Abstract
We investigated whether the eccrine sweat glands must actively produce sweat during heat acclimation if they are to adapt and increase their capacity to sweat. Eight volunteers received intradermal injections of BOTOX, to prevent neural stimulation and sweat production of the sweat glands during heat acclimation, and saline injections as a control in the contralateral forearm. Subjects performed 90 min of moderate-intensity exercise in the heat (35°C, 40% relative humidity) on 10 consecutive days. Heat acclimation decreased end-exercise heart rate (156 ± 22 vs. 138 ± 17 beats/min; P = 0.0001) and rectal temperature (38.2 ± 0.3 vs. 37.9 ± 0.3°C; P = 0.0003) and increased whole body sweat rate (0.70 ± 0.29 vs. 1.06 ± 0.50 l/h; P = 0.030). During heat acclimation, there was no measurable sweating in the BOTOX-treated forearm, but the control forearm sweat rate during exercise increased 40% over the 10 days (P = 0.040). Peripheral sweat gland function was assessed using pilocarpine iontophoresis before and after heat acclimation. Before heat acclimation, the pilocarpine-induced sweat rate of the control and BOTOX-injected forearms did not differ (0.65 ± 0.20 vs. 0.66 ± 0.22 mg·cm−2·min−1). However, following heat acclimation, the pilocarpine-induced sweat rate in the control arm increased 18% to 0.77 ± 0.21 mg·cm−2·min−1 (P = 0.021) but decreased 52% to 0.32 ± 0.18 mg·cm−2·min−1 (P < 0.001) in the BOTOX-treated arm. Using complete chemodenervation of the sweat glands, coupled with direct cholinergic stimulation via pilocarpine iontophoresis, we demonstrated that sweat glands must be active during heat acclimation if they are to adapt and increase their capacity to sweat.
Keywords: eccrine sweat gland, cholinergic sensitivity, pilocarpine, sweat rate
numerous studies have shown that following heat acclimation, there is a large increase in the secretory capacity of the eccrine sweat glands (3–5, 13, 17, 19, 20, 24). For example, chest and forearm sweat rates have been reported to increase ∼110% following heat acclimation using a controlled-hyperthermia protocol, in which the work rate was adjusted to achieve and maintain an elevated core temperature (21). Peripheral adaptations, including increased cholinergic sensitivity and hypertrophy of the sweat glands, contribute to improvement in secretory capacity (1, 5, 15, 18, 23, 28).
Adaptations of the sweat gland to repeated heat exposures are associated with morphological changes in the gland itself. Sato et al. (23) first reported that in vitro and in vivo methacholine-induced sweating in monkeys was significantly increased following 9 mo of heat acclimation. The increased sweating capacity was accompanied by significant increases in the size of the eccrine sweat glands. More recently, it has been shown that cholinergic sensitivity of human eccrine sweat glands, as measured by pilocarpine-induced sweat rate, significantly increased following 8 days of heat acclimation (5). It remains controversial, however, as to whether it is an absolute necessity for the eccrine glands to actively produce sweat during heat acclimation if these peripheral adaptations are to occur.
Fox et al. (12) and Collins et al. (9, 10) concluded that the increased sweating capacity associated with heat acclimation was a direct result of sweat gland activity during the heat exposures and was not dependent on an elevated body temperature. Conversely, Chen and Elizondo (8), using an ice-water nerve block in humans, observed that sweat capacity was increased after heat acclimation, even though secretion of the sweat glands was minimal during 9 days of heat exposure. However, the methodological approaches (e.g., direct skin cooling, indirect cooling, ice-water nerve block) used in these studies only reduced, but did not completely inhibit, sudomotor activity. Consequently, there could still have been some neural stimulation of the sweat glands causing adaptations during the heat acclimation protocols that may have confounded their findings.
To overcome the limitations of methodologies used in previous studies, the current study utilized botulinum toxin serotype A (BOTOX; Allergan, Irvine, CA) to chemically denervate the sweat glands prior to a 10-day protocol of active heat acclimation. Botulinum toxin Type A is an endopeptidase neurotoxin that enters the sympathetic nerve terminals that innervate human sweat glands. Once the neurotoxin is taken up by the presynaptic nerve terminals innervating the eccrine sweat glands, the endopeptidase cleaves SNAP-25, a SNARE protein integral to the successful release of ACh (16). Thus, unlike past studies, this methodological approach resulted in complete blockage of neural input and sweat production during heat acclimation in a localized area of the treated forearm, while the untreated control arm was allowed to freely sweat. In addition, before and after heat acclimation, the current study measured pilocarpine-induced sweat rate that is independent of direct thermoregulatory effector input. With this design, we were able to determine whether active sweating during heat acclimation is required for improvements in sudomotor functioning of the sweat gland. We hypothesized that following heat acclimation, the pilocarpine-induced sweat rate in the control arm, which was allowed to sweat freely, would increase, while it would decrease in the BOTOX-treated forearm.
METHODS
The subjects for the study were three males and five nonpregnant females (age = 29.1 ± 10.3 yr, height = 170.4 ± 7.2 cm, weight = 70.8 ± 9.9 kg). All subjects were currently engaged in a regular exercise program (≥30 min aerobic exercise at least three times weekly for the past 6 mo) to minimize the potential effects of training adaptations. No restrictions were placed on their diet, although subjects were asked to drink at least 1 liter of water 3 h before reporting to the laboratory and not to exercise in the preceding 12 h. No attempt was made to control for the menstrual phase in the women volunteers, as it has been previously shown that the sensitivity of sweat glands to cholinergic agonists is not affected by the menstrual cycle (22). The San Diego State University Institutional Review Board approved the study, and written informed consent was obtained from each subject before participation.
The experiments were conducted in San Diego, CA, between November and December 2008 (average high temperatures were 21°C and 19°C, respectively) to ensure that subjects were not naturally heat acclimatized. Subjects were given three 0.1-ml injections for a total of 12 units of BOTOX within a 5-cm2 area of the dermal layer on the flexor surface of the proximal forearm using standard sterile techniques. Saline was injected in a similar manner on the contralateral forearm, which served as the control; the treatment arms were randomly assigned. Both injection sites were outlined with an indelible ink, as subjects did not begin the heat acclimation protocol until at least 2 wk following the BOTOX injections. The delay ensured that the BOTOX completely chemodenervated the sweat glands within the treated area. All subjects completed the study within 8 wk following the BOTOX injections, which was well within the length of time that the inhibitory effects of BOTOX are reported to persist (2, 7, 11).
After subjects were injected, they completed 10 days of exercise in an environmental chamber set at 35°C and 40% relative humidity. On the first day of heat acclimation, the subjects either cycled or walked at 50% of their age-adjusted predicted heart rate reserve for 90 min. Each day, thereafter, the subjects exercised at the same absolute work rate. Heart rate was measured continuously with a telemetry transmitter strapped around the chest (Polar Electro Oy, Kempele, Finland). Subjects were required to drink a minimum of 1 liter of water each session, and the volume was recorded. Dry nude weight was measured on a balance scale before and immediately after each exercise session to determine whole body sweat rate (WBSR) after correcting for water intake. Core temperature was monitored during exercise using a sterile rectal temperature probe (YSI Series 400, Yellow Springs Instruments, Yellow Springs, OH) inserted ∼10 cm past the anal sphincter. Forearm sweat samples from the injection sites were collected during exercise for 15 min (from minutes 60 to 75) on days 1, 3, 5, 7, and 10 using macroduct sweat collectors (Wescor, Logan, UT). The skin was first cleaned with alcohol and deionized water and then dried immediately prior to securing each collector with a Velcro strap that prevented leakage and sample contamination. Forearm sweat rate was calculated by the change in volume within the sample collection tubing during the 15-min collection period.
In addition, the pilocarpine-induced sweat rate in both injection sites was determined prior to exercise on days 1 and 10 using iontophoresis. With the subject seated in a temperate environment (22–23°C), a 1% pilocarpine solution with a 1.5 mA iontophoresis current was applied for 5 min. Immediately following iontophoresis, sweat was collected from both injections sites for 15 min to determine the cholinergic-stimulated sweat rate. The pilocarpine-induced sweat rate data were collected at the same time of day for each subject. The test-retest correlation and intrasubject coefficient of variability for the pilocarpine-induced sweat rate procedure has been reported to be 0.85 and 15%, respectively (6).
End-exercise rectal temperature and heart rate, forearm sweat rate during exercise, and the WBSR on days 1, 3, 5, 7, and 10 were analyzed using one-way repeated-measures ANOVAs. The resting pilocarpine-induced sweat rate in the control and BOTOX-treated arms, before and after heat acclimation, were analyzed using a 2 × 2 repeated-measures ANOVA. Significance was set at 0.05 and, if a significant difference were detected, Tukey's post hoc analyses were performed. All results are presented as mean ± SD.
RESULTS
The end-exercise heart rate and rectal temperature decreased over the 10-day protocol, indicating that the subjects successfully heat acclimated (Table 1). End-exercise heart rate on day 1 was 156 ± 22 beats/min and was 138 ± 17 beats/min on day 10 (P = 0.0001). Similarly, the end-exercise rectal temperature decreased from 38.2 ± 0.3°C to 37.9 ± 0.3°C on days 1 and 10, respectively (P = 0.0003). In addition, there was a 51% increase in the WBSR during the 10 days of heat acclimation (P = 0.03). On day 1, the WBSR was 0.70 ± 0.29 l/h, which increased to 1.06 ± 0.51 l/h on day 10. The magnitudes of these changes were consistent with previous heat acclimation studies (5, 14, 17, 19).
Table 1.
Adaptations to 10 days of heat acclimation (n = 8)
| Day 1 | Day 3 | Day 7 | Day 7 | Day 10 | |
|---|---|---|---|---|---|
| End-exercise heart rate, beats·per min | |||||
| Combined | 156±22 | 148±23 | 146±19* | 141±19* | 138±17* |
| Men | 153±38 | 148±38 | 143±34 | 138±32 | 133±30 |
| Range | 110–181 | 104–174 | 104–168 | 103–166 | 103–162 |
| Women | 157±9 | 148±15 | 147±6 | 143±10 | 142±8 |
| Range | 148–173 | 135–173 | 142–157 | 131–157 | 135–154 |
| End-exercise core temperature, °C | |||||
| Combined | 38.2±0.3 | 38.1±0.3 | 38.0±0.3* | 38.0±0.3* | 37.9±0.3* |
| Men | 38.5±0.1 | 38.3±0.2 | 38.2±0.2 | 38.0±0.3 | 38.0±0.3 |
| Range | 38.1–38.5 | 38.0–38.4 | 38.0–38.3 | 37.8–38.3 | 37.8–38.3 |
| Women | 38.1±0.2 | 38.0±0.3 | 37.9±0.3 | 37.9±0.3 | 37.9±0.3 |
| Range | 37.8–38.4 | 37.5–38.3 | 37.5–38.2 | 37.4–38.1 | 37.4–38.1 |
| End-exercise whole-body sweat rate. l·h−1 | |||||
| Combined | 0.70±0.29 | 0.82±0.48 | 0.91±0.34 | 1.00±0.58 | 1.06±0.51* |
| Men | 0.82±0.43 | 1.04±0.78 | 1.07±0.49 | 1.43±0.82 | 1.45±0.70 |
| Range | 0.48–1.31 | 0.48–1.93 | 0.64–1.61 | 0.49–1.99 | 0.79–2.18 |
| Women | 0.63±0.20 | 0.68±0.17 | 0.81±0.21 | 0.74±0.16 | 0.83±0.15 |
| range | 0.48–0.97 | 0.52–0.97 | 0.67–1.15 | 0.67–1.02 | 0.67–0.97 |
| End-exercise forearm sweat rate, mg·cm−2·min−1 | |||||
| Combined | 0.75±0.34 | 0.71±0.25 | 0.84±0.35 | 0.95±0.49 | 1.05±0.64* |
| Men | 0.78±0.45 | 0.79±0.24 | 1.01±0.41 | 1.26±0.69 | 0.95±0.50 |
| Range | 0.25–1.04 | 0.52–0.95 | 0.54–1.29 | 0.55–1.92 | 0.55–1.51 |
| Women | 0.73±0.31 | 0.67±0.27 | 0.74±0.32 | 0.77±0.27 | 1.11±0.76 |
| Range | 0.39–1.01 | 0.43–1.10 | 0.34–1.04 | 0.54–1.12 | 0.52–2.40 |
Values are expressed as means ± SD. Note: Number of male subjects = 3; number of female subjects = 5. The end-exercise forearm sweat rate reflects the control forearm; there was no measureable sweat production in the BOTOX-treated forearm. Statistical significance was determined only on the combined data.
Different from day 1 (P<0.05).
Likewise, there was a 40% increase (P = 0.040) in the forearm sweat rate during exercise in the heat by the control arm from 0.75 ± 0.34 mg·cm−2·min−1 on day 1 to 1.05 ± 0.64 mg·cm−2·min−1 on day 10 (Table 1). There was no measurable sweat production during exercise in the heat from the area previously injected with BOTOX, consistent with the complete inhibition of neural stimulation to the sweat glands.
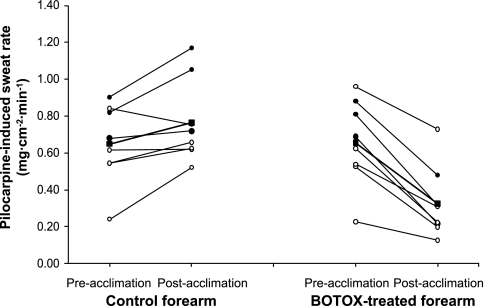
As seen in Fig. 1, before heat acclimation, the pilocarpine-induced sweat rate of the control and BOTOX-injected forearms did not differ (0.65 ± 0.20 vs. 0.66 ± 0.22 mg·cm−2·min−1). However, following heat acclimation, the pilocarpine-induced sweat rate in the control arm increased 18% to 0.77 ± 0.21 mg·cm−2·min−1 [CI95 (95th confidence interval) = 0.58–0.96; P = 0.021], but decreased 52% to 0.32 ± 0.18 mg·cm−2·min−1 (CI95 = 0.16–0.49; P < 0.001) in the BOTOX-treated arm.
Fig. 1.
The effect of heat acclimation on pilocarpine-induced sweat rate. The responses of male subjects (n = 3) are indicated by the solid circles with long-dashed lines; female subjects (n = 5) are indicated by the open circles and short-dashed lines; the combined mean response is indicated by the solid squares and solid line. There was an 18% increase of the sweat gland capacity in the control arm (P = 0.021) and a 52% decrease in the BOTOX-treated forearm (P < 0.001).
DISCUSSION
The current study used a novel research design of complete chemodenervation of the sweat glands, coupled with direct cholinergic stimulation via pilocarpine iontophoresis, to investigate whether sweat glands must be active during heat acclimation if they are to adapt and increase their capacity to sweat. Unlike previous studies that failed to totally block sweat production during heat acclimation, we were able to completely inhibit sweat gland activity. With this research design, we demonstrated that sweat glands not allowed to freely sweat failed to adapt during heat acclimation. During exercise, there was a 40% increase in sweat rate by the control forearm after heat acclimation, while the BOTOX injections prevented any measureable sweating in the treatment forearm. In addition, before and after heat acclimation, we used pilocarpine to induce sweating, so as to measure adaptations that occurred within the sweat glands, independent of any thermoregulatory effector input. In the control forearm, we observed an 18% increase in the pilocarpine-induced sweat rate, but a 52% reduction occurred in the BOTOX-treated forearm following heat acclimation (Fig. 1).
These data support and expand on the previous findings of Fox et al. (12) and Collins et al. (9, 10) that sweat glands must be active during heat acclimation if they are to increase their capacity to sweat. Fox et al. (12) investigated this issue by suppressing local sweating via cold-water immersion during whole body heat acclimation. On 14 consecutive days, they increased the core temperature of 12 subjects to 37.9°C for 2 h using vapor-barrier suits. Simultaneously, one arm was immersed in 13°C water to just above the elbow. In the forearm contained in the vapor-barrier suit, there was a 400% increase of the sweat rate following heat acclimation, but the sweat rate by the forearm immersed in cold water was unaffected. Although this procedure prevented any change of sweat rate in the arm immersed in cold water, exceptional local conditions were created that may not be applicable to actual conditions during natural heat acclimation.
Collins et al. (9, 10) tried to improve on the previous design by repeatedly raising the core and skin temperatures, while attempting to suppress sweating via indirect cooling. They immersed inactive subjects up to the waist in either 10°C or 37°C water, while the trunk was simultaneously heated with radiant heat lamps for 100 min on 10 consecutive days. While the upper body was exposed to heat, the cold-water immersion served to centrally inhibit sweating. Core temperature increased 1.2°C during both water temperature conditions. To determine whether the heat acclimation had any effect on sweat gland functioning, subjects performed 3 h of exercise at 46°C before and after the heat acclimation protocols. Following the 37°C water immersion protocol, during which the subjects sweated profusely, there was a 60% increase in the WBSR. However, there was no change in the WBSR following the 10°C water immersion protocol. This lack of change in the WBSR suggests that sweat glands must be active during heat acclimation for peripheral improvement in secretory capacity to occur. What their study was unable to determine, however, was whether the 60% increase in WBSR following immersion in 37°C water resulted from adaptations in the sweat gland or changes in the thermoregulatory effector inputs to the sweat gland.
However, the data of Chen and Elizondo (8) did not agree with the findings of Fox et al. (12) and Collins et al. (9, 10) and challenged their conclusions that sweat glands must be active if they are to adapt (8). They exercised 21 male subjects in the heat (49°C) for 90 min on 9 consecutive days. During the heat acclimation, ice water (∼2°C) was circulated around one elbow to inhibit efferent nerve conduction to the forearm, and thus forearm sweating. After heat acclimation, the forearm sweat rate increased ∼26% in both the control and nerve-blocked arms, which led them to conclude that “efferent sudomotor excitation does not participate in the modification of the sweating function” and that “sweating per se is not a necessary condition for function augmentation of the sweat gland following heat acclimation” (8). However, in the area distal to the nerve block, the sweat rate was 0.23 mg·cm−2·min−1 during the heat acclimation protocol (8); thus, the circulation of ice water around the elbow failed to completely block efferent stimulation of the forearm sweat glands. It is likely that this degree of sweating was sufficient to improve peripheral sweat gland functioning during acclimation and explains the 26% increase of sweat rate in the ice-water nerve-blocked forearm following heat acclimation.
Our observation that complete suppression of sweating by chemodenervation is accompanied by a decrease in the pilocarpine-induced sweat rate is supported by others who examined the effects of other forms of sweat gland denervation. For example, 5 days following sciatic nerve crush in mice, sweat glands in the plantar surface of the hindpaws became unresponsive to cholinergic stimulation with pilocarpine (26). Furthemore, Yaggie et al. (27) reported that the pilocarpine-induced sweat rate of the forearm of healthy subjects was 0.76 mg·cm−2·min−1 but was only 0.11 mg·cm−2·min−1 in subjects with tetraplegia.
There is the possibility that BOTOX damaged the sweat glands and was thereby responsible for reduction of the pilocarpine-induced sweat rate after heat acclimation. However, this is unlikely for two reasons. First, it has been shown from skin biopsies that intradermal injections of BOTOX do not significantly affect sweat gland morphology or innervation (25). Second, BOTOX was administered to all of our subjects between 2 and 6 wk before their preacclimation pilocarpine-induced sweat rate data were collected. It seems reasonable to assume that if BOTOX had damaged the sweat glands and inhibited their ability to respond to cholinergic agonists, then the preacclimation pilocarpine-induced sweat rate would have been lower in the BOTOX-treated forearm than in the control forearm. However, the preacclimation pilocarpine-induced sweat rates in both forearms were essentially identical (0.65 ± 0.20 mg·cm−2·min−1 vs. 0.66 ± 0.22 mg·cm−2·min−1), which strongly suggests that the BOTOX injections caused no damage to the treated sweat glands.
In conclusion, the current study used a novel research design of complete chemodenervation of the sweat glands, coupled with direct cholinergic stimulation via pilocarpine iontophoresis. Our findings clearly demonstrated that sweat glands must be active during heat acclimation if they are to adapt and increase their capacity to sweat.
ACKNOWLEDGMENTS
The authors gratefully acknowledge support from the National Institute of General Medical Sciences through the California Native American Research Center for Health Grant 3S06GM64105-01S1.
REFERENCES
- 1.Armstrong CG, Kenney WL. Effects of age and acclimation on responses to passive heat exposure. J Appl Physiol 75: 2162–2167, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Borodic GE, Ferrante R. Effects of repeated botulinum toxin injections on the orbicularis oculi muscle. J Clin Neuroophthalmol 55: 121–127, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Buono MJ, Ball KD, Kolkhorst FW. Sodium ion concentration vs. sweat rate relationship in humans. J Appl Physiol 103: 990–994, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Buono MJ, Heaney JH, Canine KM. Acclimation to humid heat lowers resting core temperature. Am J Physiol Regul Integr Comp Physiol 274: R1295–R1299, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Buono MJ, Martha SL, Heaney JH. Peripheral sweat gland function is improved with humid heat acclimation. J Therm Bio 34: 127–130, 2009 [Google Scholar]
- 6.Buono MJ, White C, Connolly KP. Pilocarpine-induced sweat rate at rest versus whole-body sweat rate during exercise. J Appl Sport Sci Res 5: 82–86, 1991 [Google Scholar]
- 7.Bushara KO, Park DM, Jones JC, Schutta HS. Botulinum-toxin a possible new treatment for axillary hyperhidrosis. Clin Exp Dermatol 21: 276–278, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Chen WY, Elizondo RS. Peripheral modification of thermoregulatory function during heat acclimation. J Appl Physiol 65: 811–814, 1974 [DOI] [PubMed] [Google Scholar]
- 9.Collins KJ, Crockford GW, Weiner JS. The local training effect of secretory activity on the response of eccrine sweat glands. J Physiol 184: 203–214, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins KJ, Crockford GW, Weiner JS. The role of glandular activity in the increased sweat response of heat acclimatization. J Physiol London 169: 12P–13P, 1963 [Google Scholar]
- 11.Davarian S, Kalantari KK, Rezasoltani A, Rahimi A. Effect and persistency of botulinum toxin iontophoresis in the treatment of palmar hyperhydrosis. Australas J Dermatol 49: 75–79, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fox RH, Goldsmith R, Hampton IFG, Lewis HE. The nature of the increase in sweating capacity produced by heat acclimatization. J Physiol 171: 368–376, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garden JW, Wilson D, Rasch PJ. Acclimation of healthy young adult males to a hot-wet environment. J Appl Physiol 21: 665–669, 1966 [DOI] [PubMed] [Google Scholar]
- 14.Houmard JA, Costill DL, Davis JA, Mitchel JB, Pascoe DD, Robergs RA. The influence of exercise intensity on heat acclimation in trained subjects. Med Sci Sports Exerc 22: 615–620, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Havenith G, Kenney LW, Loomis JL, Buskirk ER. Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. Int J Biometeorol 42: 210–216, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Kreyden OP, Scheidegger EP. Anatomy of the sweat glands, pharmacology of botulinum toxin, and distinctive syndromes associated with hyperhydrosis. Clin Dermatol 22: 40–44, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Mitchell D, Senay LC, Wyndham CH, Van Rensburg AJ, Rogers GG, Strydom NB. Acclimation in a hot, humid environment: energy exchange, body temperature, and sweating. J Appl Physiol 40: 768–778, 1976 [DOI] [PubMed] [Google Scholar]
- 18.Nadel ER, Pandolf KB, Roberts MR, Stolwijk JAJ. Mechanism of thermal acclimation to exercise and heat. J Appl Physiol 37: 515–520, 1974 [DOI] [PubMed] [Google Scholar]
- 19.Nielsen B, Strange S, Christensen N, Warberg J, Saltin B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflüger Arch 434: 49–56, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Pandolf KB, Cadarette BS, Sawka MN, Young AJ, Francesconi RP, Gonzalez RR. Thermoregulatory responses of middle-aged and young men during dry-heat acclimation. J Appl Physiol 65: 65–71, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Patterson MJ, Stocks JM, Taylor NAS. Humid heat acclimation does not elicit a preferential sweat redistribution toward the limbs. Am J Physiol Regul Integr Comp Physiol 286: R512–R518, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Sargent F, Weinman KP. Eccrine sweat gland activity during the menstrual cycle. J Appl Physiol 21: 1685–1687, 1966 [DOI] [PubMed] [Google Scholar]
- 23.Sato F, Owen E, Matthes R, Sato K, Gisolfi CV. Functional and morphological changes in the eccrine sweat gland with heat acclimation. J Appl Physiol 69: 232–236, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Strydom NB, Wyndham CH, Williams CG, Morrison GA, Benade AJS, Von Rahden M. Acclimation to humid heat and the role of physical conditioning. J Appl Physiol 21: 636–642, 1966 [DOI] [PubMed] [Google Scholar]
- 25.Swartling C, Naver H, Pihl-Lundin I, Hagforsen E, Vahlquist A. Sweat gland morphology and periglandular innervation in essential palmar hyperhydrosis before and after treatment with intradermal botulinum toxin. J Am Acad Dermatol 51: 739–745, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Vilches JJ, Rodriguez FJ, Verdú E, Valero A, Navarro X. Changes in cholinergic responses of sweat glands during denervation and reinnervation. J Auton Nerv Syst 74: 134–142, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Yaggie JA, Niemi TJ, Buono MJ. Adaptive sweat gland response after spinal cord injury. Arch Phys Med Rehabil 83: 802–805, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki F, Hamasaki K. Heat acclimation increases skin vasodilation and sweating but not cardiac baroreflex responses in heat-stressed humans. J Appl Physiol 95: 1567–1574, 2003 [DOI] [PubMed] [Google Scholar]