Abstract
Mast cells have been shown to play a role in development and persistence of various inflammatory bladder disorders. Mast cell-derived tryptase specifically activates protease-activated receptor-2 (PAR-2), and PAR-2 is known to be involved in inflammation. We investigated whether mast cells participate in increase of cyclooxygenase-2 (COX-2) protein abundance in urothelium/suburothelium of bladders of mice subsequent to cyclophosphamide (CYP)-induced bladder inflammation. We also used primary cultures of human urothelial cells to investigate cellular mechanisms underlying activation of PAR-2 resulting in increased COX-2 expression. We found that treatment of mice with CYP (150 mg/kg ip) increased COX-2 protein abundance in bladder urothelium/suburothelium 3, 6, and 24 h after CYP (P < 0.01), and increased COX-2 protein abundance was prevented by treatment of mice with the mast cell stabilizer sodium cromolyn (10 mg/kg ip) for 4 consecutive days before CYP treatment. Incubation of freshly isolated mouse urothelium/suburothelium with a selective PAR-2 agonist, 2-furoyl-LIGRLO-amide (3 μM), also increased COX-2 protein abundance (P < 0.05). We further demonstrated that 2-furoyl-LIGRLO-amide (3 μM) increased COX-2 mRNA expression and protein abundance in primary cultures of human urothelial cells (P < 0.01), and the effects of PAR-2 activation were mediated primarily by the ERK1/2 MAP kinase pathway. These data indicate that there are functional interactions among mast cells, PAR-2 activation, and increased expression of COX-2 in bladder inflammation.
Keywords: urothelium, cyclophosphamide, mouse, inflammation, bladder
painful bladder syndrome/interstitial cystitis (PBS/IC) is a chronic inflammatory disorder characterized by frequency, urgency, and bladder pain (1, 19). Although the etiology and pathogenesis of PBS/IC remain unknown, substantial evidence indicates that mast cells play a significant role in development and persistence of PBS/IC in at least some patients (20, 33, 49). Mast cells are multifunctional immune cells that contain a variety of inflammatory mediators (7), and mast cell numbers have been reported to be increased in bladder biopsies from PBS/IC patients (32, 45, 56, 57). There is also a significant increase in urinary concentrations of tryptase (a marker of mast cell activation) in PBS/IC patients (5, 43, 57).
Protease-activated receptors (PARs) are a family of four G protein-coupled receptors (34, 51, 54). PARs are activated by cleavage of their extracellular NH2-terminal domain by proteolytic enzymes, and the exposed NH2-terminal sequence acts as a tethered ligand that binds and activates the receptors, initiating signaling cascades (34, 51, 54). Mast cell tryptase specifically activates PAR-2 (7, 21, 39, 44), and activation of PAR-2 induces release of proinflammatory neuropeptides, such as calcitonin gene-related peptide and substance P, from afferent neurons (52). A recent study demonstrated that activation of PAR-2 induced colonic inflammation in mice (8). PAR-2 has been shown to be present in bladders of various species (16, 17, 39), and inflammation increased expression of PAR-2 in rat bladders (17).
Several studies have shown that PAR-2 functionally interacts with cyclooxygenase-2 (COX-2) (13, 26). PAR-2-mediated relaxation of mouse tracheal was prevented by treatment by selective COX-2 inhibitors (26). Trypsin induces itching in mice by activating PAR-2, and this effect was selectively inhibited by the COX-2 inhibitor celecoxib (13). COX-2 is a key enzyme in conversion of arachidonic acid to proinflammatory prostanoids, such as prostaglandin E2 (PGE2) (38), and PGE2 plays an important role in hyperreactivity and pain associated with bladder inflammation (35).
Chemical cystitis induced by intraperitoneal injection of cyclophosphamide (CYP) in rodents is one of the most commonly used experimental models of bladder inflammation (4, 14). CYP treatment of rats increases expression of PAR-2 (17) and COX-2, as well as production of PGE2, in the bladder (23). CYP-induced COX-2 expression in bladder appears to occur primarily in the urothelium (11, 28). In the present study, we examined the functional interactions among mast cells, PAR-2, and increased COX-2 expression in the bladders of mice treated with CYP. We also used primary cultures of human urothelial cells to investigate cellular mechanisms underlying activation of PAR-2 resulting in increased COX-2 expression.
MATERIALS AND METHODS
Animals.
C57BL/6J male mice were used at 3–6 mo of age. Experiments were conducted in accordance with National Institutes of Health guidelines, and all protocols were reviewed and approved by the Animal Care and Use Committee of the University of Wisconsin.
Induction of cystitis.
CYP (150 mg/kg) was injected intraperitoneally (ip), and this dose of CYP induced cystitis of moderate severity (61). Control mice received an equivalent volume of sterile saline (0.9%, ip) instead of CYP. At the termination of experiments, mice were deeply anesthetized with pentobarbital sodium (50 mg/kg ip) and perfused through a cannula inserted into the left ventricle with 0.9% saline. Bladders were removed, and urothelium/suburothelium was mechanically separated from detrusor using fine forceps as described previously (28). Tissues were stored at −80°C until analyzed.
Treatment with sodium cromolyn.
Sodium cromolyn stabilizes mast cell granules, thereby preventing release of chemical mediators from mast cells (53). Mice were treated with sodium cromolyn (10 mg/kg ip, given at 9:00 AM) for 4 consecutive days (13, 42, 48). Two hours after the last injection of sodium cromolyn, mice were treated with CYP or saline as described previously.
Culture of freshly isolated urothelium/suburothelium.
Normal, untreated mice were deeply anesthetized with pentobarbital sodium (50 mg/kg ip), and bladders were removed and cut into two halves longitudinally. The urothelium/suburothelium was mechanically separated from detrusor using fine forceps as described previously (28). The urothelium/suburothelium preparations were incubated in phenol red-free Ham's F12 medium (Invitrogen, Carlsbad, CA) supplemented with 2% fetal bovine serum at 37°C in a CO2 incubator. One half of urothelium/suburothelium from each bladder was used as control (vehicle treated), and the another half was treated with the selective PAR-2 agonist 2-furoyl-LIGRLO-amide (37). Before treatment, the medium was changed every 30 min for 2 h. Preparations were then treated with either vehicle (phosphate-buffered saline, PBS) or 2-furoyl-LIGRLO-amide (3 μM) for 3 h. Tissues were rinsed briefly in PBS and stored at −80°C until analyzed.
Isolation of protein from urothelium/suburothelium.
Tissues were homogenized with T-PER tissue protein extraction reagent (Thermo Scientific, Rockford, IL) containing protease inhibitors (Roche, Indianapolis, IN). Supernatants were collected by centrifugation at 10,000 g for 15 min at 4°C. Protein concentrations were determined using the BCA protein assay kit (Thermo Scientific). Protein samples were mixed 1:1 with Laemmli sample buffer (Bio-Rad, Hercules, CA), placed in boiling water for 5 min, and stored at −20°C until analyzed.
Culture of human urothelial cells.
Acquisition and use of primary human urothelial cells was reviewed and approved by the University of Wisconsin Institutional Review Board. Primary urothelial cells were derived from a bladder specimen of a patient undergoing cystoplasty in the absence of cancer, active infection, or other bladder disorders (55). Phenol red-free Ham's F12 medium (Invitrogen) supplemented with 0.1 μg/ml hydrocortisone, 5 μg/ml transferrin, 10 μg/ml insulin, 0.1 mM nonessential amino acid solution containing each amino acid at 0.1 mM, 2.7 mg/ml dextrose, 2.0 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2% fetal bovine serum was used. The cells were used between three and six passages (55). Urothelial cells were treated with the selective PAR-2 agonist 2-furoyl-LIGRLO-amide (3 μM) or PBS for various periods of time to investigate the effects of PAR-2 activation on expression of PAR-2 and COX-2. In additional experiments, cells were treated with U0126 (10 μM), a specific inhibitor of phosphorylation of extracellular signal-regulated kinase (ERK1/2) of the mitogen-activated protein kinases (MAPK) family to investigate the role of ERK1/2 MAP kinase pathway activation in PAR-2 activation-induced expression of COX-2.
For mRNA analysis, cells were plated in 12-well plates (∼50,000 cells/well). After treatment, medium was removed and cells were washed with PBS. Total RNA was extracted with Trizol reagent (Invitrogen) and treated with DNase I (Invitrogen) to remove genomic DNA. First-strand cDNA was generated using a cDNA synthesis kit according to the manufacturer's instructions (Invitrogen). For immunoblotting, cells were plated in six-well plates (∼100,000 cells/well). After treatments, medium was removed and cells were washed with PBS. M-PER mammalian protein extraction reagent (Thermo Scientific) was added, and cell lysates were collected. Supernatant were collected by centrifugation at 10,000 g for 15 min at 4°C. Protein concentrations were determined using the BCA protein assay kit (Thermo Scientific). The protein samples were mixed 1:1 with Laemmli sample buffer (Bio-Rad), placed in boiling water for 5 min, and stored at −20°C until analyzed.
Immunoblotting analysis.
Protein samples (20 μg/lane) were resolved on 10% SDS-polyacrylamide gels and transferred to Immobilon-P polyvinylidene difluoride membranes (Fisher Scientific, Itasca, IL). Membranes were blocked in 5% dry fat-free milk in 1× TBST (20 mM Tris·HCl, 137 mM NaCl, 0.05% Tween 20; pH 7.5). After being rinsed, membranes were incubated at 4°C overnight with the specific primary antibody. Membranes were then washed free of primary antibody and incubated for 1 h with appropriate secondary antibody conjugated to horseradish peroxidase at room temperature. Signals were revealed using a chemiluminescent detection reagent (Amersham, Arlington Heights, IL). Membranes were apposed to X-ray films, and films were developed. Membranes were then stripped and reblotted with a mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody as a loading control. Images were scanned and quantified using NIH Image 1.62. The primary antibodies used for immunoblotting were polyclonal rabbit anti-murine COX-2 (1:1,000) and monoclonal anti-human COX-2 (1:2,000) antibodies (Cayman Chemical, Ann Arbor, MI); monoclonal anti-GAPDH (1:5,000) antibody (Abcam, Cambridge, MA); polyclonal goat anti-PAR-2 antibody (1:500, reacts with both human and murine PAR-2); polyclonal rabbit anti-phospho-ERK1/2 (1:1,000), phospho-p38 (1:1,000), and pan-ERK1/2 (1:1,000) antibodies (Cell Signaling, Danvers, MA). The secondary antibodies were goat anti-rabbit IgG, goat anti-mouse IgG, and bovine anti-goat IgG (all conjugated to horseradish peroxidase and used at 1:20,000 dilution) (Santa Cruz, Biotechnology, Santa Cruz, CA).
Semiquantitative analysis of COX-2 mRNA in urothelial cells.
Real-time PCR was performed using an ABI 7300 thermocycler (Applied Biosystems, Foster City, CA). Samples were amplified in duplicate using the following thermal cycling conditions: 94°C for 10 min, followed by 45 cycles of amplification at 94°C for 30 s and then 60°C for 1 min to allow denaturing and annealing-extension. Abundance of PCR product was determined semiquantitatively using a standard curve for each gene. Expression of COX-2 was normalized to abundance of mRNA for S26, a constitutively expressed ribosomal protein in the same sample (55). Primer sequences are COX-2, forward 5′-CGATTGTACCCGGACAGGAT-3′, reverse 5′-AATTCCGGTGTTGAGCAGTTTT-3′; and S26, forward 5′-AGTCAGGAATCGATCTCGTGAAG-3′, reverse 5′-CAGCACCCGCAGGTCTAAAT-3′.
Histology and immunohistochemistry.
The urothelium/suburothelium preparations from two mice were fixed with 4% paraformaldehyde overnight at 4°C. They were cytoprotected with 30% sucrose in PBS and sectioned at 10 μm with a cryostat. For histology, sections were stained with hematoxylin and eosin. For immunohistochemistry, the sections were blocked with 10% normal goat serum for 1 h and then a polyclonal anti-uroplakin antibody (generously provided by Dr. Tung-Tien Sun, New York University School of Medicine; used at 1:1,500, diluted in PBS containing 0.1% BSA, 0.3% Triton-X 100) (63) was applied. Slides were kept in a humid chamber overnight at 4°C, and staining was revealed using secondary goat anti-rabbit IgG conjugated with FITC (1:1,000; Sigma, St. Louis, MO). Slides were rinsed and coverslipped with an anti-fading solution (Vector Laboratories, Burlingame, CA).
Human urothelial cells were also stained for keratin to confirm their urothelial origin (6, 50). Cells were grown in Lab-TeK slide chambers (NUNC, Rochester, NY) for 24 h and were rinsed with PBS and fixed in cold acetone. They were rinsed and blocked with 10% normal goat serum. The monoclonal anti-AE1/AE3 keratin antibody (1:100, diluted in PBS containing 0.1% BSA, 0.3% Triton-X100; Chemicon, Temecula, CA) was applied, and slides were kept in a humid chamber overnight at 4°C. Staining was revealed using secondary goat anti-mouse IgG conjugated with FITC (1:1,000; Sigma). Slides were rinsed and coverslipped with an anti-fading solution (Vector Laboratories). Slides were examined with a Nikon E600 microscope, and digital images were captured. Negative staining controls were prepared using normal mouse IgG instead of the specific antibody.
Reagents.
Sodium cromolyn (dissolved with 0.9% saline) was purchased from Sigma. U0126 (dissolved with DMSO at 10 mM as the stock solution and diluted in PBS to desired concentration) and the selective PAR-2 agonist 2-furoyl-LIGRLO-amide (dissolved in PBS) (37) were obtained from EMD Chemicals (Gibbstown, NJ).
Statistical analysis.
Data are arithmetic means ± SE. Data were analyzed using one-way ANOVA followed by Tukey's post hoc multiple comparison test (GraphPad Prism, San Diego, CA). Student's t-tests were also used when only two means were compared. A P value <0.05 was considered indicative of significant differences.
RESULTS
COX-2 protein abundance was increased in urothelium/suburothelium after induction of cystitis.
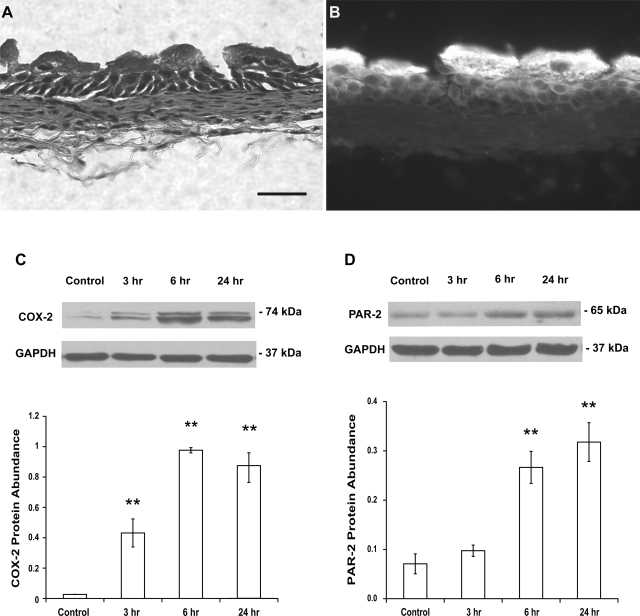
Histological appearance of urothelium/suburothelium revealed that contamination of detrusor was very limited if there was any (Fig. 1A). Superficial urothelial cells were also stained strongly with a specific uroplakin antibody (Fig. 1B).
Fig. 1.
A: histological appearance (hematoxylin and eosin staining) of urothelium/suburothelium reveals that the contamination of detrusor was very limited. Scale bar, 50 μm. B: strong staining with a specific uroplakin antibody is shown in superficial urothelial cells. C: treatment of mice with cyclophosphamide (CYP; 150 mg/kg ip) for 3, 6, and 24 h induced an increase in cyclooxygenase-2 (COX-2) protein abundance in the bladder urothelium/suburothelium, respectively. D: similarly, treatment of mice with CYP induced an increase (6 and 24 h) in protease-activated receptor-2 (PAR-2) protein abundance in the bladder urothelium/suburothelium. Control mice received saline (ip). Relative COX-2 or PAR-2 protein abundance was analyzed by immunoblotting. Values determined by densitometry were normalized to those of GAPDH (loading control) in each sample. Data are means ± SE; n = 4–5. **P < 0.01 vs. control.
COX-2 protein varies between 70 and 74 kDa on denaturing acrylamide gel, probably related to differences in glycosylated forms (28). Abundance of COX-2 protein in urothelium/suburothelium from normal control mice was relatively low, and this was consistent with previous reports (11, 28). As shown in Fig. 1C, the abundance of COX-2 protein in the urothelium/suburothelium was significantly increased 3 (16-fold), 6 (36-fold), and 24 h (32-fold) after treatment with CYP (150 mg/kg; n = 5, P < 0.01 vs. control at all time points examined), and increased COX-2 protein appeared to reach a maximum 6 h after CYP treatment.
PAR-2 protein is ∼65 kDa on denaturing acrylamide gel (36). PAR-2 was present in urothelium/suburothelium from normal, control mice. As shown in Fig. 1D, abundance of PAR-2 protein remained unchanged 3 h (P > 0.05 vs. control), but was significantly increased 6 (4-fold, P < 0.01 vs. control) and 24 h (5-fold, P < 0.01 vs. control), after CYP treatment. These results demonstrate that bladder inflammation increased abundance of COX-2 and PAR-2 protein in the urothelium/suburothelium.
Treatment with sodium cromolyn inhibited increased COX-2 protein abundance.
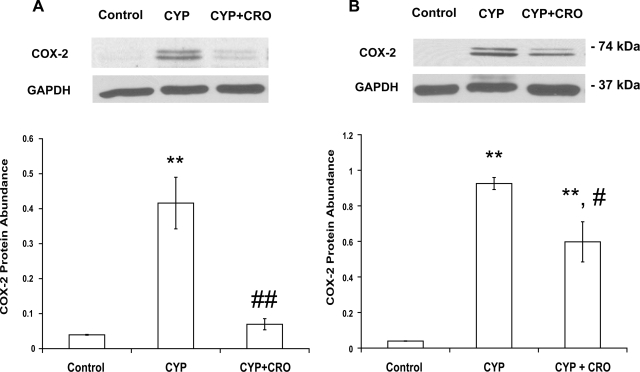
Mice were treated with sodium cromolyn (10 mg/kg) for 4 days to stabilize mast cells. Since the increase of COX-2 protein abundance reached a maximum 6 h after treatment with CYP, we examined COX-2 protein abundance 3 and 6 h after CYP treatment. As shown in Fig. 2, A and B, treatment with sodium cromolyn inhibited increased COX-2 abundance by 83 (n = 5, P < 0.01 vs. CYP-treated group) and 35% (n = 5, P < 0.05 vs. CYP-treated group) 3 and 6 h after CYP treatment, respectively. These results indicate that mast cells are involved in increased COX-2 protein abundance in response to CYP-induced bladder inflammation.
Fig. 2.
Treatment of mice with the mast cell stabilizer sodium cromolyn (CRO; 10 mg/kg) for 4 consecutive days inhibited CYP-induced increase in COX-2 protein abundance in bladder urothelium/suburothelium. Relative COX-2 protein abundance was analyzed 3 (A) and 6 h (B) after treatment with CYP. Values determined by densitometry were normalized to those of GAPDH (loading control) in each sample. Data are means ± SE; n = 4. **P < 0.01 vs. control. #P < 0.05; ##P < 0.01 vs. CYP-treated group.
Treatment with PAR-2 agonist increased COX-2 protein abundance in isolated urothelium/suburothelium.
Freshly isolated mouse urothelium/suburothelium was treated with the PAR-2 agonist 2-furoyl-LIGRLO-amide (3 μM) for 3 h. The relative COX-2 protein abundance was 0.1 ± 0.02 in the control group and 0.72 ± 0.19 in the PAR-2 agonist-treated group. 2-Furoyl-LIGRLO-amide significantly increased COX-2 protein abundance (7-fold; n = 6, P ≤ 0.01). These data indicate that PAR-2 is involved in induction of COX-2 protein.
Activation of PAR-2 increased COX-2 expression, and this effect was mediated by the ERK1/2 kinase pathway in cultured primary human urothelial cells.
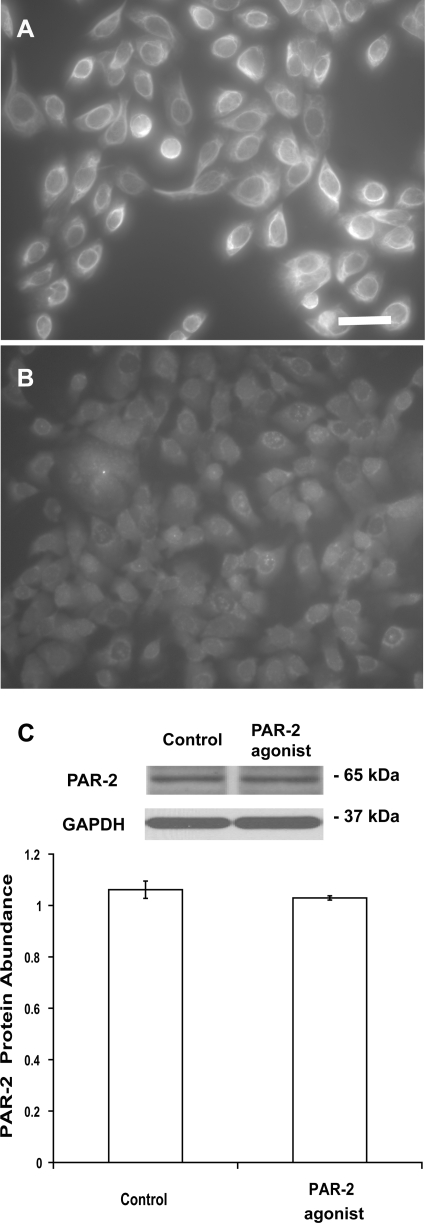
As shown in Fig. 3A, urothelial cells were stained positively with the antibody against AE1/AE3 keratin, indicating that these cells retain their epithelial characteristics in culture (6, 50). The use of normal mouse IgG instead of primary keratin antibody abolished specific staining (Fig. 3B). PAR-2 was present in urothelial cells (Fig. 3C), and PAR-2 protein abundance was unaffected by treatment with the selective PAR-2 agonist 2-furoyl- LIGRLO-amide (3 μM) for 6 h (n = 4, P > 0.05) (Fig. 3C).
Fig. 3.
A: representative images of cultured bladder urothelial cells stained with a specific monoclonal antibody against AE1/AE3 keratin. Scale bar, 50 μm. B: a normal mouse IgG was used instead of the specific antibody for immunocytochemistry as negative control. C: relative abundance of PAR-2 protein in urothelial cells was analyzed by immunoblotting. Treatment with a selective PAR-2 agonist for 6 h did not alter PAR-2 protein abundance (P > 0.05, n = 4). Values determined by densitometry were normalized to those of GAPDH (loading control) in each sample.
2-Furoyl-LIGRLO-amide (3 μM) significantly increased COX-2 mRNA expression after 30 min and 1 and 2 h of treatment (n = 6, P < 0.01 vs. control; Fig. 4A). Three and six hours after treatment with 2-furoyl-LIGRLO-amide, COX-2 mRNA expression was not different from that observed in controls (n = 6, P > 0.05 vs. control) (Fig. 4A).
Fig. 4.
A: treatment of primary cultures of human urothelial cells with a selective PAR-2 agonist (3 μM) altered expression of COX-2 mRNA with significant increase at 30 min and at 1 and 2 h. Data are means ± SE; n = 6. **P < 0.01 vs. control. B: PAR-2 agonist (3 μM) induced phosphorylation of ERK1/2, which was abolished by U0126 (10 μM), a selective ERK1/2 inhibitor. A representative example of the results of 3 independently performed experiments is shown. C: treatment with U0126 (10 μM) prevented PAR-2 agonist-induced increase in expression of COX-2 mRNA at 1 h. Data are means ± SE; n = 6. **P < 0.01 vs. control. ##P < 0.01 vs. PAR-2 agonist-treated group. D: treatment of PAR-2 agonist (3 μM) for 6 h also increased COX-2 protein abundance, and this effect of PAR-2 was prevented by U0126 (10 μM). Data are means ± SE; n = 4. **P < 0.01 vs. control. #P < 0.05 vs. PAR-2 agonist-treated group.
2-Furoyl-LIGRLO-amide (3 μM) also induced phosphorylation of ERK1/2 in a time-dependent manner (5–60 min), and this effect was abolished by pretreatment with U0126 (10 μM), an inhibitor of ERK1/2 phosphorylation, applied 15 min before 2-furoyl-LIGRLO-amide (Fig. 4B). Treatment with U0126 (10 μM) before exposure to 2-furoyl-LIGRLO-amide (3 μM) abolished increased COX-2 mRNA expression 30 min after treatment with 2-furoyl-LIGRLO-amide (n = 6, P < 0.01 vs. PAR-2 agonist-treated group) (Fig. 4C).
Similarly, treatment with 2-furoyl-LIGRLO-amide (3 μM) for 6 h increased COX-2 protein abundance by 61% (n = 6, P < 0.01 vs. control) (Fig. 4D). U0126 also inhibited PAR-2 agonist-induced increased COX-2 protein by 70% (n = 6, P < 0.05 vs. PAR-2 agonist-treated group) (Fig. 4D). These results indicate that activation of PAR-2 increased COX-2 mRNA expression and protein abundance, and this effect of PAR-2 activation was mediated by the ERK1/2 MAP kinase pathway. Treatment of cells with the PAR-2 agonist failed to induce phosphorylation of p38 MAP kinase (data not shown), and p38 MAP kinase therefore does not appear to play a role in this process.
DISCUSSION
In the present study, we found that 1) treatment of mice with CYP increased COX-2 protein abundance in bladder urothelium/suburothelium; 2) mast cells were involved in inflammation-induced increase of COX-2 protein abundance; and 3) activation of PAR-2 increased COX-2 protein abundance in mouse urothelium/suburothelium. 4) Similarly, activation of PAR-2 increased COX-2 expression in cultured primary human urothelial cells; and 5) the effect of PAR-2 activation on COX-2 expression in cultured urothelial cells was mediated by the ERK1/2 MAP kinase pathway.
Although urothelium has historically been viewed as a simple barrier separating the bladder wall from urine, increasing evidence also suggests that the urothelium plays a critical role in physiological and pathophysiological processes in the bladder (2, 59). It has been demonstrated that urothelial cells have the capacity to secrete a variety of signaling molecules such as PGE2, nerve growth factor, nitric oxide, and cytokines (59). These cells also express many signaling molecules similar to those in sensory afferent neurons, including purinergic receptors, vanilloid channels, and tyrosine kinase receptors (2, 59). Afferent nerve fibers within the bladder wall are located in close proximity to urothelial cells, suggesting that urothelial cells could be a target of neurotransmitters released from afferent nerves. Conversely, chemical mediators derived from urothelial cells could significantly influence the function of afferent nerve fibers (2, 59). Thus interaction between urothelial cells and afferent nerves may play a crucial role in the physiology and pathology of normal and pathological bladder function.
CYP is an antineoplastic alkylating agent commonly used to treat cancer patients, and an undesirable side effect of CYP is hemorrhagic cystitis (4, 14, 29, 61). CYP is metabolized by the liver to acrolein, and accumulated acrolein in urine causes cystitis (4, 14). CYP-induced cystitis has been used by many investigators to study mechanisms underlying bladder inflammation and associated visceral pain (4, 23, 29, 58, 61). COX-2 is an inducible enzyme that is upregulated during inflammation (11, 23, 38, 62); several studies have reported increased expression of COX-2 in rat bladders after treatment with CYP, and increased COX-2 protein appears to be primarily localized in the urothelium (11, 28). Furthermore, increased COX-2 protein in the bladder is accompanied by enhanced production of prostaglandins, particularly PGE2 (23), and PGE2 has been shown to cause bladder hyperreactivity by sensitizing afferent nerves (24, 36). In fact, increased bladder contractions induced by CYP, surgery, or lipopolysaccharide were reversed by a selective COX-2 inhibitor in rats (23, 30). These studies clearly demonstrated that COX-2 expression and subsequent increased PGE2 release play a role in altered bladder functions in response to inflammation. Our study provides further evidence that treatment of mice with CYP increases COX-2 protein abundance in the urothelium/suburothelium, and a CYP-induced increase of COX-2 protein abundance is at least partly mediated by mast cells.
Mast cells are multifunctional immune cells that develop from a specific bone marrow progenitor and migrate into tissue perivascular spaces (25). Previous studies have shown an increase in mast cell numbers and activation in bladders of PBS/IC patients (56). Interestingly, mast cells isolated from bladders of PBS/IC patients are more responsive to various stimuli and release increased amounts of histamine (20). Histamine and tryptase levels are increased in the urine of PBS/IC patients (49). Together, these studies suggest that mast cells play an important role in the pathogenesis of PBS/IC (32, 33, 57). Mast cells contain granule-stored, presynthesized proinflammatory molecules and rapidly secrete mediators upon allergen exposure (22, 25). Mast cells are generally activated by specific antigens through cross-linking of IgE and high-affinity surface receptors (22, 25). However, mast cells also can be activated by nonimmunologic stimuli such as bacteria, chemicals, kinins, and neuropeptides (49). Mast cells have been observed in mouse bladder submucosa and detrusor with the use of toluidine blue staining (10, 15, 31), and they are involved in mouse bladder inflammation induced by various stimuli (3, 47). In the present study, we found that treatment with sodium cromolyn (which stabilizes mast cells) before exposure to CYP achieved greater inhibition of increased COX-2 protein at 3 h than at 6 h after induction of cystitis, suggesting that mast cell-derived mediators, such as tryptase, play a particularly important role in the acute phase of bladder inflammation. Conceivably, inflammatory mediators derived from infiltrating leukocytes and/or other cellular components also may contribute to enhanced COX-2 expression as inflammation persists.
Among the four PAR subtypes identified to date, PAR-2 is highly expressed in afferent neurons, making PAR-2 a promising therapeutic target for treatment of inflammatory disorders and pain (12, 52, 54). Whereas other PARs are primarily activated by thrombin, PAR-2 is specifically activated by mast cell tryptase (51). Nakahara et al. (40) reported that tryptase and selective PAR-2 agonist induced contractions of isolated rat bladder strips, and the contractions were prevented by removal of the urothelium. Furthermore, the contractions were inhibited by indomethacin, a nonselective COX inhibitor (40). In another study by the same group, activation of PAR-2-induced detrusor contractions and release of PGE2 from urothelium were enhanced in rats treated with CYP (41). These results strongly suggest that 1) activation of PAR-2 increases release of prostaglandins from urothelium; 2) released prostaglandins cause detrusor contractions; and 3) PAR-2 mediated contractions are augmented during bladder inflammation. Our observations that PAR-2 was present in the urothelium and that treatment with CYP increased PAR-2 and COX-2 protein abundance are consistent with these findings. We further demonstrated that activation of PAR-2 increased COX-2 protein abundance in urothelium. In addition, activation of PAR-2 increased COX-2 mRNA expression and protein abundance in cultured human urothelial cells. Therefore, our results reveal that there are functional interactions between PAR-2 activation and COX-2 expression.
The MAPK are a group of protein serine/threonine kinases that mediate signal transduction from the cell surface to the nucleus in response to a variety of extracellular stimuli (9). The MAPK consist of three major families of protein kinases, including p38, ERK and c-Jun NH2-terminal kinase (9, 18), and activity of MAPK depends on their phosphorylation status (9, 18). Interestingly, treatment of rats with CYP induced phosphorylation of ERK1/2 in bladder (46), suggesting ERK1/2 kinase is involved in regulating bladder function. In human lung-derived A549 epithelial cells, activation of PAR-2 increased expression of COX-2 and enhanced release of PGE2 (27), and these effects were inhibited by both selective ERK1/2 and p38 inhibitors (27). Similarly, PAR-2-mediated relaxation of isolated mouse tracheal was prevented by selective ERK1/2 and p38 inhibitors (26). These studies suggest that both ERK1/2 and p38 pathways are involved in PAR-2-mediated expression of COX-2. In the present study, a PAR-2 agonist induced phosphorylation of ERK1/2, but not p38, in urothelial cells. Furthermore, increased COX-2 mRNA expression and protein abundance following PAR-2 activation were prevented by the selective ERK1/2 phosphorylation inhibitor U0126 (26). Therefore, our results indicate that PAR-2-induced expression of COX-2 is primarily mediated by the ERK1/2 MAP kinase pathway in urothelial cells. These results also suggest that participation of MAP kinase pathways in PAR-2 signaling varies among different tissues or species.
Perspectives and Significance
Our findings demonstrate that COX-2 protein abundance is increased in mouse urothelium/suburothelium after induction of cystitis by CYP treatment and that the increase of COX-2 protein abundance is at least partially mediated by mast cells. We further demonstrate that activation of PAR-2 increases expression of COX-2 in primary cultures of human urothelial cells and mouse urothelium/suburothelium. Furthermore, the effects of PAR-2 activation in urothelial cells appear to be mediated primarily by the ERK1/2 MAP kinase pathway. These data indicate that there are functional interactions among mast cells, PAR-2 activation, and increased expression of COX-2. The results of the current study also raise intriguing questions about the convergent role of PAR-2 and COX-2 in bladder inflammatory diseases and suggest that both PAR-2 and COX-2 are likely therapeutic targets for the treatments of patients with PBS/IC. It should be noted that bladder inflammation is a complicated process and that other inflammatory mediators, such as cytokinins, neuropeptides and neurotrophic factors, also may contribute to regulating COX-2 expression, and this must be the focus of future studies.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK066349.
REFERENCES
- 1.Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology 49, Suppl 5A: 52–57, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4: 46–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorling DE, Jerde TJ, Zine MJ, Busser BW, Saban MR, Saban R. Mast cells mediate the severity of experimental cystitis in mice. J Urol 162: 231–236, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bon K, Lanteri-Minet M, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: a c-fos and Krox-24 study at telencephalic levels, with a note on pituitary adenylate cyclase activating polypeptide (PACAP). Exp Brain Res 122: 165–174, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Boucher W, el-Mansoury M, Pang X, Sant GR, Theoharides TC. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol 76: 94–100, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat 214: 516–559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 217: 141–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol 170: 4296–4300, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Chen MC, Blunt LW, Pins MR, Klumpp DJ. Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol 175: 754–759, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chuang YC, Yoshimura N, Huang CC, Wu M, Chiang PH, Chancellor MB. Intravesical botulinum toxin A administration inhibits COX-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur Urol 56: 159–167, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Coelho AM, Ossovskaya V, Bunnett NW. Proteinase-activated receptor-2: physiological and pathophysiological roles. Curr Med Chem Cardiovasc Hematol Agents 1: 61–72, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, Quintão NL, Juliano L, Brain SD, Calixto JB. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol 154: 1094–1103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox PJ. Cyclophosphamide cystitis–identification of acrolein as the causative agent. Biochem Pharmacol 28: 2045–2049, 1979 [DOI] [PubMed] [Google Scholar]
- 15.D'Andrea MR, Saban MR, Gerard NP, Wershil BK, Saban R. Lack of neurokinin-1 receptor expression affects tissue mast cell numbers but not their spatial relationship with nerves. Am J Physiol Regul Integr Comp Physiol 288: R491–R500, 2005 [DOI] [PubMed] [Google Scholar]
- 16.D'Andrea MR, Saban MR, Nguyen NB, Andrade-Gordon P, Saban R. Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder. Am J Pathol 162: 907–923, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dattilio A, Vizzard MA. Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol 173: 635–639, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol 20: 55–72, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Erickson DR, Davies MF. Interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct 9: 174–183, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Frenz AM, Christmas TJ, Pearce FL. Does the mast cell have an intrinsic role in the pathogenesis of interstitial cystitis? Agents Actions 41: C14–C15, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Frungieri MB, Weidinger S, Meineke V, Köhn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma: possible relevance to human fibrotic disorders. Proc Natl Acad Sci USA 99: 15072–15077, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol 117: 1285–1291, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol 284: R574–R585, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka O, Mattiasson A, Andersson KE. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: involvement of tachykinins? J Urol 153: 2034–2038, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol 9: 1215–1223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawabata A, Kubo S, Ishiki T, Kawao N, Sekiguchi F, Kuroda R, Hollenberg MD, Kanke T, Saito N. Proteinase-activated receptor-2-mediated relaxation in mouse tracheal and bronchial smooth muscle: signal transduction mechanisms and distinct agonist sensitivity. J Pharmacol Exp Ther 311: 402–410, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kawao N, Nagataki M, Nagasawa K, Kubo S, Cushing K, Wada T, Sekiguchi F, Ichida S, Hollenberg MD, MacNaughton WK, Nishikawa H, Kawabata A. Signal transduction for proteinase-activated receptor-2-triggered prostaglandin E2 formation in human lung epithelial cells. J Pharmacol Exp Ther 315: 576–589, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Laird JM, Souslova V, Wood JN, Cervero F. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. J Neurosci 22: 8352–8356, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lecci A, Birder LA, Meini S, Catalioto RM, Tramontana M, Giuliani S, Criscuoli M, Maggi CA. Pharmacological evaluation of the role of cyclooxygenase isoenzymes on the micturition reflex following experimental cystitis in rats. Br J Pharmacol 130: 331–338, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Evanoff DP, Chen X, Luo Y. Urinary bladder epithelium antigen induces CD8+ T cell tolerance, activation, and autoimmune response. J Immunol 178: 539–546, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundeberg T, Liedberg H, Nordling L, Theodorsson E, Owzarski A, Ekman P. Interstitial cystitis: correlation with nerve fibres, mast cells and histamine. Br J Urol 71: 427–429, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Lynes WL, Flynn SD, Shortliffe LD, Lemmers M, Zipser R, Roberts LJ 2nd, Stamey TA. Mast cell involvement in interstitial cystitis. J Urol 138: 746–752, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev 53: 245–282, 2001 [PubMed] [Google Scholar]
- 35.Maggi CA, Giuliani S, Conte B, Furio M, Santicioli P, Meli P, Gragnani L, Meli A. Prostanoids modulate reflex micturition by acting through capsaicin-sensitive afferents. Eur J Pharmacol 145: 105–112, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Matej R, Mandáková P, Netíková I, Poucková P, Olejár T. Proteinase-activated receptor-2 expression in breast cancer and the role of trypsin on growth and metabolism of breast cancer cell line MDA MB-231. Physiol Res 56: 475–484, 2007 [DOI] [PubMed] [Google Scholar]
- 37.McGuire JJ, Saifeddine M, Triggle CR, Sun K, Hollenberg MD. 2-Furoyl-LIGRLO-amide: a potent and selective proteinase-activated receptor 2 agonist. J Pharmacol Exp Ther 309: 1124–1131, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JA, Evans TW. Cyclooxygenase-2 as a therapeutic target. Inflamm Res 47: S88–S92, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Moffatt JD. Proteinase-activated receptors in the lower urinary tract. Naunyn Schmiedebergs Arch Pharmacol 375: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Nakahara T, Kubota Y, Mitani A, Maruko T, Sakamoto K, Ishii K. Protease-activated receptor-2-mediated contraction in the rat urinary bladder: the role of urinary bladder mucosa. Naunyn Schmiedebergs Arch Pharmacol 367: 211–213, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Nakahara T, Kubota Y, Saito M, Sakamoto K, Ishii K. Protease-activated receptor-2-mediated contraction of urinary bladder is enhanced in cyclophosphamide-treated rats. Naunyn Schmiedebergs Arch Pharmacol 369: 212–219, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci USA 105: 18053–18057, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161: 438–441, 1999 [PubMed] [Google Scholar]
- 44.Palmer HS, Kelso EB, Lockhart JC, Sommerhoff CP, Plevin R, Goh FG, Ferrell WR. Protease-activated receptor 2 mediates the proinflammatory effects of synovial mast cells. Arthritis Rheum 56: 3532–3540, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Peeker R, Enerbäck L, Fall M, Aldenborg F. Recruitment, distribution and phenotypes of mast cells in interstitial cystitis. J Urol 163: 1009–1015, 2000 [PubMed] [Google Scholar]
- 46.Qiao LY, Gulick MA. Region-specific changes in the phosphorylation of ERK1/2 and ERK5 in rat micturition pathways following cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 292: R1368–R1375, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Saban R, Saban MR, Nguyen NB, Hammond TG, Wershil BK. Mast cell regulation of inflammation and gene expression during antigen-induced bladder inflammation in mice. Physiol Genomics 7: 35–43, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer 107: 159–163, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 69, Suppl 4: 34–40, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Southgate J, Masters JRW, Trejdosiewicz Culture of human urothelium. In: Culture of Epithelial Cells, edited by Freshney RI, Freshney MG.New York: Wiley-Liss, 2002, p. 381–399 [Google Scholar]
- 51.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 6: 151–158, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev 26: 1–43, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. J Asthma 42: 79–89, 2005 [PubMed] [Google Scholar]
- 54.Surprenant A. Pain TRP-ed up by PARs. J Physiol 578: 631, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teng J, Wang ZY, Prossnitz ER, Bjorling DE. The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation. Endocrinology 149: 4024–4034, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA, Jr, Meares EM., Jr Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. J Urol 153: 629–636, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology 57: 47–55, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Vera PL, Iczkowski KA, Wang X, Meyer-Siegler KL. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association. PLoS One 3: e3898, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res 152: 97–115, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Wen S, Bunnett NW, Leduc R, Hollenberg MD, MacNaughton WK. Proteinase-activated receptor-2 induces cyclooxygenase-2 expression through beta-catenin and cyclic AMP-response element-binding protein. J Biol Chem 283: 809–815, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Wheeler MA, Yoon JH, Olsson LE, Weiss RM. Cyclooxygenase-2 protein and prostaglandin E2 production are up-regulated in a rat bladder inflammation model. Eur J Pharmacol 417: 239–248, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Wu RL, Osman I, Wu XR, Lu ML, Zhang ZF, Liang FX, Hamza R, Scher H, Cordon-Cardo C, Sun TT. Uroplakin II gene is expressed in transitional cell carcinoma but not in bilharzial bladder squamous cell carcinoma: alternative pathways of bladder epithelial differentiation and tumor formation. Cancer Res 58: 1291–1297, 1998 [PubMed] [Google Scholar]