK1/2 phosphorylation were determined in hBRIE 380i-P2Y5 and hBRIE 380i-vector cells by Western blot and densitometry analysis as described in C. Percent inhibition is the ratio of the fold induction from P2Y5 transfected cells to empty vector transfected cells normalized to that of the control (bar 1; 5 μM LPA alone without inhibitors), which is arbitrarily set to 100. Bars are means ± SD (n = 3). aP < 0.05, vs. bar 1. Inset: the endogenous expression of EGFR in hBRIE 380i cells was determined by Western blot as described in materials and methods. Depicted is a representative image, and the arrow indicates the molecular mass of 170 kDa.
EGFR transactivation is involved in the P2Y5-mediated ERK1/2 phosphorylation.
LPA can also increase ERK1/2 phosphorylation through the transactivation of receptor tyrosine kinase (RTK) by LPA-responsive GPCRs (69); therefore, we examined the potential involvement of tyrosine kinase in the phosphorylation of ERK1/2. Genistein, a tyrosine kinase inhibitor (1), at 10 μM significantly decreased P2Y5-induced ERK1/2 phosphorylation (Fig. 3D), suggesting the involvement of tyrosine kinase. One member of the RTK family that is transactivated by LPA receptors and expressed endogenously in hBRIE 380i cells (Fig. 3D, inset) is EGFR. A specific EGFR inhibitor (AG 1478) at doses from 5 to 50 nM significantly inhibited the P2Y5-induced ERK1/2 phosphorylation in response to LPA (Fig. 3D), suggesting that LPA-activated P2Y5 could transactivate EGFR through the Gαi family, resulting in an increase of ERK1/2 phosphorylation in hBRIE 380i cells.
LPA induces SRE-linked luciferase reporter activity in P2Y5-overexpressing cells.
Most LPA-responsive GPCRs can couple to Gα12/13 (33, 41, 71). The aequorin-based calcium assay of cells cotransfected with promiscuous Gα proteins cannot be used to determine the coupling of the Gα12/13 family by activated GPCRs because a construct of Gαq that has been shown to couple to GPCRs that only associate with Gα12/13 family has yet to be developed. Therefore, changes of SRE reporter activity [a commonly used indicator for Gα12/13 family activation (52)] in response to LPA was determined in P2Y5-overexpressing cells. SRE reporter activity can also be induced by the activation of Gαq-coupled GPCRs. Since P2Y5 does not couple with Gαq, the induction of SRE reporter activity by P2Y5 activation would indicate the activation of a Gα12/13-mediated signal pathway.
Treatment of P2Y5-transfected hBRIE 380i cells with LPA for 6 h increased SRE-linked reporter activity in a dose-dependent manner. At a 10 μM concentration, LPA increased the SRE reporter activity by 18.2-fold (compared with a 4.4-fold increase in the empty vector transfected cells) (Fig. 4A). The EC50 value was 3.4 μM for 18:1-LPA-induced SRE reporter activity that was similar to the EC50 value determined by the aequorin-based calcium assay in hBRIE 380i cells. A dose lower than the EC50 value of 18:1-LPA, 1 μM, was used to compare the potencies of different LPAs on the SRE reporter activation. The 18:1-LPA was still the most effective agonist for the SRE reporter induction compared with the other LPAs tested (Fig. 4B), and the rank of potencies among different LPAs for the SRE reporter induction was similar to the rank of potencies determined by the aequorin-based calcium assay. These studies indicate that P2Y5 is coupled to the Gα12/13 family proteins.
Fig. 4.
Serum response element (SRE) luciferase reporter activity is induced in response to LPA and farnesyl pyrophosphate (FPP) in P2Y5-overexpressing hBRIE 380i cells. A: hBRIE 380i cells were transfected with SRE-linked luciferase reporter plasmid plus the empty vector or P2Y5 cDNA and treated with various doses of 18:1-LPA as indicated in the figure for 6 h. Fold induction is the ratio of the value from each sample to the value from the control (no LPA treatment), which was arbitrarily designated as 1. Data points are means ± SD (n = 3). B: hBRIE 380i cells were transfected as described in A and treated with 1 μM of 14:0-LPA, 16:0-LPA, 18:0-LPA, or 18:1-PA for 6 h. Bars are means ± SD (n = 3). Normalized fold induction is the ratio of the fold induction from P2Y5-transfected cells to empty vector transfected cells normalized to that of the control (bar 1; no treatment), which is arbitrarily set to 1. aP < 0.05, relative to bar 1. C: hBRIE 380i cells were transfected as described in A and treated with 5 μM of 18:1-LPA (LPA), FPP, geranylgeranyl diphosphate (GGP), geranyl diphosphate (GPP), or farnesol (FOH) for 6 h. Bars are means ± SD (n = 3). Normalized fold induction is as described in B. aP < 0.05, relative to bar 1. D: hBRIE 380i cells were transfected as described in A and treated with different doses of FPP as indicated in the figure for 6 h. Data points are means ± SD (n = 3). Fold induction is as described in A.
FPP increases SRE reporter activity through P2Y5, in hBRIE 380i cells, without activating Gαi-mediated signal cascades.
FPP is an endogenous ligand that acts as an antagonist to the activation of EDG family receptors by LPA (36) and an agonist to GPR93 activation by LPA (48). FFP did not act as an antagonist to LPA on the induction of [Ca2+]i in P2Y5 and GαΔ6qi5myr-cotransfected CHO cells or to LPA-induced SRE reporter activity in P2Y5-transfected hBRIE 380i cells (data not shown). FPP alone (up to 10 μM) did not induce [Ca2+]i flux in CHO or hBRIE 380i cells overexpressing P2Y5 with (or without) Gα15 or GαΔ6qi5myr and ERK1/2 phosphorylation and the CRE reporter activity in both vector- and P2Y5-transfected hBRIE 380i cells (data not shown). However, FPP and geranylgeranyl diphosphate (GGPP) at 5 μM significantly induced SRE reporter activity in P2Y5 transfected hBRIE 380i cells by 5.2- and 2.9-fold, respectively (Fig. 4C), suggesting that FPP and GGPP could activate the Gα12/13 family through P2Y5. A dose-response curve was determined for FPP on the induction of SRE reporter activity by using vector- and P2Y5-transfected hBRIE 380i cells (Fig. 4D). The EC50 value for the induction of SRE reporter activity in response to FPP in cells overexpressing P2Y5 was 2.9 μM. These results suggest that P2Y5 expressed in hBRIE 380i cells was able to activate only the Gα12/13 family in response to FPP.
The induction of SRE reporter activity in P2Y5-overexpressing cells is mediated through Gα12/13 family, Rho family GTPase, and Rho kinase.
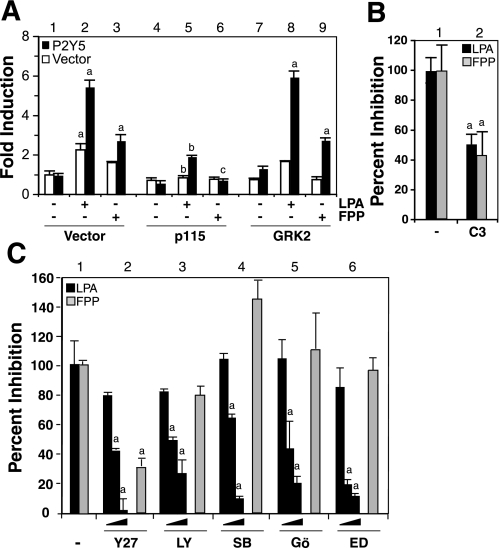
The regulator of G protein signaling (RGS) domain of p115RhoGEF binds and enhances the intrinsic GTPase activity of the Gα12/13 family, leading to the deactivation of the Gα12/13 family (16). Because RGS inhibits the Gα12/13-mediated SRE reporter transactivation in HeLa cells (58), we investigated the effects of the RGS domain of p115RhoGEF on the induction of SRE reporter activity by LPA and FPP in HeLa cells overexpressing P2Y5 to determine the contribution of Gα12/13 family in P2Y5 signal transduction. Cotransfection of the RGS domain of p115RhoGEF with P2Y5 in HeLa cells significantly reduced 65% of the LPA induced SRE reporter activity (Fig. 5A, black bars 2 vs. 5) and blocked FPP induced SRE reporter activity (Fig. 5A, black bars 3 vs. 6). Cotransfection of the RGS domain of GPCR kinase 2 (GRK2) with P2Y5, in HeLa cells, had no effect on LPA- and FPP-induced SRE reporter activity (Fig. 5A), since the RGS domain of GRK2 targets the Gαq but not the Gα12/13 family (16). These data verified that the induction of SRE reporter activity in response to LPA-activated and FPP-activated P2Y5 was mediated by the Gα12/13 family.
Fig. 5.
P2Y5-induced increases in SRE luciferase reporter activity are mediated through the Gα12/13 family. A: HeLa cells were transfected with SRE-linked luciferase reporter plasmid plus vector or P2Y5 cDNA, with or without the RGS domains from p115RhoGEF or GRK2, and treated with 10 μM LPA or 5 μM FPP for 6 h. Fold induction is the ratio of the value from each sample to the value from the control (open bar 1), which was arbitrarily designated as 1. Bars are means ± SD (n = 3). aP < 0.05 vs. corresponding color in bar 1. bP < 0.05 vs. corresponding color in bar 2. cP < 0.05 vs. corresponding color in bar 3. B: hBRIE 380i cells were transfected with SRE-linked luciferase reporter plasmid plus P2Y5 cDNA, with or without the C3 exotoxin cDNA, and treated with 10 μM LPA or 5 μM FPP for 6 h. Percent inhibition is the ratio of the fold induction from P2Y5 transfected cells to empty vector transfected cells normalized to that of the control (bar 1 of the corresponding color; 10 μM LPA or 5 μM FPP alone), which is arbitrarily set to 100. Bars are means ± SD (n = 3). aP < 0.05, relative to corresponding color in bar 1. C: effects of Y-27632 (Y27; 0.1, 1, or 10 μM), LY 294002 (LY; 5, 10, or 50 μM), SB 202190 (SB; 2, 10 or 20 μM), Gö 6976 (Go; 0.2, 1 or 2 μM), or edelfosine (ED; 5, 10, or 20 μM) on the induction of SRE reporter in P2Y5-overexpressing hBRIE 380i cells in response to 10 μM LPA or 5 μM FPP for 6 h was determined as described in materials and methods. Percent inhibition is as described in B. Bars are means ± SD (n = 3). aP < 0.05, relative to bar 1.
Activation of the Gα12/13 family can induce the expression of SRE regulated genes through the activation of Rho and ROCK (9, 52). Overexpression of C3 exoenzyme [a toxin that inactivates Rho A, B, and C small GTPases (68)] significantly reduced the effects of 10 μM LPA and 5 μM FPP on the SRE reporter activity in P2Y5-transfected hBRIE 380i cells by 50 and 57%, respectively (Fig. 5B, bar 1 vs. bar 2). A specific inhibitor of ROCK, Y-27632, at doses from 1 to 10 μM, significantly reduced the induction of SRE reporter activity in response to LPA in the P2Y5-transfected hBRIE 380i cells (Fig. 5C). Y-27632 at 1 μM (the lowest dose producing significant inhibition of the LPA-induced SRE reporter activity) also significantly reduced the FPP-induced SRE reporter activity in the P2Y5-transfected hBRIE 380i cells (Fig. 5C). This data suggests that the Rho-ROCK pathway was involved in the transactivation of SRE reporter activity in response to LPA- and FPP-activated P2Y5 in hBRIE 380i cells. During the preparation of this manuscript, Yanagida et al. (72) reported that LPA activates the G12/13 Rho signaling pathways in P2Y5-overexpressing B103 rat neuroblastoma cells.
Phosphoinositide-3 kinase (PI3K) and p38 MAP kinase (MAPK) are downstream effectors of the Gα12/13 family that can mediate the induction of the SRE reporter activity (46, 66). Treatments with LY294002 (a PI3K inhibitor) and SB202190 (a p38 MAPK inhibitor) significantly reduced LPA-mediated induction of SRE reporter activity in P2Y5-transfected hBRIE 380i cells, in a dose-dependent manner, up to 75 and 90%, respectively (Fig. 5C) and suggested the involvement of PI3K and p38 MAPK in P2Y5 signaling. However, the 5 μM FPP induction of SRE reporter activity observed in P2Y5-overexpressing hBRIE 380i cells was insensitive to both LY294002 (50 μM) and SB202190 (20 μM), indicating that the LPA induction of the SRE reporter activity might be mediated through different effector pathways than the induction by FPP.
Changes in [Ca2+]i can modulate SRE reporter activity (37, 54); therefore the involvement of calcium-related signal pathways was investigated. Gö 6976 (an inhibitor of PKC isoforms) and edelfosine [a phosphatidylinositol (PI)-specific phospholipase C (PLC) inhibitor] significantly inhibited the SRE reporter activity induced in P2Y5-overexpressing hBRIE 380i cells treated with 10 μM LPA, in a dose-dependent manner. This suggests the involvement of [Ca2+]i-related signal pathways (Fig. 5C). However, treatments of 2 μM Gö 6976 and 20 μM edelfosine did not affect the 5 μM FPP-induced SRE reporter activity in P2Y5-overexpressing hBRIE 380i cells, suggesting that only LPA-activated P2Y5 could initiate [Ca2+]i-related signal pathways that lead to the induction of SRE reporter activity.
ERK1/2 is a downstream signal molecule that can mediate the induction of SRE reporter activity and can be activated by LPA. We observed that P2Y5 activation by LPA increased ERK1/2 phosphorylation through a PTX-sensitive pathway (Fig. 3). PD98059 at 50 μM did not inhibit the P2Y5-mediated SRE transactivation (in response to LPA and FPP) in hBRIE 380i cells (data not shown). This result was consistent with data demonstrating that PTX treatment did not block LPA- and FPP-induced SRE reporter activity in P2Y5-overexpressed hBRIE 380i cells (data not shown).
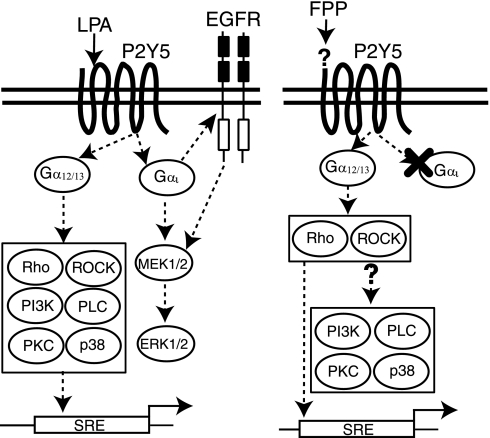
FPP can be used metabolically as a source for 15 carbon isoprenyl lipid during the protein farnesylation process catalyzed by farnesyl transferase (FTase) (5). Some members of the Rho GTPase family are farnesylated, which affects the function of small GTPases such as RhoB (5). We tested whether the farnesylation of small GTPases could account for increased activity of FPP activated P2Y5. Treatment with an FTase inhibitor, FTI-277 (10 μM), did not alter the effects of FPP and LPA on the transactivation of SRE reporter in hBRIE 380i cell overexpressing P2Y5 (data not shown), suggesting that protein farnesylation was not likely to be involved in P2Y5-mediated SRE transactivation. A summary of our results for LPA- and FPP-induced signaling pathways by P2Y5 activation is shown in Fig. 6.
Fig. 6.
Potential LPA- and FPP-initiated intracellular signaling pathways in hBRIE 380i cells overexpressing P2Y5. Potential LPA (left)- and FPP (right)-initiated intracellular signaling cascades through P2Y5 are illustrated in this diagram. LPA-activated P2Y5 can initiate the activation of Gαi and Gα12/13 families, leading to increased phosphorylation of ERK1/2 (through MEK) that is sensitive to EGFR inhibitor and SRE reporter activity that is sensitive to inhibitors targeting Rho small GTPase, ROCK, p38 MAPK, PI3K, PKC, and PLC. FPP can also activate P2Y5 and activate Gα12/13 but not Gαi family-mediated signal pathways. Furthermore, unlike LPA, the FPP-activated P2Y5-induced SRE reporter activity is sensitive to inhibitors targeting Rho small GTPase and ROCK but not p38 MAPK, PI3K, PKC, and PLC.
LPA reduces hBRIE 380i cell adhesion through activation of P2Y5.
LPA can modulate intestinal epithelial cell adhesion and deadhesion (or motility) processes through Gαi- and Gα12/13-coupled GPCRs (22, 26, 60, 70). Therefore, we examined the effects of LPA activated P2Y5 on the hBRIE 380i cell adhesion. A treatment with LPA (at 1 and 10 μM) significantly reduced the adhesiveness of P2Y5-transfected hBRIE 380i cells compared with vector-transfected cells (Fig. 7A), indicating that LPA-activated P2Y5 could reduce hBRIE 380i cell adhesion. The extent of the decrease in adhesion was similar to what was observed with hBRIE 380i cells exposed to NPY and PYY (34).
Fig. 7.
LPA- and FPP-activated P2Y5 reduces hBRIE 380i cell adhesion. A: adhesiveness of hBRIE 380i-P2Y5 and hBRIE 380i-vector cells to collagen I-coated wells in the presence or absence of LPA was determined as described in materials and methods. Percent adhesion is the ratio of the value from each sample to the value from the control (no LPA treatment), which was arbitrarily designated as 100. Data points are means ± SD (n = 3). aP < 0.05, relative to value of hBRIE 380i-vector cells of the same treatment. B: effect of AG 1478 (5, 50, or 250 nM), Gö 6976 (Go; 0.2, 1 or 2 μM), or edelfosine (ED; 5, 10, or 20 μM) was determined by pretreating cells with each inhibitor for 2 h. Ten μM LPA was used as a stimulus. Bars are means ± SD (n = 3). Percent adhesion is as described in A. aP < 0.05, relative to bar 1 (10 μM LPA treatment without inhibitors). C: adhesiveness of hBRIE 380i-P2Y5 and hBRIE 380i-vector cells to collagen I-coated wells in the presence or absence of FPP was determined as described in materials and methods. Bars are means ± SD (n = 3). aP < 0.05, relative to the value from hBRIE 380i-vector cells for the same treatment.
The activation of EGFR can result in the modulation of cell adhesion, migration through the activation of p38 MAPK, and alteration of the interaction between E-cadherin and the actin cytoskeleton (12, 20, 38). Since LPA effects can be mediated by EGFR activation (21), we tested whether LPA-activated P2Y5 might also reduce cell adhesion via transactivation of EGFR. AG 1478 at 50 and 250 nM displayed a significant and dose-dependent inhibition of the reduced adhesion observed in P2Y5-overexpressing cells treated with LPA (Fig. 7B), suggesting the involvement of EGFR.
The [Ca2+]i-related signal pathways (e.g., the activation of PKC and PLC) are often involved in the regulation of cell adhesion and migration (29, 50). We therefore tested whether changes in [Ca2+]i were involved in reduction of intestinal cell adhesion resulting from LPA activation of P2Y5. The presence of 1 μM Gö 6976 or 10 μM edelfosine significantly inhibited the LPA-mediated reduction of adhesion in P2Y5-overexpressing hBRIE 380i cells (Fig. 7B). The possible involvement of [Ca2+]i-related signal pathways was consistent with our SRE reporter assay data (Fig. 5C). However, up to 10 μM U-73122 [a inhibitor of PLCβ often activated by Gαq-coupled GPCRs or Gβγ dimers of Gαi-coupled GPCRs (28)] had no effect on the LPA-mediated reduction of P2Y5-overexpressing hBRIE 380i cell adhesion (data not shown). This indicates that activation of PLCβ was not likely involved.
Rho can reduce cell adhesion though the activation of ROCK (56). The ROCK inhibitor, Y-27632, at 10 μM did not affect P2Y5-mediated reduction of cell adhesion by LPA (data not shown). This suggests that ROCK was not involved in LPA-regulated hBRIE 380i cell adhesion through P2Y5. We also investigated the effects of FPP on hBRIE 380i cell adhesion. At 10 μM, FPP treatment of P2Y5-overexpressing hBRIE 380i cells caused a small but significant reduction (18%) of cell adhesion in (Fig. 7C). Since FPP did not seem to activate EGFR, PLC, or PKC through P2Y5 in hBRIE 380i cells (Fig. 5C), the effects of these inhibitors on the FPP-mediated reduction in hBRIE 380i cell adhesion were not pursued.
P2Y5 overexpression increases specific [3H]18:1-LPA binding to the plasma membrane.
To investigate whether LPA could directly interact with P2Y5, we examined the binding of [3H]18:1-LPA to plasma membranes isolated from P2Y5-overexpressing hBRIE 380i cells. These isolated membranes bound to [3H]18:1-LPA in a dose-responsive fashion, and the binding was higher in P2Y5-transfected hBRIE 380i cells than in vector-transfected hBRIE 380i cells (Fig. 8A), suggesting that LPA bound to P2Y5 directly. The apparent Kd and Bmax calculated from the saturation plot were 23.5 nM and 4.51 fmol/μg protein, respectively, which were within the range reported for other LPA receptors (31, 47).
Fig. 8.
There are increases in the specific [3H]LPA and [3H]FPP binding in membrane fractions prepared from P2Y5-transfected cells. A: plasma membrane was isolated from hBRIE 380i cells stably transfected with P2Y5 or the empty vector, and 30 μg of the membrane fraction was incubated with various doses of [3H]18:1-LPA for 30 min at 25°C with gentle shaking. Nonspecific binding was determined in the presence of 10 μM nonradiolabeled 18:1-LPA. The specific binding was calculated by subtracting the nonspecific binding from the total binding. Data points are means ± SD (n = 3). The value of apparent Kd and Bmax were calculated as described in materials and methods. B: competition between 10 nM [3H]18:1-LPA and various doses of unlabeled ATP binding to P2Y5-overexpressed hBRIE 380i membrane was determined as described in materials and methods. Nonspecific binding was determined as described in A. Percent binding is the ratio of 10 nM [3H]18:1-LPA specific binding to plasma membrane in the presence of the competitors to that without the competitors, which is arbitrarily set to 100. Bars are means ± SD (n = 3). C: plasma membrane was isolated from hBRIE 380i cells stably transfected with P2Y5 or the empty vector, and 30 μg of the membrane fraction was incubated with 100 nM of [3H]FPP. The experiment proceeded as described in A. Bars are the means of counts per minute (cpm) ± SD (n = 3). *P < 0.05 vs. the open bar.
Low-affinity agonists to a particular GPCR do not always have higher EC50 values compared with higher affinity agonists for the same receptor (31). Therefore, the relative affinities of different LPAs were also quantified. Various concentrations of unlabeled 18:1-LPA, 18:0-LPA, or 16:0-LPA were used to compete with 10 nM of [3H]18:1-LPA in a competition binding assay. The calculated Ki values are listed in Table 5. The 18:0-LPA had a higher affinity to P2Y5 than 16:0-LPA but 16:0-LPA was more effective in the induction of [Ca2+]i. These results suggest that the affinities of the agonists for P2Y5 might not always correlate with their potencies, which is consistent with the reported nucleotide binding and potency data for P2Y5 (high affinity but no potency) (35, 67). Up to 1 μM ATP had no effect on the [3H]18:1-LPA binding to P2Y5 (Fig. 8B), suggesting ATP and LPA might bind to different regions of P2Y5.
Table 5.
Competition between an LPA analog and [3H]18:1-LPA determined by binding experiments using P2Y5 overexpressing hBRIE 380i cell membrane
| Name | Ki (nM) |
|---|---|
| 18:1 LPA | 25 |
| 18:0 LPA | 43 |
| 16:0 LPA | 66 |
The specific binding of 10 nM [3H]18:1-LPA in the presence or absence of each of the competitors and the Ki values were determined as described in the materials and methods.
FPP did not function as an antagonist of LPA in the calcium mobilization assay (Table 4), but FPP could activate SRE reporter activity, suggesting that FPP and LPA might interact with different structural domains of P2Y5. Therefore, the competition binding experiments with FPP against [3H]18:1-LPA might not have revealed whether FPP could directly interact with P2Y5; hence, the binding of [3H]FPP to hBRIE 380i cells overexpressing P2Y5 was examined. At 100 nM FPP, membrane preparations from hBRIE 380i cells transfected with P2Y5 retained a small but significantly higher (87.6%) amount of [3H]FPP compared with the empty vector (0.59 ± 0.06 fmol [3H]FPP/μg protein vs. 0.31 ± 0.07 fmol [3H]FPP/μg protein; Fig. 8C). The relative minor amount of labeled FPP binding to the P2Y5 membrane preparation, compared with labeled LPA, raises the possibility that FPP might not directly interact with P2Y5.
DISCUSSION
The epithelial cells lining the intestinal lumen are exposed to molecules from the external environment through their apical surface, as well as molecules from peripheral tissues through their basolateral surface. These molecules can modulate events that are essential for the maintenance of mucosal functional and structural integrity such as cell migration along the crypt-to-villus axis, axial and spatial differentiation, and anoikis. P2Y5 could act as a receptor that helps coordinate environmental and systemic cues on intestinal cell activities because its ligand, LPA, can be present in the intestinal lumen and also released from peripheral sources. Although P2Y5 transcript was observed to be distributed throughout the intestinal mucosa, the expressed receptor could exert a specific regional effect along the proximal to distal intestine, or along the crypt-to-villus axis, by differentially responding to more than one agonist at a given time.
We determined that FPP is also an agonist of P2Y5, thus making this receptor the second known cell surface receptor activated by FPP after GPR93 (48). FPP is an intermediate in the isoprenoid biosynthetic pathway (23), also present in the circulation (55, 61), and an endogenous antagonistic ligand of LPA2 and LPA3 (36). Unlike with LPA, the treatment of P2Y5-overexpressing cells with FPP did not result in an induction of [Ca2+]i mobilization and ERK1/2 phosphorylation.
Both LPA and FPP activation of P2Y5 induced the Rho GTPase, ROCK pathway, and SRE reporter activity through the coupling of Gα12/13; however, LPA and FPP might utilize different downstream effectors of SRE reporter activity. FPP-induced SRE reporter activity was not affected by inhibitors targeting p38 MAPK, PI3K, PLC, or PKC. Therefore, despite being P2Y5 agonists, LPA and FPP might be able to elicit distinct effector responses. Furthermore, the simultaneous presence of LPA and FPP in vivo might inhibit the activity of LPA2 and LPA3, but not that of P2Y5 and GPR93. Our finding presents an additional point of regulation for the signal cascades activated by LPA-responsive GPCRs. The specific effects of P2Y5 activation may not only be dependent on the expression pattern of the receptor but also on the combination of agonists at a given time.
Recently, Hypotrichosis simplex (a group of hereditary isolated alopecia) was correlated to a truncated mutation of P2Y5 by genomewide linkage analysis (49). LPA treatments of human P2Y5-overexpressing CHO cells led to a PTX-insensitive induction of CRE reporter activity and did not reduce forskolin-elevated CRE reporter activity. It was therefore concluded that P2Y5 activation by LPA was coupled to Gαs and not to Gαi (49). However, CRE can be activated by both [Ca2+]i- and cAMP-mediated pathways (2). Following LPA activation of P2Y5 in CHO cells, we detected neither changes in [Ca2+]i, even with coexpression of Gα15 (a G protein that can enhance [Ca2+]i mobilization by activated GPCRs coupled to Gαs), nor increases in intracellular cAMP levels, which indicates that P2Y5 is likely not coupled to Gαs. However, a possibility remains that P2Y5 activation might result in a latent increase in [Ca2+]i that could modulate CRE reporter activity. We observed that P2Y5-induced SRE reporter activity was sensitive to both PI-PLC and PKC inhibitors, suggesting the involvement of a Gα12/13-linked PLC isoform, such as PLCε (52), in a delayed elevation of [Ca2+]i following LPA stimulation.
The median effective concentration (i.e., EC50 values) of P2Y5 agonists is not strictly correlated to the strength of ligand binding (i.e., Kd values) to the receptor. P2Y5 exhibited a higher affinity to 18:0-LPA than to 16:0-LPA, but 16:0-LPA was a more effective agonist for the induction of [Ca2+]i. Similarly, P2Y5 activation by FPP resulted in a higher SRE reporter activity than by LPA, even though there was a greater total binding of LPA than FPP to the membrane fraction of P2Y5-overexpressing cells. Those observations could be a reflection of the difficulties in using standard receptor binding assays for lipid ligands; however, the possibility exists that liganded P2Y5 interacts with other accessory proteins such as GPCR kinase, β arrestin, or other 7TMR (which are either coupled or uncoupled to G proteins), causing an enhancement of downstream signaling pathways (8, 27, 57). Therefore, the physiological actions of LPA and FPP in the intestine might not be simply the result of an increase in binding of a particular ligand.
LPA induces migration of intestinal cells in culture through the activation of Gαi (22, 60). Other PTX-sensitive receptors have been demonstrated to induce intestinal cell migration that is linked to a decrease cell adhesion. For example, the activation of the NPY receptor family [a Gαi-coupled receptor family (43)] increases hBRIE 380i cell motility through a process that is linked to a decrease in cell adhesion by decreasing the adhesion molecule CD63, increasing matrix metalloproteinase 3 and through the action of small GTPase cdc42 (34). P2Y5 might activate similar downstream signal cascades to reduce epithelial cell adhesiveness. P2Y5 may also alter cell adhesion through RTK-mediated pathways since our results indicate that P2Y5 transactivates EGFR and EGFR activation in the intestine has been reported to modulate cell migration and adhesion (14).
The involvement of P2Y5 in the LPA-mediated decrease in intestinal cell adhesion could be important for maintaining intestinal epithelia integrity in vivo, for example by restitution or anoikis (18, 34, 60). Furthermore, the differential activation of P2Y5 by various forms of LPA and FPP could result in distinct Gα coupling, activation of downstream effector molecules (e.g., p38 MAPK, PI3K, PLC, and PKC), and signaling events. P2Y5 may play a significant role in the maintenance of mucosal homeostasis along the crypt-to-villus axis by acting as a means for the epithelium to respond to a range of extracellular cues, which can reflect the state of cell-to-cell integrity, cell metabolism, or the content of molecules in the lumen including those derived from the diet or intestinal flora.
GRANTS
This work was supported by a grant from the University of California Cancer Research Coordinating Committee and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK58592.
ACKNOWLEDGMENTS
We thank Amy Shiu for technical assistance.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592–5595, 1987 [PubMed] [Google Scholar]
- 2.Andrisani OM. CREB-mediated transcriptional control. Crit Rev Eukaryot Gene Expr 9: 19–32, 1999 [PubMed] [Google Scholar]
- 3.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277: 48737–48744, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Aponte GW, Keddie A, Hallden G, Hess R, Link P. Polarized intestinal hybrid cell lines derived from primary culture: establishment and characterization. Proc Natl Acad Sci USA 88: 5282–5286, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res 47: 15–31, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chiu TT, Leung WY, Moyer MP, Strieter RM, Rozengurt E. Protein kinase D2 mediates lysophosphatidic acid-induced interleukin 8 production in nontransformed human colonic epithelial cells through NF-κB. Am J Physiol Cell Physiol 292: C767–C777, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol 292: G98–G112, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Defea K. Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol 153, Suppl 1: S298–S309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutt P, Kjoller L, Giel M, Hall A, Toksoz D. Activated Galphaq family members induce Rho GTPase activation and Rho-dependent actin filament assembly. FEBS Lett 531: 565–569, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Fang X, Yu S, LaPushin R, Lu Y, Furui T, Penn LZ, Stokoe D, Erickson JR, Bast RC, Jr, Mills GB. Lysophosphatidic acid prevents apoptosis in fibroblasts via G(i)-protein-mediated activation of mitogen-activated protein kinase. Biochem J 352: 135–143, 2000 [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, Bautista D, Parrill AL, Tigyi G. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol 60: 776–784, 2001 [PubMed] [Google Scholar]
- 12.Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279: 44513–44521, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J 12: 1589–1598, 1998 [PubMed] [Google Scholar]
- 14.Goke M, Podolsky DK. Regulation of the mucosal epithelial barrier. Baillieres Clin Gastroenterol 10: 393–405, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Grossmann J. Molecular mechanisms of “detachment-induced apoptosis–Anoikis”. Apoptosis 7: 247–260, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Hains MD, Siderovski DP, Harden TK. Application of RGS box proteins to evaluate G-protein selectivity in receptor-promoted signaling. Methods Enzymol 389: 71–88, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hallden G, Aponte GW. Evidence for a role of the gut hormone PYY in the regulation of intestinal fatty acid-binding protein transcripts in differentiated subpopulations of intestinal epithelial cell hybrids. J Biol Chem 272: 12591–12600, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Hallden G, Hadi M, Hong HT, Aponte GW. Y receptor-mediated induction of CD63 transcripts, a tetraspanin determined to be necessary for differentiation of the intestinal epithelial cell line, hBRIE 380i cells. J Biol Chem 274: 27914–27924, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Hallden G, Holehouse EL, Dong X, Aponte GW. Expression of intestinal fatty acid binding protein in intestinal epithelial cell lines, hBRIE 380 cells. Am J Physiol Gastrointest Liver Physiol 267: G730–G743, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem 273: 9078–9084, 1998 [DOI] [PubMed] [Google Scholar]
- 21.He D, Natarajan V, Stern R, Gorshkova IA, Solway J, Spannhake EW, Zhao Y. Lysophosphatidic acid-induced transactivation of epidermal growth factor receptor regulates cyclo-oxygenase-2 expression and prostaglandin E(2) release via C/EBPbeta in human bronchial epithelial cells. Biochem J 412: 153–162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hines OJ, Ryder N, Chu J, McFadden D. Lysophosphatidic acid stimulates intestinal restitution via cytoskeletal activation and remodeling. J Surg Res 92: 23–28, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Holstein SA, Hohl RJ. Isoprenoids: remarkable diversity of form and function. Lipids 39: 293–309, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Huang C, Hujer KM, Wu Z, Miller RT. The Ca2+-sensing receptor couples to Gα12/13 to activate phospholipase D in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol 286: C22–C30, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Janssens R, Boeynaems JM, Godart M, Communi D. Cloning of a human heptahelical receptor closely related to the P2Y5 receptor. Biochem Biophys Res Commun 236: 106–112, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Jacamo R, Zhukova E, Sinnett-Smith J, Rozengurt E. RNA interference reveals a differential role of FAK and Pyk2 in cell migration, leading edge formation and increase in focal adhesions induced by LPA in intestinal epithelial cells. J Cell Physiol 207: 816–828, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen R, Holliday ND, Hansen JL, Vrecl M, Heding A, Schwartz TW, Elling CE. Characterization of G-protein coupled receptor kinase interaction with the neurokinin-1 receptor using bioluminescence resonance energy transfer. Mol Pharmacol 73: 349–358, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kelley GG, Kaproth-Joslin KA, Reks SE, Smrcka AV, Wojcikiewicz RJ. G-protein-coupled receptor agonists activate endogenous phospholipase Cepsilon and phospholipase Cbeta3 in a temporally distinct manner. J Biol Chem 281: 2639–2648, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koivunen J, Aaltonen V, Koskela S, Lehenkari P, Laato M, Peltonen J. Protein kinase C alpha/beta inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res 64: 5693–5701, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kostenis E. Is Galpha16 the optimal tool for fishing ligands of orphan G-protein-coupled receptors? Trends Pharmacol Sci 22: 560–564, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Sillard R, Owman C, Leeb-Lundberg FL, Olde B. Lysophosphatidic acid binds to and activates Gpr92, a receptor highly expressed in gastrointestinal. J Pharmacol Exp Ther 318: 619–628, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem 282: 4310–4317, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13 and Gq coupled lysophosphatidic increases cAMP: LPA5. J Biol Chem 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lee M, Hadi M, Hallden G, Aponte GW. Peptide YY and neuropeptide Y induce villin expression, reduce adhesion, and enhance migration in small intestinal cells through the regulation of CD63, matrix metalloproteinase-3, and Cdc42 activity. J Biol Chem 280: 125–136, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Schachter JB, Harden TK, Nicholas RA. The 6H1 orphan receptor, claimed to be the p2y5 receptor, does not mediate nucleotide-promoted second messenger responses. Biochem Biophys Res Commun 236: 455–460, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Liliom K, Tsukahara T, Tsukahara R, Zelman-Femiak M, Swiezewska E, Tigyi G. Farnesyl phosphates are endogenous ligands of lysophosphatidic acid receptors: inhibition of LPA GPCR and activation of PPARs. Biochim Biophys Acta 1761: 1506–1514, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin K, Wang D, Sadee W. Serum response factor activation by muscarinic receptors via RhoA. Novel pathway specific to M1 subtype involving calmodulin, calcineurin, and Pyk2. J Biol Chem 277: 40789–40798, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Mariner DJ, Davis MA, Reynolds AB. EGFR signaling to p120-catenin through phosphorylation at Y228. J Cell Sci 117: 1339–1350, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Marrache AM, Gobeil F, Zhu T, Chemtob S. Intracellular signaling of lipid mediators via cognate nuclear G protein-coupled receptors. Endothelium 12: 63–72, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Matsuo A, Matsumoto S, Nagano M, Masumoto KH, Takasaki J, Matsumoto M, Kobori M, Katoh M, Shigeyoshi Y. Molecular cloning and characterization of a novel Gq-coupled orphan receptor GPRg1 exclusively expressed in the central nervous system. Biochem Biophys Res Commun 331: 363–369, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Meyer Zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta 4: 4, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Moolenaar WH, Kranenburg O, Postma FR, Zondag GC. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol 9: 168–173, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Mullins DE, Zhang X, Hawes BE. Activation of extracellular signal regulated protein kinase by neuropeptide Y and pancreatic polypeptide in CHO cells expressing the NPY Y(1), Y(2), Y(4) and Y(5) receptor subtypes. Regul Pept 105: 65–73, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Murakami M, Shiraishi A, Tabata K, Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun 371: 707–712, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Nakane S, Tokumura A, Waku K, Sugiura T. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: distinct molecular species compositions. Lipids 36: 413–419, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, Takagahara S, Turner JH, Kozasa T, Kobayashi H, Sato Y, Kawanishi T, Inoue R, Nagao T, Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem 280: 18434–18441, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem 278: 25600–25606, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Oh da Y, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O'Dell DK, Walker JM, Na HS, Lee MG, Kwon HB, Kim K, Seong JY. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem 283: 21054–21064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet 40: 329–334, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology 114: 493–502, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Qian L, Xu Y, Arai H, Aoki J, McIntyre TM, Prestwich GD. Synthesis of migration-resistant hydroxyethoxy analogues of lysophosphatidic acid. Org Lett 5: 4685–4688, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci 26: 146–154, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol 160: 25–46, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Sagi SA, Seasholtz TM, Kobiashvili M, Wilson BA, Toksoz D, Brown JH. Physical and functional interactions of Galphaq with Rho and its exchange factors. J Biol Chem 276: 15445–15452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saisho Y, Morimoto A, Umeda T. Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal Biochem 252: 89–95, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell 18: 3429–3439, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz TW, Holst B. Ago-allosteric modulation and other types of allostery in dimeric 7TM receptors. J Recept Signal Transduct Res 26: 107–128, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Shi CS, Sinnarajah S, Cho H, Kozasa T, Kehrl JH. G13alpha-mediated PYK2 activation. PYK2 is a mediator of G13alpha-induced serum response element-dependent transcription. J Biol Chem 275: 24470–24476, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Smyth SS, Cheng HY, Miriyala S, Panchatcharam M, Morris AJ. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim Biophys Acta 1781: 563–570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sturm A, Sudermann T, Schulte KM, Goebell H, Dignass AU. Modulation of intestinal epithelial wound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology 117: 368–377, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Szkopinska A, Plochocka D. Farnesyl diphosphate synthase; regulation of product specificity. Acta Biochim Pol 52: 45–55, 2005 [PubMed] [Google Scholar]
- 62.Thomson AB, Drozdowski L, Iordache C, Thomson BK, Vermeire S, Clandinin MT, Wild G. Small bowel review: normal physiology, part 1. Dig Dis Sci 48: 1546–1564, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T, Satouchi K, Waku K, Fukuzawa K. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. Biochem J 365: 617–628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomura H, Mogi C, Sato K, Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal 17: 1466–1476, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Graeler MH, Goetzl EJ. Physiological sphingosine 1-phosphate requirement for optimal activity of mouse CD4+ regulatory T Cells. FASEB J 18: 1043–1045, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Falasca M, Schlessinger J, Malstrom S, Tsichlis P, Settleman J, Hu W, Lim B, Prywes R. Activation of the c-fos serum response element by phosphatidyl inositol 3-kinase and rho pathways in HeLa cells. Cell Growth Differ 9: 513–522, 1998 [PubMed] [Google Scholar]
- 67.Webb TE, Kaplan MG, Barnard EA. Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun 219: 105–110, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Wilde C, Genth H, Aktories K, Just I. Recognition of RhoA by Clostridium botulinum C3 exoenzyme. J Biol Chem 275: 16478–16483, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Wu J, Cunnick JM. Trans-regulation of epidermal growth factor receptor by lysophosphatidic acid and G protein-coupled receptors. Biochim Biophys Acta 1582: 100–106, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Wu SS, Chiu T, Rozengurt E. ANG II and LPA induce Pyk2 tyrosine phosphorylation in intestinal epithelial cells: role of Ca2+, PKC, and Rho kinase. Am J Physiol Cell Physiol 282: C1432–C1444, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J Biol Chem 282: 5814–5824, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem 284: 17731–17741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H, Bialkowska A, Rusovici R, Chanchevalap S, Shim H, Katz JP, Yang VW, Yun CC. Lysophosphatidic acid facilitates proliferation of colon cancer cells via induction of Kruppel-like factor 5. J Biol Chem 282: 15541–15549, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu L, Liu Y, Forte JG. Ezrin oligomers are the membrane-bound dormant form in gastric parietal cells. Am J Physiol Cell Physiol 288: C1242–C1254, 2005 [DOI] [PubMed] [Google Scholar]