Abstract
We have recently shown that membrane association of neuronal nitric oxide synthase-α (nNOSα) is critical in the regulation of synthesis of NO during nitrergic neurotransmission. The purpose of this study was to examine the role of the synapse-associated proteins (SAPs) in membrane association of nNOSα. Varicosities (swellings on terminal axons) were isolated from mice gastrointestinal tract and examined for nNOSα, postsynaptic density protein 95 (PSD95), and membrane interactions by coimmunoprecipitation and SDS-PAGE. Our results show that PSD95 protein was present in the membrane fraction of the nerve varicosity, whereas both PSD95 and SAP97 were present in the cytosol. nNOSα was associated with PSD95 but not SAP97. nNOSα-PSD95 complex was bound to the membrane via palmitoylation of PSD95. Depalmitoylation of PSD95 with 2-bromopalmitate dislocates nNOSα and PSD95 from the varicosity membrane and abolishes NO production. These studies show that palmitoylation of PSD95 anchors nNOSα to the varicosity membrane and that it is obligatory for NO production by the enzyme. Palmitoylation of PSD95 may provide a novel target for regulation of nitrergic neurotransmission.
Keywords: postsynaptic density protein, palmitoylation, synapse-associated protein, neuronal nitric oxide synthase, membrane-bound nNOS
nitric oxide (no) is a major inhibitory neurotransmitter involved in peristalsis, sphincter relaxation, and other inhibitory reflexes in the gut, and abnormalities of nitrergic neurotransmission have been implicated in major clinical disorders of gastrointestinal motility (15, 16, 33, 35). During nitrergic inhibitory neurotransmission, NO is produced de novo by NO synthase (NOS) in the nerve varicosities (31). The type of NOS that synthesizes NO during inhibitory neurotransmission has been shown to be neuronal NOS-α (nNOSα) dimer that is associated with varicosity membrane (1, 21, 37). Upon nerve stimulation, the active nNOSα dimer binds with calmodulin (CaM) in a calcium-dependent fashion to produce NO (26, 31). Membrane localization may allow association of nNOSα with the regulatory enzymes and voltage-dependent calcium channels that expose active nNOSα to high local concentrations of calcium during Ca2+ influx after stimulation of the varicosities (1, 4, 6, 25, 37). However, the molecular basis of membrane binding of nNOSα with the presynaptic or prejunctional varicosity membrane in gut is not well understood.
The nNOS isoforms, nNOSα and nNOSμ, have been reported to be associated with the postsynaptic membranes via scaffolding proteins (1, 32). For example, the localization of nNOSμ to the postsynaptic striated muscle membranes of motor end plates involves a scaffolding protein, syntrophin-1α (4). On the other hand, membrane association of nNOSα to postsynaptic densities in the postsynaptic dendrites in the forebrain synapses involves a family of scaffolding proteins called postsynaptic density (PSD) proteins or synapse-associated proteins (SAPs), particularly PSD95 (4, 25). At some sites, nNOS has also been reported to associate with the membranes via PSD95-independent interaction (27).
PSD proteins are characterized by PDZ domain (PDZ is an acronym for PSD95, Drosophila septate junction protein Discs-large, and the epithelial tight junction protein ZO-1). They belong to a superfamily of proteins called membrane-associated guanylate kinases, which contain a SH3 domain and a guanylate kinase-like domain in addition to one or more PDZ domains (19). The four main PSD proteins, namely, PSD93/chapsyn110, PSD95/SAP90, SAP97, and SAP102, are differentially distributed in tissues, particularly the neural synapses (2). The specific scaffolding proteins that are involved in the membrane association of nNOSα in the presynaptic or prejunctional nitrergic varicosities in the gut are not well understood.
The purpose of the present study, in the isolated mice enteric varicosities, was to investigate 1) the nature of SAPs in membrane and cytosolic fractions, 2) nNOSα binding to SAPs that were identified in the varicosities, 3) the role of palmitoylation of PSD95, which was found to be membrane bound, in PSD95-nNOSα binding to the varicosity membrane, and 4) the effect of depalmitoylation by 2-bromopalmitate (2-BP) on membrane binding of the PSD-nNOSα complex and NO production from the varicosities in vitro.
MATERIALS AND METHODS
The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Veterans Affairs Boston Healthcare System.
Antibodies and Chemicals
The following antibodies were used: anti-PSD93 (mouse monoclonal, clone N18/30; NeuroMab/Antibody), anti-PSD95 (mouse monoclonal, Clone 7E3–1B8; Affinity Bioreagent), anti-SAP97 (Affinity Bioreagent), anti-SAP102 (mouse monoclonal, clone N19/2; NeuroMab/Antibody), anti-nNOS1–20 (rabbit polyclonal against NH2-terminal sequences of nNOSα; Santa Cruz Biotechnology), and anti-c-kit (CD117) antibody (Abcam). All species-appropriate secondary antibodies were obtained from Santa Cruz Biotechnology and used at a five-/tenfold dilution to the primary. 2-BP (2-bromohexadecanoic acid) and palmitate (hexadecanoic acid) (used as 100 μM solution) were obtained from Sigma.
Tissue Extract
Tissue extracts from the mice were prepared as described before (6, 26). The total mass of the gastrointestinal tract tissue from six mice processed for each study was ∼16.8 g. All experiments were performed in triplicate sets of varicosity extracts (n = 6 mice, 3 independent groups).
Subcellular Fractionation to Obtain Purified Varicosities
The crude varicosity from the gut tissue extract was prepared by ultracentrifugation using protocols that have been used both for brain as well as gut tissues (6, 17, 26). The purified varicosity fraction was obtained from the crude extract by unequal density gradient centrifugation using sucrose medium. This protocol is similar to that which has been extensively used for isolating purified synaptosomes from the brain or the gut tissues (6, 17). Regardless of the tissue, the purified synaptosomal fraction collects near the interphase of 0.8–1.2 M sucrose. The purified synaptosomal fraction contains presynaptic varicosities represented by sealed plasma membrane containing synaptic vesicles and small mitochondria (39). The vesicle membranes may show electron-dense structures and may be associated with postsynaptic membranes, the neurosomes (18). Electron microscopic examination of this fraction in the mice gut has been shown to contain exclusively synaptosomes (26).
Separation of Membrane and Cytosolic Fractions of Purified Varicosities
The intact varicosities were disrupted by hypo-osmotic shock. The purified varicosity fraction was incubated in a two-volume solution of 0.5 mM sodium phosphate (using a high pH of 8.1, to prevent adsorption of soluble enzymes to membranes) and 0.1 mM magnesium sulfate to facilitate nonsealing of membranes for 6 h on ice. The lysate was then subjected to high-velocity differential centrifugation at a speed of 100,000 revolution/min (Optima TLX UltraCentrifuge, Beckman Coulter) for 1 h at 4°C. The supernatant represented the cytosolic fraction and was aspirated and stored, whereas the pellet represented membranes of the varicosities including the active zone and the nNOSα-associated membranes.
Isolation of the Varicosity Membranes
The varicosity membrane fraction presumably included mixture of membranes of all types of varicosities including cholinergic and nitrergic motor varicosities and sensory nerve endings. nNOSα-bound varicosity membranes were purified by immunoprecipitation with anti-nNOS1–20 antibody.
Palmitoylation Status of PSD95
Acyl biotin exchange (ABE) reactions were performed to assess palmitoylation of PSD95 using protocols described for ABE chemistry (11, 36). Briefly, this protocol includes three steps. Preexisting, free sulfhydryl groups were alkylated by N-ethylmaleimide. The fatty acyl palmitate group was then removed by hydroxylamine that leaves the cysteine residue, leaving a free sulfhydryl group. The third step labeled the newly created free sulfhydryl group with biotin-N-(6-(Biotinamido)hexyl)-3′-(2′-pyridyldithio)-propionamide, and biotin tag was later removed by β-mercaptoethanol treatment after purification on streptavidin-agarose beads. Repetitive chloroform-methanol precipitation was used to separate proteins from excess reagent in between steps (38). Half of all samples did not receive the second hydroxylamine treatment and served as internal controls. The final eluates were run on SDS-PAGE and immunoblotted with anti-PSD95 antibody for detection of signals.
Dissolution of Varicosity Membrane From the Bound Proteins
Interaction between membrane-associated PSD95 and nNOSα was investigated by dissolving the varicosity membranes in 1% (wt/vol) SDS in 50 mM Tris·HCl buffer, pH 7.4 (samples kept on ice). The final concentration of SDS is 0.01%. This procedure completely dissolved the lipidic membranes and avoided nonspecific protein-to-protein interactions.
Coimmunoprecipitation Studies
Coimmunoprecipitation experiments, as described earlier (6), were used to study protein interactions of nNOS and PSD protein because of the advantages this technique offers for studies in small quantities of proteins found in the endogenous systems. In brief, solubilized precleared varicosity extracts (both membrane and cytosol) were incubated with protein A/G agarose-immobilized antibody, followed by washing. In the final steps, cold elution of the supernatant on ice was performed by >1-h incubation in SDS and β-mercaptoethanol-containing buffer and subsequent centrifugation. The beads were boiled at 95°C for 10 min and the whole eluate electrophoresed. Double coimmunoprecipitation experiments were performed in the same fashion using the first coimmunoprecipitate as the starting material. For the double coimmunoprecipitation experiments, the beads were eluted in cold condition after being incubated for greater than 30 min with 2× SDS buffer and being spun at high speeds. This was to ensure the retention of secondary structures of the proteins to be studied.
Pitfalls of nonspecific protein-to-protein interactions were avoided by taking the following precautions. 1) All antibodies used had documented compatibility for immunoprecipitation experiments, and whenever possible affinity purified monoclonal antibodies were used. 2) Compatibility of antibody isotype binding with protein A/G agarose was checked for the antibodies used. 3) The SDS detergent-solubilized membranes were diluted with a buffered salt solution containing Nonidet, before addition of antibody to decrease nonspecific adsorption, while maintaining antigen-antibody interactions. The experiments were accompanied with nonspecific IgG controls using antibodies belonging to the same species and the same Ig subclass as the experimental antibody. Apart from these controls, additional functional studies were performed to document functional importance of the protein-to-protein interactions.
SDS-PAGE
The extracts were processed at a low temperature (4°C) as described earlier (6). Electrophoresis was carried out with Bio-Rad mini-protean Tetra electrophoresis gel casting system, and immunolabeled blots were developed as described earlier (6).
In Vitro NO Production
We used a fluorescent system using diaminofluorescein as an indicator for NO production as described earlier (6, 26).
Statistics
Unpaired t-test was used to compare means between two groups, and ANOVA was used to compare means between multiple groups.
RESULTS
Types of SAP in the Varicosities
As seen in Fig. 1, Western blots of the purified whole-varicosity extracts showed presence of ∼90-kDa PSD95 and ∼140-kDa SAP97. Signals for PSD93 and SAP102 were not identified even though positive signals of 110-kDa PSD93 and ∼100-kDa SAP102 were identified in mouse brain extract. Moreover, another monoclonal PSD93 antibody also failed to detect any PSD93 signal. In contrast, PSD93 signals were identified in the crude extracts of gut tissues, suggesting that, whereas PSD93 is present in the gut tissues, it is not present in the purified varicosities. Cytosol of the purified varicosities showed signals for both PSD95 and SAP97. However, the membrane fraction of the purified varicosities showed the presence of PSD95 only, and no signal for SAP97 was detected. Western blotting with c-kit antibody showed a 117-kDa band in whole gut samples that was absent in varicosity extracts. This shows that PSD95 from the interstitial cells of Cajal did not contribute to PSD95 identified in the varicosities.
Fig. 1.
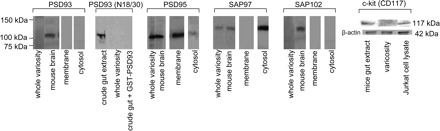
Immunoblots showing distribution of postsynaptic density (PSD) proteins in different fractions of purified varicosity. Note that PSD95 was present in both the membrane and the cytosolic factions and synapse-associated protein 97 (SAP97) was present only in the cytosolic fraction. PSD93 was not detected in varicosity but identified in the whole gut extract using a monoclonal antibody (clone N18/30), and signal was not seen when the crude gut extract was incubated with a 33-kDa control glutathione-S-transferase (GST)-fusion protein of the PSD93 antigen. Also note that SAP102 was not detected in the varicosity. Also note the lack of 117-kDa c-kit signal in varicosity extract but its presence in the whole gut extract and Jurkat cell extract (positive control).
PSD95 But Not SAP97 Binds With nNOSα
To examine association of nNOSα with PSD proteins in varicosity membrane and cytosol, respective extracts were immunoprecipitated with anti-nNOS1–20 antibody, and the immunoprecipitates were probed with the specific PSD protein antibodies. As shown in Fig. 2A, PSD95 and nNOSα association was seen in both the cytosolic and the membrane fractions of the varicosities. SAP97 yielded negative results. PSD93 and SAP102 were negative as expected. These observations show that PSD95 associates with nNOS in membranes and cytosol of the purified varicosities, but SAP97 does not associate with nNOS in any of these fractions. The specificity of the anti-nNOS antibody is shown in Fig. 2B. The membrane and cytosolic lysates, positive controls, and IgG negative controls were run on the same gel.
Fig. 2.
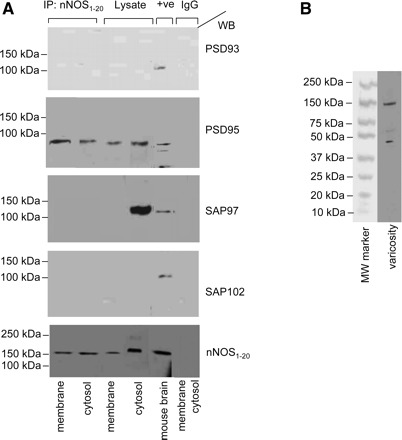
PSD95 is present in gut nerve varicosity membrane and coimmunoprecipitates with anti-neuronal nitric oxide synthase (nNOS)1–20 antibody. A: membrane and cytosolic fractions of purified varicosities were immunoprecipitated (IP) with anti-nNOS1–20 antibody, eluted by boiling the beads, and SDS-PAGE of the immunoprecipitated protein samples were blotted with monoclonal antibodies against PSD proteins on the same membrane. Both membrane-bound and cytosolic nNOS associated with PSD95 but not with SAP97. Mouse brain extract and boiled lysates of membrane and cytosolic samples (20 μg in each lane) are shown. Representative blots of IgG controls using both membrane and cytosolic fractions are also shown. Note the 155-kDa monomeric nNOSα signals in boiled samples of varicosity membrane and cytosol in the lowermost panel. B: specificity of anti-nNOS1–20 antibody (sc-1025, Lot No.J1606, Santa Cruz Biotechnology) is depicted in a whole lane. Kaleidoscope MW marker (Bio-Rad) was used. +ve, positive control; WB, Western blot.
Nature of nNOSα Associated With PSD95 in the Varicosity Membrane
The purified varicosity extract has been shown to contain various isoforms of nNOS, including nNOSα and nNOSβ. However, nNOSα isoform is the main constituent of the membrane-associated nNOS (6). To detect whether PSD95 in the varicosity membrane associates with nNOSα, the purified varicosity membrane samples were immunoprecipitated with anti-PSD95 antibody, and Western blots of pulled-down proteins were probed with anti-nNOS1–20 antibody that specifically detects nNOSα. As shown in Fig. 3, a 320-kDa band is seen in the anti-PSD95 immunoprecipitate, indicating that nNOSα dimer is bound to PSD95 in the membrane (Fig. 3).
Fig. 3.
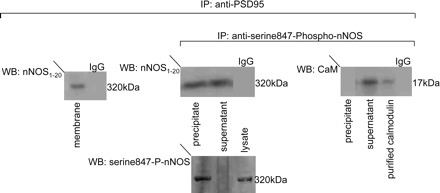
PSD95 in the varicosity membrane associates with both serine847-phospho-nNOSα dimer and dephospho-nNOSα dimer. Purified varicosity membrane samples were immunoprecipitated with anti-PSD95 antibody. The immunoprecipitate was immunoblotted with anti-nNOS1–20 antibody. 320-kDa band of nNOSα dimer was seen, suggesting that PSD95 is associated with nNOSα dimer in the varicosity membrane. Incubation with IgG served as negative control. Furthermore, varicosity membrane samples immunoprecipitated with anti-PSD95 was again immunoprecipitated with anti-serine847-phospho-nNOS antibody. The resulting immunoprecipitate and supernatant were separately loaded for SDS-PAGE and probed with anti-nNOS1–20 antibody. Both the immunoprecipitate and the supernatant showed 320-kDa bands, suggesting that the PSD95 in varicosity membrane is associated with both serine847-phospho-nNOSα dimer and dephospho-nNOSα dimer. When probed with anti-calmodulin (CaM) antibody, in the presence of Ca2+, only the supernatant showed a 17-kDa band. IgG negative control and purified bovine brain CaM as a positive control for the 17-kDa signal were used for the coimmunoprecipitation experiments.
Varicosity membrane-associated nNOS has been shown to exist as catalytically inactive serine847-P-nNOS and active nonphosphorylated nNOSα(6). The nonphosphorylated nNOSα binds with CaM in a calcium-dependent fashion. We examined whether both forms of nNOSα were associated with PSD95 in the varicosity membrane. In these studies, varicosity membrane proteins immunoprecipitated with anti-PSD95 were again immunoprecipitated with anti-serine847-phospho-nNOS antibody. The resulting second immunoprecipitate and supernatant were separated, loaded for SDS-PAGE, and probed with anti-nNOS1–20 antibody. Both the immunoprecipitate and the supernatant showed 320-kDa bands, indicating that the PSD95-bound nNOSα dimer in the membrane is present in both forms, serine847-phosphorylated as well as unphosphorylated at serine847 (Fig. 3). Furthermore, when probed with anti-CaM antibody, only the supernatant showed a 17-kDa band, indicating that the serine847-dephospho-nNOSα dimer was CaM bound (Fig. 3). The efficiency of pull down with the phospho-specific antibody is also shown in Fig. 3.
Varicosity Membrane-Associated But Not Cytosolic PSD95 is Palmitoylated
The soluble protein PSD95 is known to bind to the lipid membranes via palmitoylation. We examined the palmitoylation status of membrane-bound and cytosolic PSD95 using ABE chemistry, where palmitoylated proteins yield signals in hydroxylamine-treated samples (11). Palmitoylation signals were seen in varicosity membrane-bound PSD95 but not in the cytosolic PSD95 (Fig. 4).
Fig. 4.
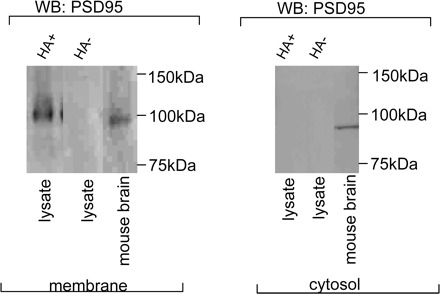
Membrane-bound PSD95 is palmitoylated. Immunoblots showing that varicosity membrane-bound PSD95 is palmitoylated (hydroxylamine treated, HA+), whereas cytosolic PSD95 is not palmitoylated. Hydroxylamine-omitted (HA- samples) were internal controls. Mouse brain extract served as positive controls while probing with anti-PSD95 antibody.
Effect of 2-BP Treatment
2-BP treatment is known to deacylate palmitate covalently linked to protein and thus reverses protein palmitoylation (11). Whole purified varicosities were treated with 2-BP (100 μM) for 2 h to examine the effect of depalmitoylation on membrane association of PSD95 and nNOSα and enzymatic and physiological function of nNOSα. In all these experiments, palmitate treatment, which does not affect protein acylation, served as controls. Untreated samples served as double controls.
Effects of 2-BP on membrane association of PSD95, palmitoyl-PSD95, and nNOSα.
2-BP treatment resulted in total loss of PSD95 from membrane (Fig. 5B) and loss of palmitoylation signals in the PSD95 (Fig. 5A). On the other hand, palmitate treatment had no effect on palmitoylation status of membrane-bound PSD95 (Fig. 5A). The samples in which hydroxylamine treatment was omitted during ABE chemistry steps served as controls for all these experiments (data not shown). 2-BP treatment, compared with palmitate treatment, also resulted in total loss of nNOSα dimer from the membrane (Fig. 5C). Palmitate and untreated varicosities served as controls. 2-BP treatment had no effect on PSD95 and nNOSα in the varicosity cytosolic fractions (Fig. 5, B and C).
Fig. 5.
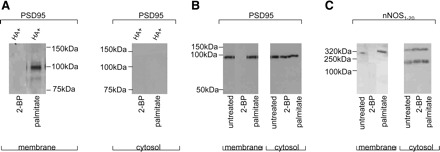
Depalmitoylation by 2-bromopalmitate (2-BP) dislocates palmitoyl-PSD95, PSD95, and nNOSα from varicosity membrane. A: 100 μM 2-BP causes depletion of palmitoyl-PSD95 from the varicosity membrane. B: depalmitoylation by 2-BP (100 μM) completely dissociates PSD95 from the membrane. C: 2-BP (100 μM) causes dislocation of 320-kDa nNOSα dimer from the membrane. The nNOS immunoblots were developed by cold SDS-PAGE. 2-BP treatment did not have any effect on nNOSα monomer and dimer in the varicosity cytosolic fractions. Untreated lysates or palmitate treatment served as controls.
Effect of 2-BP on NO production during in vitro assay.
As shown in Fig. 6, the purified varicosity membrane fractions produced NO during an in vitro assay system. This is consistent with association of catalytically active nNOSα with the varicosity membrane. 2-BP treatment that dissociates PSD95-nNOSα from the membrane significantly inhibited NO production in membrane fractions in vitro (P < 0.00001, F = 787.87, one-way ANOVA between different groups, experiments performed in triplicate). In contrast, palmitate treatment that does not dissociate PSD95-nNOSα from the membrane had no adverse effect on the NO production. Figure 4 also shows that the cytosolic fraction, either before or after 2-BP treatment, does not produce significant amounts of NO, suggesting that, upon dissociation from the membrane, nNOSα is rendered catalytically inactive.
Fig. 6.
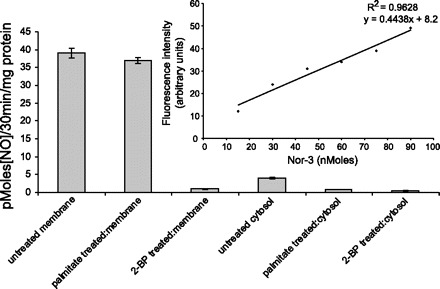
In vitro NO production in varicosity membrane lysates is significantly diminished by 2-BP. 100 μM 2-BP treatment significantly inhibited NO production in varicosity membrane fraction compared with untreated or palmitate-treated controls. All cytosolic fractions produced scant quantities of NO. Inset: standard curve of NO production with known concentrations of NO donor Nor-3.
DISCUSSION
The present studies show that in the enteric nerve varicosities 1) SAPs, PSD95, and SAP97 are present, and PSD93 and SAP102 are not detectable; 2) PSD95 is associated with varicosity membrane as well as cytosol, whereas SAP97 is present only in the cytosol; 3) PSD95 but not SAP97 binds with nNOSα; 4) PSD95 binds to the varicosity membrane by palmitoylation; and 5) depalmitoylation by 2-BP leads to dislocation of nNOSα-PSD complex from the membrane and loss of NO production in the varicosities. These results are summarized in Fig. 7.
Fig. 7.
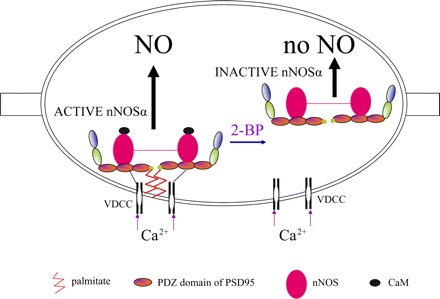
Cartoon summarizing the critical role of palmitoylation of PSD95 in catalytical activity of nNOS. The scaffolding protein PSD95 has 3 PDZ binding domains, a SH3, and a guanylate kinase domain. PDZ is an acronym for PSD95, Drosophila septate junction protein Discs-large, and the epithelial tight junction protein ZO-1. PSD95 is attached to the varicosity membrane by palmitoylation at the NH2-terminal end of PDZ1. nNOSα is attached to the PDZ2 domain of PSD95. PSD95 is also anchored to voltage-dependent calcium channels (VDCC) although the exact site of this interaction is not known. These interactions cluster nNOSα with VDCCs. Calcium influx causes CaM binding and activation of nNOS to produce NO. Treatment with depalmitoylating agent 2-BP results in dissociation of PSD95 and nNOSα from the varicosity membrane, leading to inactivation of nNOSα, causing loss of NO production.
The four main PSD proteins, namely PSD93, PSD95, SAP97, and SAP102, are differentially distributed in the neural synapses (9, 12). Membrane or cytosolic localization of the specific SAPs in isolated varicosities has not been previously reported. However, with the use of a nonspecific antibody that detects PDZ domains of PSD93, PSD95, and SAP97, PSD95 and PSD93 have been shown to be predominantly expressed in the interstitial cells of Cajal (3). mRNA for PSD95 has also been reported to be expressed in the gut, and mRNA for PSD93 has been reported to be present in the whole gut extract and in the myenteric plexus neurons (3, 5, 29). Using specific antibodies, we found that PSD95 and SAP97 but not PSD93 or SAP102 are present in the enteric varicosities. Moreover, mainly PSD95 was identified in the nitrergic varicosity membranes though both PSD95 and SAP97 were present in the cytosolic fraction of the purified varicosity extract. These studies show that PSD95 is the main SAP that is associated with the varicosity membrane.
The presence of SAP97 or SAP102 has not been previously examined in the gut. Our studies show that SAP97 is localized to the cytosol of the enteric varicosities. SAP97 lacks amino acid cysteine in the NH2-terminal critical region that is required for palmitoylation and attachment to the varicosity membrane. Therefore, SAP97 may not directly bind with the varicosity membrane nNOS. SAP97 has been identified in the presynaptic nonnitrergic nerve terminals in hippocampus and cerebellum (14, 22). Because our varicosity preparation represented mixture of nitrergic and nonnitrergic varicosities, SAP97 may be derived from nitrergic or nonnitrergic or both types of the varicosities. We did not detect SAP102 in the varicosities. The lack of finding of SAP102 in the varicosities in the adult mice is consistent with the fact that SAP102 does not associate with nNOS and is mainly involved in synaptic development (8, 28, 37).
One of the unexpected findings was the lack of PSD93 protein in the purified varicosity fraction. mRNA for PSD93 has been previously shown to be present in whole gut extract and has been localized to the interstitial cells of Cajal (3). Moreover, PSD93 has been shown to be present in the myenteric neurons, and it is possible that, like elsewhere, PSD93 is associated with cholinergic nicotinic receptors in the postsynaptic densities in the dendrites of myenteric neurons(24). Brenman et al. (5) reported that PSD93 is associated with glutamate receptors in the nonnitrergic dendrites of the Purkinje γ-amino butyric acid neurons in the cerebellum. Although PSD93 can associate with nNOSα in vitro and is likely present in the nitrergic postsynaptic dendrites, its presence in presynaptic or nonsynaptic/prejunctional nitrergic varicosities has not been reported. Further studies are needed to examine selective targeting of PSD93 to the postsynaptic dendrites in the myenteric neurons.
Our studies show that PSD95 is the main PSD protein in the prejunctional nitrergic varicosities. PSD95 has been shown to be the main scaffolding protein of nitrergic postsynaptic densities in the postsynaptic dendrites in the forebrain where PSD95 is aggregated together with other PSD proteins and actin to form microscopic density called postsynaptic density. The postsynaptic densities are composed of a number of PSD proteins and up to 300 different proteins (23). Although synaptic microscopic membrane densities have not been described in the presynaptic, nonsynaptic, or junctional varicosities, the present studies show that PSD95 is present in the enteric nerve varicosities. Our studies suggest that PSD95 was present both in association with membrane and cytosol of enteric varicosity. The membrane-associated PSD95 was found to be palmitoylated, suggesting that PSD95 was bound to membrane via palmitoylation as has been reported previously (13). Palmitoylation has been shown to cause clustering of PSD95 with other membrane-associated proteins.
Membrane-bound nNOSα has been shown to be an important component of the postsynaptic densities at glutaminergic excitatory synapse. However, nNOS has also been localized to a number of presynaptic, nonsynaptic, and prejunctional membranes of neurons including postganglionic parasympathetic neurons to cerebral and penile blood vessels, myenteric inhibitory motor neurons in gut, and stellate cells and basket cells in the cerebellum (34). At the postsynaptic densities, nNOSα binds with PSD95 that in turn binds to the plasma membrane, thereby binding nNOSα to the membrane. However, the mechanism underlying binding of nNOSα to presynaptic or nonsynaptic varicosities remains poorly understood (2). Our observations using coimmunoprecipitation experiments document binding of PSD95 with nNOSα. Although it is possible that this association can be direct or indirect via another intermediate linking protein, it is well known that nNOS binds with PSD95 via specific PDZ-to-PDZ interaction involving PDZ2 domain of the PSD95 (7, 30, 32).
It has been shown that 2-BP inhibits enzymatic palmitoylation (13). If so, 2-BP may cause dissociation of PSD95 and nNOSα from the varicosity membrane. We found that depalmitoylation with 100 μM 2-BP caused dislocation of PSD95 and nNOSα from the varicosity membrane, supporting the role of palmitoylation of PSD95 in the association of nNOSα with the varicosity membrane. Further proof that membrane binding of PSD95-nNOSα complex is functionally important was demonstrated by dislocating PSD95 and nNOSα from the membrane that resulted in the loss of NO production by the varicosities. These observations provide strong evidence for the importance of palmitoylation in nitrergic neurotransmission in the gut.
nNOS has been identified in several types of enteric neurons including the following: 1) inhibitory motor neuron to the circular muscle, 2) inhibitory motor neuron to the longitudinal muscle, 3) noncholinergic secretomotor/vasodilator submucosal neuron, 4) descending interneuron responsible for the so-called occult reflex, 5) some descending interneurons, to inhibitory motor neurons, and 6) some intestinofugal neurons (10, 20). Varicosities of intestinofugal neurons are present in the sympathetic ganglia and are not included in our preparation. Moreover, the nature and the membrane targeting of nNOS to the varicosities remain unknown in many of the neuron populations. In some of the neurons, nNOS may be directed to the dendrites and not the varicosities and may not be included in our preparation. Further studies are needed to determine which of the nitrergic neurons are affected by depalmitoylation.
Loss of NO-related neurotransmission has been shown to cause diseases affecting all parts of the gastrointestinal tract, resulting in difficulty in swallowing, gastric stasis, intestinal stasis, and megacolon. In some of these disorders, on the basis of studies of nNOS content and immunohistochemistry, importance of impaired nitrergic neurotransmission is underestimated. The present study shows that disorders of PSD95 and its palmitoylation may affect nitrergic neurotransmission without changes in nNOS content in the varicosities.
In summary, the present study shows that, in the gut nerve terminals, soluble nNOSα uses palmitoyl-PSD95 to associate with lipidic membranes to optimize neurotransmitter function. Disruption of palmitoylated PSD95 may result in loss of nitrergic neurotransmission. Palmitoylation may provide an important target for regulation of nitrergic neurotransmission.
GRANTS
This work was supported by a NIDDK grant (DK 062867) to R. K. Goyal.
ACKNOWLEDGMENTS
The authors thank Dr. Hong Zhen He for technical assistance.
REFERENCES
- 1. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse 40: 239–257, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol 493: 193–206, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84: 757–767, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Brenman JE, Christopherson KS, Craven SE, McGee AW, Bredt DS. Cloning and characterization of postsynaptic density 93, a nitric oxide synthase interacting protein. J Neurosci 16: 7407–7415, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhury A, Rao YM, Goyal RK. PIN/LC8 is associated with cytosolic but not membrane-bound nNOS in the nitrergic varicosities of mice gut: implications for nitrergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 295: G442–G451, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem 274: 27467–27473, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, Garman D, Rabinowitz J, Lu PS, Tymianski M. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J Neurosci 27: 9901–9915, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeGiorgis JA, Galbraith JA, Dosemeci A, Chen X, Reese TS. Distribution of the scaffolding proteins PSD-95, PSD-93, and SAP97 in isolated PSDs. Brain Cell Biol 35: 239–250, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132: 1912–1924, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Drisdel RC, Green WN. Labeling and quantifying sites of protein palmitoylation. Biotechniques 36: 276–285, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron 52: 307–320, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron 44: 987–996, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Garner CC, Kindler S. Synaptic proteins and the assembly of synaptic junctions. Trends Cell Biol 6: 429–433, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol 42: 610–619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyal RK, Hirano I. The enteric nervous system. N Engl J Med 334: 1106–1115, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat 96: 79–88, 1962 [PMC free article] [PubMed] [Google Scholar]
- 18. Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J Neurosci 5: 2240–2253, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771–781, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res 302: 59–72, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest 98: 8–13, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muller BM, Kistner U, Veh RW, Cases-Langhoff C, Becker B, Gundelfinger ED, Garner CC. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neurosci 15: 2354–2366, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci 34: 503–518, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Parker MJ, Zhao S, Bredt DS, Sanes JR, Feng G. PSD93 regulates synaptic stability at neuronal cholinergic synapses. J Neurosci 24: 378–388, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rameau GA, Chiu LY, Ziff EB. Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J Biol Chem 279: 14307–14314, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Rao YM, Chaudhury A, Goyal RK. Active and inactive pools of nNOS in the nerve terminals in mouse gut: implications for nitrergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 294: G627–G634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saitoh F, Tian QB, Okano A, Sakagami H, Kondo H, Suzuki T. NIDD, a novel DHHC-containing protein, targets neuronal nitric-oxide synthase (nNOS) to the synaptic membrane through a PDZ-dependent interaction and regulates nNOS activity. J Biol Chem 279: 29461–29468, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci 20: 1260–1271, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saur D, Neuhuber WL, Gengenbach B, Huber A, Schusdziarra V, Allescher HD. Site-specific gene expression of nNOS variants in distinct functional regions of rat gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 282: G349–G358, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Stricker NL, Christopherson KS, Yi BA, Schatz PJ, Raab RW, Dawes G, Bassett DE, Jr, Bredt DS, Li M. PDZ domain of neuronal nitric oxide synthase recognizes novel C-terminal peptide sequences. Nat Biotechnol 15: 336–342, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Thatte HS, He XD, Goyal RK. Imaging of nitric oxide in nitrergic neuromuscular neurotransmission in the gut. PLoS One 4: e4990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tochio H, Mok YK, Zhang Q, Kan HM, Bredt DS, Zhang M. Formation of nNOS/PSD-95 PDZ dimer requires a preformed beta-finger structure from the nNOS PDZ domain. J Mol Biol 303: 359–370, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Toda N, Herman AG. Gastrointestinal function regulation by nitrergic efferent nerves. Pharmacol Rev 57: 315–338, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Toda N, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev 55: 271–324, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis—a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol 4: 336–346, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nat Protoc 2: 1573–1584, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Watanabe Y, Song T, Sugimoto K, Horii M, Araki N, Tokumitsu H, Tezuka T, Yamamoto T, Tokuda M. Postsynaptic density-95 promotes calcium/calmodulin-dependent protein kinase II-mediated Ser847 phosphorylation of neuronal nitric oxide synthase. Biochem J 372: 465–471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138: 141–143, 1984 [DOI] [PubMed] [Google Scholar]
- 39. Whittaker VP. The morphology of fractions of rat forebrain synaptosomes separated on continuous sucrose density gradients. Biochem J 106: 412–417, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]


