Abstract
Presence of taste receptor families in the gastrointestinal mucosa suggests a physiological basis for local and early detection of a meal. We hypothesized that luminal l-glutamate, which is the primary nutrient conferring fundamental umami or proteinaceous taste, influences mucosal defense mechanisms in rat duodenum. We perfused the duodenal mucosa of anesthetized rats with l-glutamate (0.1–10 mM). Intracellular pH (pHi) of the epithelial cells, blood flow, and mucus gel thickness (MGT) were simultaneously and continuously measured in vivo. Some rats were pretreated with indomethacin or capsaicin. Duodenal bicarbonate secretion (DBS) was measured with flow-through pH and CO2 electrodes. We tested the effects of agonists or antagonists for metabotropic glutamate receptor (mGluR) 1 or 4 or calcium-sensing receptor (CaSR) on defense factors. Luminal l-glutamate dose dependently increased pHi and MGT but had no effect on blood flow in the duodenum. l-glutamate (10 mM)-induced cellular alkalinization and mucus secretion were inhibited by pretreatment with indomethacin or capsaicin. l-glutamate effects on pHi and MGT were mimicked by mGluR4 agonists and inhibited by an mGluR4 antagonist. CaSR agonists acidified cells with increased MGT and DBS, unlike l-glutamate. Perfusion of l-glutamate with inosinate (inosine 5′-monophosphate, 0.1 mM) enhanced DBS only in combination, suggesting synergistic activation of the l-glutamate receptor, typical of taste receptor type 1. l-leucine or l-aspartate had similar effects on DBS without any effect on pHi and MGT. Preperfusion of l-glutamate prevented acid-induced cellular injury, suggesting that l-glutamate protects the mucosa by enhancing mucosal defenses. Luminal l-glutamate may activate multiple receptors and afferent nerves and locally enhance mucosal defenses to prevent subsequent injury attributable to acid exposure in the duodenum.
Keywords: intracellular alkalinization, mucus secretion, bicarbonate secretion, monosodium glutamate, taste receptor
the duodenal mucosa has multilayered, multistep defense mechanisms to counter mucosal injury attributable to constant exposure to luminal concentrated acid and high Pco2 attributable to gastric acid and secreted HCO3− (24). These mechanisms coordinately regulate premucosal, mucosal, and submucosal components, including mucus and HCO3− secretion, intracellular pH (pHi) regulation and cellular buffering, and submucosal neuronal activation and blood flow response. Because duodenal luminal pH rapidly changes between 2 and 7 as a result of the constant mixture of secreted HCO3− with jets of antrally propelled gastric acid, the duodenal mucosa must rapidly adjust its defense mechanisms according to luminal pH (23).
We have examined the upper gastrointestinal (GI) defense factors in response to luminal acid, using fluorescent microscopy and Doppler flowmetry where epithelial pHi, mucosal blood flow, and mucus gel thickness (MGT), corresponding to mucus secretion rate, are simultaneously measured in the gastroduodenum of living rats (6, 7, 9, 25, 34, 43). In duodenum, luminal acid is sensed by the “capsaicin pathway,” consisting of acid-induced intracellular acidification, H+ secretion via the basolateral Na+/H+ exchanger-1, activation of capsaicin-sensitive afferent nerves, the release of vasoactive mediators, and an increase of mucosal blood flow and mucus secretion, followed by delayed cyclooxygenase (COX)/prostaglandin (PG)-dependent mucus and HCO3− secretion (24). These data demonstrate that the duodenal mucosa “tastes” luminal acidity using epithelial ion transporters and neuronal acid sensors. Furthermore, luminal CO2 is also sensed by the capsaicin pathway, facilitated by epithelial carbonic anhydrases (5, 31), suggesting that luminal H+ and CO2 provide equivalent acid loads that signal protective effector mechanisms.
Although the five basic tastes (sweet, salty, sour, bitter, and umami) have been recognized for centuries, taste receptor families have only recently been cloned from the lingual taste buds (26). Of interest, these receptors are also present in the GI tract. In addition to salty taste detected by epithelial Na+ channels and sour taste by H+-gated ion channels, a heterodimer of the taste receptor, type 1, member 2 (T1R2) and 3 (T1R3) is a sweet receptor, expressed in the small intestinal mucosa (19, 28, 29). Bitter taste receptors consisting of the taste receptor, type 2 (T2R) family are expressed in the GI tract (47). This suggests that the intestinal mucosa, in addition to the tongue, also senses the luminal content to presumably detect nutrients and expel unfavorable substances to maintain physiological processes such as digestion, absorption, secretion, and motility.
The receptor for l-glutamate (l-Glu), the primary nutrient conferring umami taste, is a heterodimer of T1R1 and T1R3 (32, 48) or alternately the metabotropic l-Glu receptor (mGluR) 1 or 4 (15, 44). These receptors belong to G protein-coupled receptor superfamily. These receptors and their cognate G proteins, α-gustducin, are localized in the epithelial cells in the GI tract (28, 29, 38, 40), suggesting that the mucosa directly taste luminal content to release mediators or conduct the luminal information to internal milieu. Indeed, luminal l-Glu stimulates gastric vagal afferents with release of nitric oxide and 5-hydroxytryptamine (5-HT) with the involvement of 5-HT3 receptor (46), suggesting a nutrient-sensing pathway for l-Glu in the upper GI tract related to known protective efferent neural circuitry. Thus we hypothesized that luminal l-Glu affects mucosal defense mechanisms via l-Glu receptors in the upper GI tract, similar to the acid-sensing pathways.
Here we show that luminal l-Glu enhances duodenal mucosal defenses mediated via multiple l-Glu receptors and activation of capsaicin and COX/PG pathways and that l-Glu protects the duodenal mucosa from acid-induced injury.
MATERIALS AND METHODS
Chemicals and animals.
2′,7′-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) acid, its acetoxy methyl ester (BCECF-AM), and propidium iodide (PI) were obtained from Molecular Probes (Eugene, OR). Two-micron pink fluorescent microspheres were obtained from Bangs Laboratories (Fishers, IN). VU0155041 (cis-2-[[(3,5-dichlorophenyl)amino]carbonyl]cyclohexane carboxylic acid) sodium salt was obtained from Tocris Bioscience (Ellisville, MO). Monosodium l-Glu, d-glutamate (d-Glu), l-aspartate (l-Asp), l-alanine (l-Ala), l-leucine (l-Leu), inosine 5′-monophosphate (IMP), spermine, l-2-amino-4-phosphonobutyric acid (l-AP4), (S)-2-amino-2-methyl-4-phosphonobutyric acid (MAP4), (S)-3,5-dihydroxyphenylglycine (S-DHPG), 1-amino-2,3-dihydro-1H-indene-1,5-dicarboxylic acid (AIDA), HEPES and other chemicals were obtained from Sigma Chemical (St. Louis, MO). Krebs solution contained (in mM) 136 NaCl, 2.6 KCl, 1.8 CaCl2, and 10 HEPES at pH 7.0. Osmolarity was adjusted to isotonic by reducing NaCl concentration. pH of Krebs solution after dissolving the compound was adjusted to pH 7.0. All solutions were prewarmed at 37°C in a water bath, and temperature was maintained by a heating pad. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee (VA IACUC). Male Sprague-Dawley rats weighing 200–250 g (Harlan, San Diego, CA) were fasted overnight but had free access to water.
Measurements of pHi, blood flow, and MGT in duodenum.
Simultaneous measurements of pHi of duodenal epithelial cells using fluorescent ratio technique, duodenal blood flow with laser Doppler flowmetry, and MGT with fluorescent microspheres were performed as described elsewhere (7, 9, 30). Under isoflurane anesthesia (2%), the exposed, chambered duodenal mucosa was incubated with 10 μM BCECF-AM in pH 7.0 Krebs buffer for 15 min to load the duodenal epithelial cells. After BCECF loading, fluorescent microspheres diluted to 0.05% wt/vol with prewarmed Krebs buffer were placed over the mucosa to delineate the luminal surface of the gel layer. Blood flow was measured via serosally placed right angle probe with laser Doppler flowmetry (Transonic, Ithaca, NY).
BCECF fluorescence was visualized with 495- and 450-nm excitation and 515-nm emission (green filter set; Chroma, Brattleboro, VT), whereas fluorescent microspheres were visualized with 575-nm excitation and 600-nm emission (red filter set) using a ×10 objective mounted on a Zeiss MPS microscope and recorded with a cooled charge-coupled device video camera (Hamamatsu Orca-EN; Hamamatsu USA, Bridgewater, NJ). Images were captured, digitized, recorded, and analyzed using Apple G4 microcomputer and image analysis software (OpenLab; Improvision, Lexington, MA). pHi was calculated from fluorescence intensity ratio and in vitro calibration curve as previously described (9). MGT was determined by measuring traveling distance between focal planes of the green fluorescent cell surface and the red fluorescent microspheres using a digital z-axis measuring device (Quick-Check; Metronics, Bedford, NH) (7).
After BCECF was loaded, fluorescent microspheres were applied on the gel surface, and blood flow was stabilized with pH 7.0 Krebs buffer perfusion for ∼30 min, the time was set as t = 0. The proximal duodenal mucosa was isolated by the chamber and topically perfused, preventing the contamination of pancreatic secretion. The chambered duodenal mucosa was perfused with pH 7.0 Krebs from t = 0 min until t = 10 min (basal period), followed by with pH 7.0 Krebs with or without chemicals from t = 10 min until t = 20 min (challenge period), then with pH 7.0 Krebs from t = 20 min until t = 35 min (recovery period). All measurements were conducted every 5 min.
Measurement of duodenal HCO3− secretion.
Duodenal loops were prepared and perfused as previously described (10, 31). In brief, under isoflurane anesthesia (2%), the proximal duodenal loop (perfused length 2 cm) was perfused with pH 7.0 normal saline by using a peristaltic pump (Fisher Scientific, Pittsburgh, PA) at 1 ml/min. The perfusate was bubbled with 100% O2 and stirred and warmed at 37°C with a heating stirrer (Barnstead International, Dubuque, IA). The pH of the perfusate was kept constant at pH 7.0 with a pH stat (models PHM290 and ABU901; Radiometer Analytical, Lyon, France). Furthermore, to eliminate the buffer action of agonists or antagonists, which would over- or underestimate the titration volume using pH stat, two sets of flow through pH and CO2 electrodes (Lazar Research Laboratories, Los Angeles, CA) were connected in the perfusion loop where pH and [CO2] were simultaneously and continuously measured. Because the input (perfusate) [CO2] is ∼0, the effluent [CO2] and pH were used to calculate the total CO2 output equivalent to the secreted HCO3− as previously described (10, 31). To prevent contamination of the perfusate from bile or pancreatic juice, the pancreaticobiliary duct was ligated just proximal to its insertion into the duodenal wall and cannulated with a PE-10 tube to drain the juice. After stabilization with continuous perfusion of pH 7.0 saline for ∼30 min, the time was set as t = 0. The duodenal loop was perfused with pH 7.0 saline from t = 0 min until t = 10 min (basal period). The perfusate was then changed to pH 7.0 Krebs buffer from t = 10 min until t = 35 min (challenge period), with or without chemicals. At t = 10 min, the system was gently flushed so as to rapidly change the composition of the perfusate.
Experimental protocol.
To examine the effect of luminal l-Glu on mucosal defense mechanisms, l-Glu (0.1–10 mM) was luminally perfused over the duodenal mucosa while pHi, blood flow, and MGT were measured. d-Glu (10 mM) was used as a negative control. In duodenal HCO3− secretion experiments, l-Glu (10 mM) was perfused through a duodenal loop during the challenge period.
Some animals were pretreated with high dose of capsaicin (125 mg/kg sc) 10–14 days before the experiments to selectively denervate the afferent nerves or were pretreated with indomethacin (5 mg/kg ip) 1 h before the experiments to inhibit COX activity as previously described (6). The duodenal mucosa of capsaicin- or indomethacin-treated rats was superfused with l-Glu (10 mM) as described above.
To evaluate the involvement of T1Rs as an amino acid receptor in l-Glu effects, the effects of l-Asp, l-Ala, or l-Leu (each 10 mM) on pHi/blood flow/MGT or HCO3− secretion were examined. Because the luminal concentration of l-Ala (1.9 mM) or l-Leu (3 mM) in the human jejunum 3 h after a protein-rich meal is similar to that of l-Glu (2.6 mM) (1), we chose l-Ala and l-Leu in addition to another excitatory amino acid l-Asp to compare their effects with l-Glu. Because l-Glu-stimulated lingual umami perception is synergistically enhanced by the addition of IMP (41), and because the T1R1/T1R3 heterodimer is synergistically activated with amino acid + IMP (32), the additional effect of IMP on mucosal defense factors (pHi/blood flow/MGT or HCO3− secretion) was examined. IMP (0.1 mM) alone or IMP with l-Glu, l-Asp, l-Ala, or l-Leu (each 10 mM) was superfused or perfused during the challenge period.
To test the role of mGluRs in l-Glu effects, we examined the effects of a selective group I mGluR (mGluR1 and 5) agonist S-DHPG (0.1 mM) or a selective group III mGluR (mGluR4, 6, 7, and 8) agonist l-AP4 (0.1 mM) on mucosal defense factors. The effects of coperfusion of a selective group I mGluR antagonist AIDA (0.1 mM) or a selective group III mGluR antagonist MAP4 (0.1 mM) with l-Glu (10 mM) were also examined. l-AP4 and MAP4 were predicted to be selective for mGluR4. Furthermore, a highly selective mGluR4 agonist was tested. VU0155041 (10 μM), which is a potent allosteric modulator of mGluR4, but also acts as a highly selective allosteric agonist of mGluR4 (35), was superfused.
To investigate the role of calcium-sensing receptor (CaSR) in l-Glu effects, the effects of perfusion of high Ca2+ Krebs solution (4 mM) or a CaSR agonist spermine (1 mM) (16) on mucosal defense factors were examined.
Measurement of duodenal epithelial cell injury.
Epithelial cellular injury was assessed by in vivo in situ PI staining as previously reported (4). The mucosa was superfused with a PI-containing Krebs solution (1 μM) to stain injured cell nuclei. After BCECF images were captured, PI fluorescence was visualized by illuminating the mucosa at 535 nm with emission detected at 590 nm. PI-positive dots corresponding to injured cell nuclei were counted in each image (microscopic field) observed with ×10 objective lens. The mean of the number obtained from three microscopic fields was defined as the number for the given time period. We have correlated PI-positive cell number in vivo with a cellular injury scale on the basis of the examination of hematoxylin-eosin conventionally stained sections (4). After BCECF loading followed by the stabilization with pH 7.0 Krebs superfusion, the mucosa was superfused with pH 7.0 Krebs for 10 min with or without l-Glu (10 mM) during the basal period (t = 0–10 min), followed by superfusion of pH 1.8 acid solution for 5 min (challenge period, t = 10–15 min), then with pH 7.0 Krebs for 15 min (recovery period, t = 15–30 min). PI images were captured every 5 min.
Expression of l-Glu receptors in rat tissues.
To investigate the expression of l-Glu receptors in the rat tissues, RT-PCR analysis was performed for T1R isoforms, mGluR isoforms, and CaSR. Three rats were euthanized by the terminal exsanguinations under sodium pentobarbital anesthesia (50 mg/kg ip). The lower esophagus, fundus and antrum of stomach, proximal duodenum, Th1-Th8 dorsal root ganglia (DR), and nodose ganglia (NG) were removed and used for RT-PCR as previously described (5). Furthermore, to sublocalize the presence of the target mRNA in the esophagus, stomach, and duodenum, these tissues were separated from the muscle layers and the mucosa by sharp dissection under a Zeiss stereomicroscope in ice-cold RNA stabilizing solution (RNA-Later; Qiagen, Valencia, CA) followed by RNA extract. The PCR primers used in the present study were listed in Table 1, and β-actin was used as an internal control. An aliquot of the RT reaction product served as a template in 40 cycles with 30 s at 95°C, 30 s at 58–64°C, and 30 s at 72°C. Furthermore, after dissection of villi, crypt, and muscle layer of the duodenum under a stereomicroscope, real-time PCR was performed with SYBR green fluorescence using a thermal cycler (MyiQ single-color real-time PCR detection system; Bio-Rad, Hercules, CA). Threshold cycle (Ct) value of each target mRNA was compared with Ct value of β-actin using ΔCt method for reference gene. The expression level of each receptor was presented as fold induction per 103 copies of β-actin. Each primer set showed 89–98% PCR efficiency in brain or tongue tissue as positive controls, except for T1R2 (∼70%), presumably attributable to lower expression.
Table 1.
PCR primers used
| Sequence | Product Size, bp | Accession Number | |
|---|---|---|---|
| T1R1 | |||
| sense | 5′-TTGGTCTACTGCTGGGCTTT-3′ | 414 | NM053305 |
| antisense | 5′-AGCGTGGTCAGTGTTGTCAG-3′ | ||
| T1R2 | |||
| sense | 5′-AACAACACGGTCCCTGTCTC-3′ | 439 | XM001074791 |
| antisense | 5′-GGCAGAAGCATGAGAAGACC-3′ | ||
| T1R3 | |||
| sense | 5′-GAGGCCACTCTCAACCAGAG-3′ | 477 | NM130818 |
| antisense | 5′-CCAGCTGAAATTCTGCAACA-3′ | ||
| mGluR1 | |||
| sense | 5′-GCTCCTGGATTTCCTCATCA-3′ | 216 | NM017011 |
| antisense | 5′-CGTACCATTCCGCTTTTGTT-3′ | ||
| mGluR4 | |||
| sense | 5′-GATATTGTCCGAGCCCTCAA-3′ | 255 | NM022666 |
| antisense | 5′-CTCCAACACCCTCCTGATGT-3′ | ||
| CaSR | |||
| sense | 5′-TCTTGTGGAGTGGGTTCTCC-3′ | 353 | NM016996 |
| antisense | 5′-GGCCTTGACGATAGGTGTGT-3′ | ||
| β-actin | |||
| sense | 5′-GTGGGCCGCCCTAGGCACCA-3′ | 241 | NM031144 |
| antisense | 5′-TGGCCTTAGGGTTCAGAGGG-3′ |
T1R, taste receptor type 1; mGluR, metabotropic glutamate receptor; CaSR, calcium-sensing receptor.
Statistics.
All data are expressed as means ± SE. Data were derived from six rats in each group. Comparisons between groups were made by one-way ANOVA followed by Fischer's least-significant difference test. P values of 0.05 were taken as significant.
RESULTS
Effect of luminal l-Glu on duodenal mucosal defense factors.
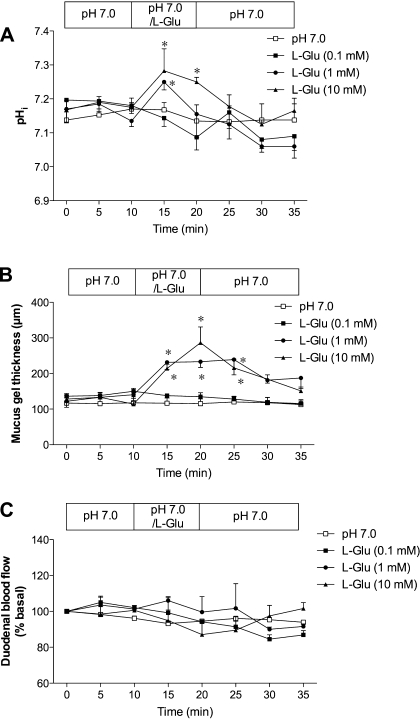
Duodenal perfusion with pH 7.0 Krebs buffer solution alone showed the stable pHi, MGT, and blood flow (Fig. 1, A–C). Luminal perfusion with l-Glu (0.1–10 mM) dose dependently increased pHi (Fig. 1A). Luminal l-Glu also increased MGT, followed by the gradual decrease of gel thickness after l-Glu removal (Fig. 1B), consistent with the increased mucus secretion (7). In contrast, luminal l-Glu had no effect on blood flow (Fig. 1C). These results suggest that luminal l-Glu enhances duodenal defense mechanisms.
Fig. 1.
Effect of luminal l-glutamate (l-Glu) on mucosal defense factors in rat duodenum. Intracellular pH (pHi) of epithelial cells, mucus gel thickness, and blood flow were simultaneously measured in vivo using fluorescence microscopy and flowmetry. Luminal perfusion of l-Glu (0.1–10 mM) dose dependently increased pHi (A) and mucus gel thickness (B) but had no effect on duodenal blood flow (C). Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group.
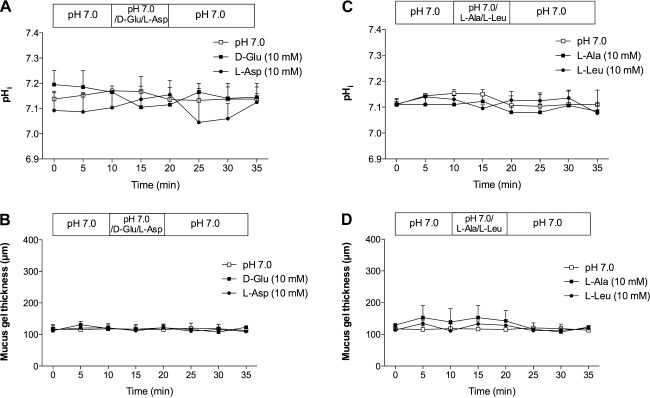
Luminal perfusion of d-Glu or l-Asp (10 mM) had no effect on pHi or MGT in the duodenum (Fig. 2, A and B). Furthermore, l-Ala or l-Leu (10 mM) also had no effect on pHi or MGT (Fig. 2, C and D). None had any effect on blood flow. These results suggest that l-Glu is a potent effector on pHi and MGT.
Fig. 2.
Effect of luminal amino acids on pHi and mucus gel thickness in rat duodenum. d-glutamate (d-Glu, 10 mM), l-aspartate (l-Asp, 10 mM), l-alanine (l-Ala, 10 mM), or l-leucine (l-Leu, 10 mM) had no effect on pHi (A and C) and mucus gel thickness (B and D). Each data point represents mean ± SE (n = 6 rats).
Effect of capsaicin or indomethacin pretreatment on l-Glu-induced effects in rat duodenum.
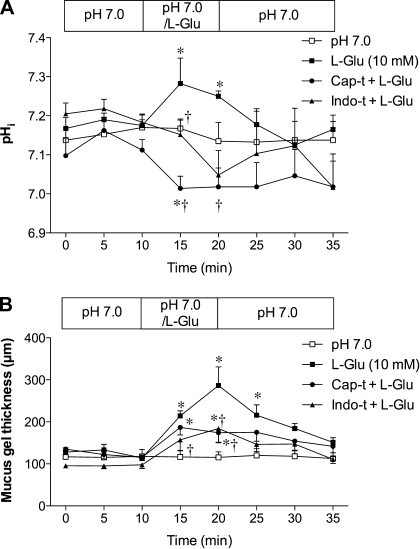
Because the duodenal mucosal defense mechanisms involve the activation of capsaicin-sensitive afferent nerves and/or COX (24), we next examined the effect of l-Glu on the duodenal mucosal defenses in capsaicin- or indomethacin-pretreated rats. Either treatment had no effect on pHi and MGT during the basal period (Fig. 3, A and B). Pretreatment with indomethacin inhibited l-Glu-induced cellular alkalinization (Fig. 3A) and reduced l-Glu-induced mucus secretion (Fig. 3B). Pretreatment with capsaicin actually decreased pHi during l-Glu perfusion (Fig. 3A) and reduced l-Glu-induced mucus secretion (Fig. 3B). Blood flow was unchanged in any groups (data not shown). These results suggest that cellular alkalinization and mucus secretion induced by luminal l-Glu are dependent on capsaicin-sensitive afferent nerves and COX activity in rat duodenum.
Fig. 3.
Effect of afferent denervation or cyclooxygenase inhibition on l-Glu-induced intracellular alkalinization and mucus secretion in rat duodenum. Rats were pretreated with capsaicin (Cap-t, 125 mg/kg sc) or indomethacin (Indo-t 5 mg/kg, sc). A: pHi. Cap-t and Indo-t inhibited l-Glu-induced intracellular alkalinization, while l-Glu acidified the cells in Cap-t rats. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu group. B: mucus gel thickness. Cap-t and Indo-t reduced l-Glu-induced mucus secretion. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu group.
Expression of l-Glu receptors in the rat tissues.
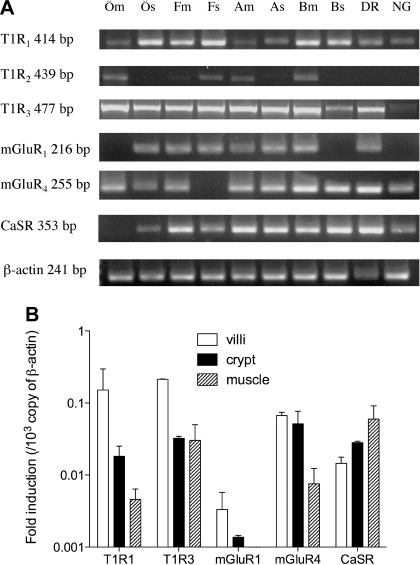
RT-PCR revealed high T1R1 and T1R3 expression in esophageal, gastric, and duodenal mucosa and in sensory neurons, with less T1R2 expressed (Fig. 4A). mGluR1 and 4 and CaSR were also expressed in the fundic and duodenal mucosa. Furthermore, real-time PCR revealed that T1R1 and 3, mGluR1 and 4, and CaSR were expressed in the duodenal villi (Fig. 4B).
Fig. 4.
Expression of l-Glu receptors in rats. A: RT-PCR analysis for taste receptor 1 subtypes (T1R1, 2, 3), metabotropic glutamate receptor subtypes (mGluR1, 4), and calcium-sensing receptor (CaSR) in the mucosa (m) and muscle layer (s) of the esophagus (Ö), fundic stomach (F), gastric antrum (A), proximal duodenum (B), dorsal root ganglia (DR) and nodose ganglia (NG) in rats. 1 of 3 experiments is represented. B: real-time PCR for T1Rs, mGluRs, and CaSR in the villi, crypt, and muscle layer of the proximal duodenum. Relative expression of each receptor for β-actin was calculated from threshold cycle (Ct) values. Each data point presents mean ± SE (n = 3 rats).
Effect of luminal l-Glu on duodenal HCO3− secretion.
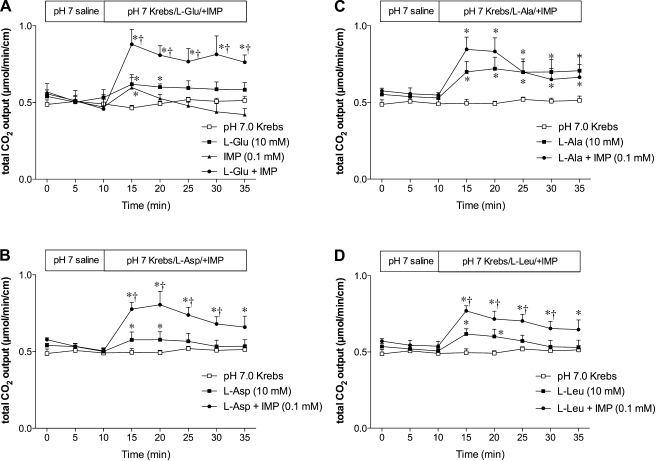
Because HCO3− secretion is one of the most important, well-studied defense factors against luminal acid in duodenum, we hypothesized that luminal l-Glu stimulates duodenal HCO3− secretion. Luminal perfusion of l-Glu (10 mM) significantly but slightly increased HCO3− secretion (Fig. 5A), which was robustly enhanced by the addition of IMP (0.1 mM) to l-Glu. IMP alone had a little effect on HCO3− secretion. These results are consistent with the synergistic effect of l-Glu and IMP, well-described for l-Glu sensing by the taste buds (33). Furthermore, l-Asp or l-Leu also modestly increased HCO3− secretion with robust enhancement by the addition of IMP (Fig. 5, B and D), similar to l-Glu. These results suggest that l-Glu-associated HCO3− secretion is likely mediated via T1R1/T1R3. In contrast, l-Ala alone increased HCO3− secretion without further significant enhancement by IMP (Fig. 5C). Lack of synergy of l-Ala with IMP may be due to a different affinity for T1R1/T1R3 (32), submaximal effect of l-Ala alone, or a nonspecific effect.
Fig. 5.
Effect of amino acid and inosine monophosphate (IMP) on duodenal HCO3− secretion in rats. Duodenal HCO3− secretion is expressed as total CO2 output. Perfusion of l-Glu (10 mM) or IMP (0.1 mM) had a little effect on HCO3− secretion, while l-Glu coperfused with IMP synergistically augmented HCO3− secretion (A). Similar synergism was observed with perfusion of l-Asp (10 mM) (B) or l-Leu (10 mM) (D), whereas l-Ala (10 mM) increased HCO3− secretion without the synergy with IMP (C). Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. the corresponding amino acid group.
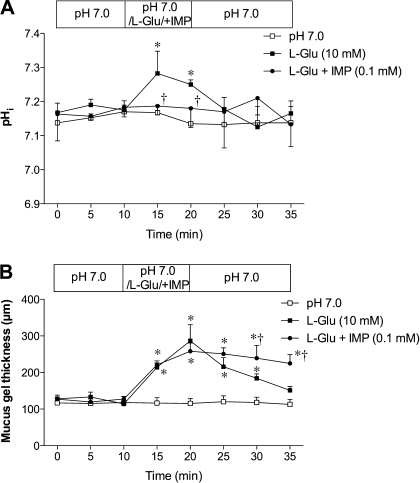
The synergy between l-Glu and IMP prompted us to examine the effect of l-Glu and IMP on pHi and MGT. The addition of IMP inhibited l-Glu-induced cellular alkalinization (Fig. 6A), suggesting that l-Glu-induced pHi modulation is closely linked with HCO3− secretion. The addition of IMP to l-Glu did not augment the peak value of MGT (Fig. 6B). Nevertheless, the addition of IMP with l-Asp, l-Leu, or l-Ala had no effect on pHi, MGT, or blood flow (data not shown), further suggesting that the activation of T1R1/T1R3 does not directly affect pHi or MGT.
Fig. 6.
Effect of l-Glu and IMP on pHi and mucus gel thickness in rats. A: coperfusion of IMP (0.1 mM) with l-Glu (10 mM) inhibited l-Glu-induced intracellular alkalinization. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu group. B: IMP had no enhancing effect on l-Glu-induced mucus secretion. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu group.
Effect of mGluR agonists or antagonists on duodenal mucosal defense factors.
Next, we examined the effect of mGluR agonists on pHi and MGT or the effect of mGluR antagonists on l-Glu-induced cellular alkalinization and mucus secretion in the duodenum. Perfusion of the mGluR4 agonist l-AP4 (0.1 mM) increased both pHi and MGT (Fig. 7, A and B), mimicking the effects of l-Glu. In contrast, the mGluR1/5 agonist S-DHPG (0.1 mM) temporally increased pHi but had no effect on MGT (Fig. 7, A and B). Coperfusion of the mGluR4 antagonist MAP4 (0.1 mM) with l-Glu abolished l-Glu-induced cellular alkalinization and inhibited l-Glu-induced mucus secretion, whereas the mGluR1/5 antagonist AIDA (0.1 mM) partially inhibited l-Glu-induced alkalinization but had no effect on l-Glu-induced mucus secretion (Fig. 7, D and E). These results suggest that l-Glu-induced pHi and MGT increases are mediated mainly via mGluR4 and that mGluR1/5 is partially involved in only l-Glu-induced cellular alkalinization.
Fig. 7.
Effect of mGluR agonists or antagonists on duodenal mucosal defense factors. A and B: effects of (S)-3,5-dihydroxyphenylglycine (S-DHPG) (0.1 mM), l-2-amino-4-phosphonobutyric acid (l-AP4) (0.1 mM), or VU0155041 (10 μM) on pHi of epithelial cells (A) and mucus gel thickness (B) were examined in rat duodenum. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. C: duodenal loop was perfused, and HCO3− secretion was measured with pH and CO2 electrodes. HCO3− secretion is expressed as total CO2 output. Each data point represents mean ± SE (n = 6 rats). D and E: effects of 1-amino-2,3-dihydro-1H-indene-1,5-dicarboxylic acid (AIDA) (0.1 mM), or (S)-2-amino-2-methyl-4-phosphonobutyric acid (MAP4) (0.1 mM) on l-Glu (10 mM)-induced pHi (D) and mucus gel thickness (E) increases were examined. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu group.
To further confirm the role of mGluR4 in l-Glu effects, we tested the highly selective compounds for mGluR4. Cells were alkalinized by a highly selective mGluR4 agonist VU0155041 (10 μM) (Fig. 7A). VU0155041 also stimulated mucus secretion (Fig. 7B). Another highly selective mGluR4/8 agonist (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (0.1 mM) also mimicked l-Glu effects on pHi and MGT (data not shown). These results further suggest that l-Glu increases pHi and MGT most likely via mGluR4.
We also tested the effects of the mGluR1/5 agonist S-DHPG (0.1 mM) or the mGluR4 agonist l-AP4 (0.1 mM) on duodenal HCO3− secretion. S-DHPG or l-AP4 had no effect on HCO3− secretion (Fig. 7C), suggesting that, at least, mGluR1 and 4 were not involved in the regulation of duodenal HCO3− secretion.
Effect of CaSR agonists on duodenal mucosal defense factors.
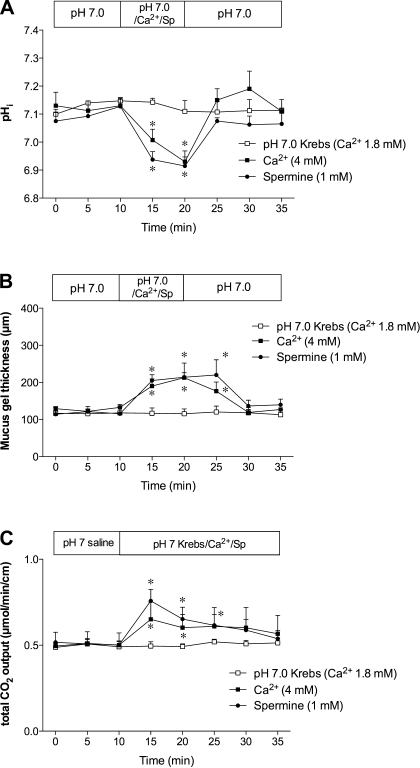
We next examined the effect of CaSR agonists on pHi, blood flow, and MGT. Perfusion of high Ca2+ Krebs solution (4 mM) or spermine (1 mM) decreased pHi (Fig. 8A) but increased MGT (Fig. 8B) and blood flow (data not shown), mimicking the effect of luminal acid (2) but unlike l-Glu. Furthermore, perfusion of high Ca2+ or spermine increased duodenal HCO3− secretion (Fig. 8C).
Fig. 8.
Effect of CaSR agonists on duodenal mucosal defense factors. A and B: effects of perfusion of high Ca2+ solution (4 mM) or spermine (Sp) (1 mM) on pHi of epithelial cells (A) and mucus gel thickness (B) were examined in rat duodenum. pH 7.0 Krebs solution containing 1.8 mM Ca2+ was perfused as control. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. C: effect of Ca2+ (4 mM) or spermine (1 mM) on duodenal HCO3− secretion measured via duodenal loop perfusion was examined. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group.
Effect of l-Glu on acid-induced epithelial injury in duodenum.
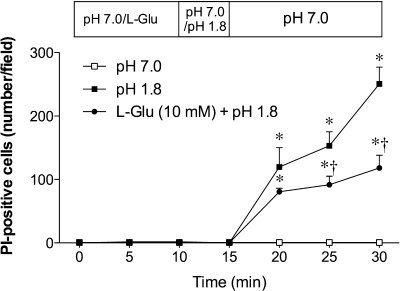
Because luminal l-Glu enhanced mucosal defense mechanisms, we examined the protective effect of l-Glu on acid-induced injury. Acute epithelial injury was invoked in a microscopic model by perfusion of a superphysiological pH 1.8 acid solution for 5 min with injury measured in vivo in situ PI staining (4). Exposure to a pH 1.8 solution progressively increased the number of PI-positive nuclei within the mucosa (Fig. 9). Preperfusion of l-Glu (10 mM) reduced the increase of PI-positive nuclei, suggesting that luminal l-Glu protects the mucosa from acid-induced injury in the duodenum.
Fig. 9.
Effect of l-Glu on acid-induced epithelial injury in rat duodenum. Epithelial injury was assessed in vivo in situ by propidium iodide (PI) staining. Pretreatment with luminal l-Glu (10 mM) reduced pH 1.8 acid-induced increase of PI-positive cell number. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu group.
DISCUSSION
We demonstrated that luminal l-Glu increased pHi and MGT, but not blood flow, in the duodenal mucosa. l-Glu-induced cellular alkalinization and mucus secretion were mediated by capsaicin- and indomethacin-sensitive pathways in the duodenum, corresponding to the activation of capsaicin-sensitive afferent nerves and COX activity, respectively. Luminal l-Glu enhanced mucosal defense mechanisms via multiple l-Glu receptors (Fig. 10). l-Glu-induced pHi and MGT increases were mimicked by mGluR4 agonists and inhibited by an mGluR4 antagonist. mGluR1/5 was partially involved in the l-Glu effect on pHi. In contrast, the synergy between l-Glu and IMP for augmentation of duodenal HCO3− secretion implicates the involvement of the T1R1/T1R3 heterodimer. This is the first study to demonstrate the involvement of l-Glu sensing by the upper GI mucosa in the enhancement of mucosal defense mechanisms and in the protection of the mucosa from acid-induced injury.
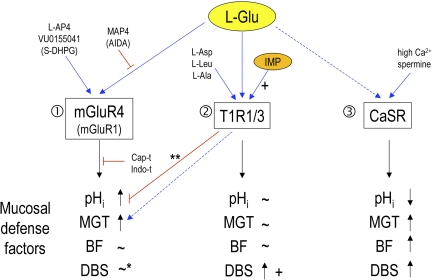
Fig. 10.
Summary diagram: effects of luminal l-Glu on mucosal defense factors in rat duodenum. 1) l-Glu increases pHi and mucus gel thickness (MGT) mainly via mGluR4 without changes of blood flow (BF), mimicked by l-AP4 and VU0155041, inhibited by MAP4, capsaicin pretreatment (Cap-t), or indomethacin (Indo-t). l-Glu-induced effects may involve mGluR1, partially mimicked by S-DHPG and reduced by AIDA. *Note that l-Glu does not likely increase duodenal bicarbonate secretion (DBS) via mGluRs. 2) l-Glu increases DBS, enhanced by IMP, probably via the T1R1/3 heterodimer, mimicked by l-Asp, l-Leu, or l-Ala. l-Glu-induced changes in pHi and MGT are unlikely to be mediated by T1R1/3. **Note that IMP inhibits l-Glu-induced pHi increase and sustained l-Glu-induced MGT increase. 3) CaSR activation by high Ca2+ solution or spermine decreases pHi and increases MGT, BF, and DBS, not mirroring l-Glu-induced effects. Blue arrows, stimulation; red lines, inhibition; +, positive allosteric effect by IMP; dashed line, possible involvement; parenthesis, partial involvement.
Cellular alkalinization is an important defense factor against luminal acid. Cellular alkalinization attributable to epithelial cell basolateral HCO3− uptake before HCO3− secretion protects the epithelial cells from acid-induced injury, as demonstrated using models of cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction (4, 8, 22). We demonstrated that l-Glu-induced cellular alkalinization contributes to the protection of epithelial cells from acid-induced injury. Mucus secretion is another mucosal defense factor, which is thought to create a premucosal buffering zone, damping direct acid exposure to the epithelial cells (20). Our study also implicates l-Glu-induced mucus secretion in the mucosal protection.
Mucosal defense mechanisms in the upper GI tract are mediated by capsaicin-sensitive afferent nerves and COX (24). Capsaicin and PGE2 stimulates HCO3− secretion with the augmented epithelial cellular buffering and stimulates mucus secretion in the duodenum (3, 42), consistent with our result that l-Glu-induced increase of pHi and MGT was inhibited by pretreatment with capsaicin or indomethacin in the duodenum. These two pathways also increase blood flow in the duodenum (6, 27), inconsistent with the observed lack of effect of l-Glu on blood flow. Because intracellular acidification, not alkalinization, is correlated with blood flow increase (9), l-Glu-induced cellular alkalinization may fail to increase blood flow, despite the involvement of capsaicin and COX pathways.
Possible candidates for the l-Glu receptor are taste receptor heterodimer (T1R1 and 3) (32, 48) or mGluR (1 or 4) (15, 44), as reported in the taste buds for umami perception, or possibly the CaSR (17). The T1R1/3 detects amino acids including l-Glu, l-Asp, l-Ala, and l-Leu with differing affinity synergistically enhanced by IMP (32). The mGluR recognizes l-Glu selectively, yet synergism with IMP has not been confirmed. CaSR nonselectively binds l-type amino acids including l-Glu and l-Asp but is not synergistic with IMP (17). The relatively specific effects of l-Glu on pHi and mucus secretion in the duodenum suggest that mGluRs are most likely l-Glu receptors. Our results suggest that mGluR4 mainly mediates l-Glu-induced pHi and MGT increases, whereas mGluR1 may have partial role in pHi regulation. In contrast, the synergistic effect of l-Glu, l-Asp, or l-Leu with IMP on duodenal HCO3− secretion suggests that T1R1/3 is the corresponding receptor. Nevertheless, l-Asp, l-Leu, or l-Ala alone had no effect on pHi and MGT, unlike l-Glu, suggesting that T1R1/3 is only involved in the regulation of HCO3− secretion. Furthermore, lack of effect of l-Asp, l-Leu, or l-Ala with IMP on pHi and MGT suggests that T1R1/3 activation is indirectly involved in pHi and MGT regulation. In contrast, CaSR agonists acidified cells and increased MGT, blood flow, and HCO3− secretion, like luminal acid, but unlike l-Glu. It is still possible that the CaSR is involved in l-Glu-induced mucus and HCO3− secretion. The selective inhibitors for T1R1/T1R3 and CaSR will clarify their contribution to l-Glu-induced augmentation in mucosal defense factors.
Inhibition of cellular alkalinization by l-Glu with IMP is still unclear. Because our results suggest that the modulation of pHi and HCO3− secretion by luminal l-Glu is differently mediated via mGluRs and T1Rs, respectively, the close link between pHi and HCO3− secretion may explain this phenomenon. A rapid increase in the rate of HCO3− secretion may reduce cellular HCO3− concentration, resulting the inhibition of cellular alkalinization. l-Glu and mGluR agonists had discrepant effects on pHi and HCO3− secretion. Although both agonists alkalinized cells, l-Glu increased HCO3− secretion, whereas mGluR agonists did not. Cellular alkalinization may not have been of sufficient magnitude to support measurable HCO3− secretion, which may also need the participation of other secretory components such as CFTR (4). The mechanism by which CaSR stimulation acidified cells accompanied by increased mucus and HCO3− secretion is also unclear. Acid superfusion of the mucosa acidifies epithelial cells, presumably via CO2 entry while increasing mucus secretion and blood flow (7). Because CaSR activation produces comparable effects, we can speculate that the observed cellular acidification is attributable to augmented HCO3− secretion with reduction of cellular HCO3− concentration or to independent effects of CaSR activation. Activation of CaSR acidifies in vitro microperfused mouse medullary thick ascending limb cells, which express CaSR at the basolateral membrane (11), consistent with our finding. This suggests that intracellular acidification by CaSR activation triggers mucus and HCO3− secretion, similar to the effect of luminal acid.
l-Glu receptors have not been fully localized in the gut. Because intestinal endocrine cells as well as enterocytes are functionally implicated in luminal chemosensing (28, 29, 37, 38), l-Glu receptors are likely to be expressed in the intestinal endocrine cells or enterocytes. Nevertheless, the localization of T1Rs in the GI tract is controversial. T1R1 and T1R3 are located on the apical membrane of rat jejunal enterocytes (28), whereas T1R3 is only expressed in the endocrine cells in human and mouse duodenum (29). Although each expression was detected by PCR in this study, lack of specific antibodies for T1Rs limits to clearly localize T1Rs in the GI tract. In contrast, CaSR has been localized mainly on the basal surface of enterocytes, gastric surface cells, and parietal cells as well as in the myenteric plexus (14, 18, 39). Furthermore, all groups of mGluRs are expressed in DR and NG (12, 13, 36), suggesting the mGluR expression on the afferent nerves in the GI tract, consistent with our findings that l-Glu effects on pHi and MGT are mediated via mGluRs and capsaicin-sensitive afferent nerves. Detailed localization of these receptors in the GI tract may further clarify the mechanisms underlying l-Glu-induced effects on mucosal defense factors.
Luminal l-Glu signaling is of uncertain functional significance. Because l-Glu is the most predominant amino acid in proteins (21), a sharp rise of luminal l-Glu concentration can signal protein ingestion, readying the gut for gastric and pancreatic secretion of peptidases (45). Although the luminal concentration of some other amino acids reached the similar level of l-Glu after a protein-rich meal in human jejunum (1), l-Asp, l-Ala, or l-Leu had no effect on pHi and MGT. The specificity for luminal l-Glu among the 20 amino acid in the stimulation of rat gastric vagal afferents accompanied by the release of 5-HT and nitric oxide (46) also support the observed protective effects of l-Glu on pHi and MGT. In contrast, these amino acids including l-Glu likely share the regulation of HCO3− secretion, suggesting cooperative mucosal protection during protein digestion. Because luminal l-Glu enhanced mucosal defenses and reduced acid-induced epithelial injury in the duodenum, luminal l-Glu signaling including l-Glu-specific and -nonspecific amino acid sensing may precondition or prime the mucosa for subsequent acid exposure and presumably protein digestion.
In conclusion, luminal l-Glu enhanced the mucosal defense mechanisms in the duodenum, via activation of most likely mGluR4 and T1R1/3, capsaicin-sensitive afferent nerves and COX pathway, protecting the mucosa from injury attributable to luminal acid. The umami taste receptors may be involved in the mucosal physiological responses for luminal amino acids, especially l-Glu.
GRANTS
This work was supported by a research grant from Ajinomoto, Japan (Y. Akiba), Department of Veterans Affairs Merit Review Award, NIH-NIDDK R01 DK54221 (J. Kaunitz), and the animal core of NIH-NIDDK P30 DK0413 (J. E. Rozengurt).
ACKNOWLEDGMENTS
We thank Jenifer Kugler for assistance with manuscript preparation.
REFERENCES
- 1.Adibi SA, Mercer DW. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest 52: 1586–1594, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acute adaptive cellular base uptake in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 280: G1083–G1092, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Sensory pathways and cyclooxygenase regulate mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol 280: G470–G474, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Sassani P, Dukkipatis R, Pushkin A, Kurtz I, Kaunitz JD. Cellular bicarbonate protects rat duodenal mucosa from acid-induced injury. J Clin Invest 108: 1807–1816, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, Swenson ER, Kaunitz JD. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology 131: 142–152, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol Gastrointest Liver Physiol 277: G268–G274, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol 279: G437–G447, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Akiba Y, Jung M, Ouk S, Kaunitz JD. A novel small molecule CFTR inhibitor attenuates HCO3− secretion and duodenal ulcer formation in rats. Am J Physiol Gastrointest Liver Physiol 289: G753–G759, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Akiba Y, Kaunitz JD. Regulation of intracellular pH and blood flow in rat duodenal epithelium in vivo. Am J Physiol Gastrointest Liver Physiol 276: G293–G302, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol 293: G1223–G1233, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Aslanova UF, Morimoto T, Farajov EI, Kumagai N, Nishino M, Sugawara N, Ohsaga A, Maruyama Y, Tsuchiya S, Takahashi S, Kondo Y. Chloride-dependent intracellular pH regulation via extracellular calcium-sensing receptor in the medullary thick ascending limb of the mouse kidney. Tohoku J Exp Med 210: 291–300, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Azkue JJ, Murga M, Fernandez-Capetillo O, Mateos JM, Elezgarai I, Benitez R, Osorio A, Diez J, Puente N, Bilbao A, Bidaurrazaga A, Kuhn R, Grandes P. Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J Comp Neurol 430: 448–457, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol 501: 780–789, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am J Physiol Gastrointest Liver Physiol 274: G122–G130, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 3: 113–119, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology 126: 148–158, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. l-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol 291: G753–G761, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Dufner MM, Kirchhoff P, Remy C, Hafner P, Muller MK, Cheng SX, Tang LQ, Hebert SC, Geibel JP, Wagner CA. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol 289: G1084–G1090, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33: 302–305, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Engel E, Guth PH, Nishizaki Y, Kaunitz JD. Barrier function of the gastric mucus gel. Am J Physiol Gastrointest Liver Physiol 269: G994–G999, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Giacometti T. Free and bound glutamate in natural products. In: Glutamic Acid: Advances in Biochemistry and Physiology, edited by Filer LJ., Jr New York: Raven, 1979, p. 25–34 [Google Scholar]
- 22.Hirokawa M, Takeuchi T, Chu S, Akiba Y, Wu V, Guth PH, Engel E, Montrose MH, Kaunitz JD. Cystic fibrosis gene mutation reduces epithelial cell acidification and injury in acid-perfused mouse duodenum. Gastroenterology 127: 1162–1173, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kaunitz JD, Akiba Y. Duodenal carbonic anhydrase: mucosal protection, luminal chemosensing, and gastric acid disposal. Keio J Med 55: 96–106, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kaunitz JD, Akiba Y. Acid-sensing protective mechanisms of duodenum. J Physiol Pharmacol 54: 19–26, 2003 [PubMed] [Google Scholar]
- 25.Kaunitz JD, Nishizaki Y, Kaneko K, Guth PH. Effect of orogastric nicotine on rat gastric mucosal gel thickness, surface cell viability, and intracellular pH. J Pharmacol Exp Ther 265: 948–954, 1993 [PubMed] [Google Scholar]
- 26.Lindemann B. Receptors and transduction in taste. Nature 413: 219–225, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lugea A, Salas A, Guarner F, Malagelada JR. Adaptive cytoprotection of the rat duodenum is not dependent on nitric oxide-induced changes in blood flow. Am J Physiol Gastrointest Liver Physiol 264: G994–G1000, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizumori M, Choi Y, Guth PH, Engel E, Kaunitz JD, Akiba Y. CFTR inhibition augments NHE3 activity during luminal high CO2 exposure in rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol 294: G1318–G1327, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y. Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 573: 827–842, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr 130: 950S–953S, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Nishizaki Y, Guth PH, Quintero E, Del Rivero M, Bover J, Kaunitz JD. Prostaglandin E2 enhances gastric defense mechanisms against acid injury in uremic rats. Gastroenterology 107: 1382–1389, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol 74: 1345–1358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page AJ, Young RL, Martin CM, Umaerus M, O'Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology 128: 402–410, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Raybould HE. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 277: G751–G755, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 291: G171–G177, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Rutten MJ, Bacon KD, Marlink KL, Stoney M, Meichsner CL, Lee FP, Hobson SA, Rodland KD, Sheppard BC, Trunkey DD, Deveney KE, Deveney CW. Identification of a functional Ca2+-sensing receptor in normal human gastric mucous epithelial cells. Am J Physiol Gastrointest Liver Physiol 277: G662–G670, 1999 [DOI] [PubMed] [Google Scholar]
- 40.San Gabriel AM, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett 581: 1119–1123, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sato M, Yamashita S, Ogawa H. Potentiation of gustatory response to monosodium glutamate in rat chorda tympani fibers by addition of 5′-ribonucleotides. Jpn J Physiol 20: 444–464, 1970 [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi K, Matsumoto J, Ueshima K, Okabe S. Role of capsaicin-sensitive afferent neurons in alkaline secretory response to luminal acid in the rat duodenum. Gastroenterology 101: 954–961, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Tanaka S, Taché Y, Kaneko H, Guth PH, Kaunitz JD. Central vagal activation increases mucus gel thickness and surface cell intracellular pH in rat stomach. Gastroenterology 112: 409–417, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res 313: 29–35, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Uneyama H, Gabriel AS, Kawai M, Tomoe M, Torii K. Physiological role of dietary free glutamate in the food digestion. Asia Pac J Clin Nutr, 17Suppl 1: 372–375, 2008 [PubMed] [Google Scholar]
- 46.Uneyama H, Niijima A, San GA, Torii K. Luminal amino acid sensing in the rat gastric mucosa. Am J Physiol Gastrointest Liver Physiol 291: G1163–G1170, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99: 2392–2397, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003 [DOI] [PubMed] [Google Scholar]