Abstract
Dietary sodium restriction coupled with axotomy of the rat chorda tympani nerve (CTX) results in selectively attenuated taste responses to sodium salts in the contralateral, intact chorda tympani nerve. Converging evidence indicates that sodium deficiency also diminishes the activated macrophage response to injury on both the sectioned and contralateral, intact sides of the tongue. Because a sodium-restricted diet causes a robust increase in circulating aldosterone, we tested the hypothesis that changes in neurophysiological and immune responses contralateral to the CTX could be mimicked by aldosterone administration instead of the low-sodium diet. Taste responses in rats with CTX and supplemental aldosterone for 4–6 days were similar to rats with CTX and dietary sodium restriction. Responses to sodium salts were as much as 50% lower compared with sham-operated and vehicle-supplemented rats. The group-related functional differences were eliminated with lingual application of amiloride, suggesting that a major transduction pathway affected was through epithelial sodium channels. Consistent with the functional results, few macrophages were observed on either side of the tongue in rats with CTX and aldosterone. In contrast, macrophages were elevated on both sides of the tongue in rats with CTX and the vehicle. These results show that sodium deficiency or administration of aldosterone suppresses the immune response to neural injury, resulting in attenuation of peripheral gustatory function. They also show a potential key link among downstream consequences of sodium imbalance, taste function, and immune activity.
Keywords: taste, sodium, chorda tympani nerve, osmotic pumps, electrophysiology
the gustatory system has long been recognized as a model to study neural plasticity in the peripheral nervous system (8, 9, 30, 36, 37). In addition to substantial evidence for structural and functional plasticity during development (25, 34, 35), there are also clear examples demonstrating the peripheral gustatory system remains plastic throughout adulthood (9, 47). In particular, experimental manipulations instituted while taste buds and taste nerves regenerate following axotomy provide a powerful tool to examine the functional plasticity of the peripheral nervous system in adults (22, 33, 39).
Sectioning the chorda tympani nerve leads to a well-described loss of normal taste bud morphology from the anterior tongue. Within weeks after nerve section, the chorda tympani reinnervates lingual epithelia and restores taste bud structure and function (8, 9, 39). However, if rats are fed a sodium-restricted diet during the period of nerve regeneration, taste function is altered in a specific and dramatic way upon reappearance of taste buds (33, 39). Taste responses to sodium salts from the regenerated chorda tympani nerve are selectively attenuated by as much as 65%, and the decreased response persists for 80+ days after nerve section, as long as the rats are maintained on the sodium-restricted diet. This effect is achieved only by combining chorda tympani nerve sectioning with dietary sodium restriction. That is, dietary sodium restriction alone or nerve section alone does not alter the regenerated chorda tympani nerve function (33, 39).
In addition to the depressed sodium salt response following chorda tympani nerve regeneration in sodium-restricted rats, other novel functional effects are observed. In the same rats, the contralateral, intact chorda tympani nerve becomes supersensitive to sodium salts after about 40 days after nerve section. The hypersensitivity develops progressively following an initial and profoundly low response that is seen within days after axotomy (39). This phenomenon is not limited to sectioning of the chorda tympani nerve; injury to other nerves innervating the tongue or cellular damage to the tongue coupled with a low-sodium diet results in attenuated sodium taste responses in the intact chorda tympani (22). The closer the damage is to the contralateral, intact chorda tympani nerve, the greater the functional impairment. This finding suggests that local factors mediate functional changes, and the source of these factors may be mediated through the immune system (see below). Although nerve cut alone produces an early, transient (24 h) functional effect in the contralateral nerve (46), the progression whereby the nerve changes from responding relatively low to sodium salt taste stimuli (at 4–6 days postsection) to a supernormal sodium salt taste response (>40 days postsection) occurs only when the contralateral nerve section is coupled with dietary sodium restriction.
Clearly, unilateral sectioning the chorda tympani nerve coupled with a low-sodium diet produces a myriad of short- and long-term functional effects. Unfortunately, a clearly identified molecular/cellular mechanism(s) has not been found that separates or links these phenomena. However, some of these effects appear to be regulated through corresponding alterations in the immune system (e.g., short-term effects contralateral to the nerve section), while others may involve other physiological systems (e.g., long-term effects on regenerated and contralateral chorda tympani nerve). It is possible that the endocrine system is also a contributor to the effects because of the myriad of circulating factors, including hormones, that is released when animals are fed a low-sodium diet (1, 12, 16, 18, 28, 42).
Coupling unilateral chorda tympani section with dietary sodium restriction appears to target only the transduction pathway primarily responsible for sodium salt taste, the amiloride-sensitive epithelial sodium channel (ENaC) (21, 50). Evidence suggests this change in sodium taste responses and sensitivity to amiloride is at least partially due to a suppressed immune system in sodium-restricted rats (6, 7, 32, 33). In rats fed a sodium-replete diet, chorda tympani nerve transection increases activated macrophages in lingual epithelium, while sodium-restricted rats fail to show the transection-induced macrophage response. Upregulation of immune function with LPS eliminates both diet-related differences in sodium responsiveness and the number of activated macrophages (6, 39). Thus, the functional plasticity observed with chorda tympani transection and sodium-restricted diet is associated with a suppressed immune response to nerve injury.
As of yet, the link between suppressed sodium taste responses, the immune system, and dietary sodium restriction has not been shown. Candidate circulating factors that may play a role linking sodium-restricted diets with altered functional taste responses and immune function include those involved in the renin-angiotensin-aldosterone system. In particular, circulating levels of angiotensin, atrial natriuretic peptide, renin, cortisol, and aldosterone are influenced by dietary sodium (1, 12, 16, 18, 28, 42). Aldosterone is an especially attractive candidate as a factor involved in the pathway(s) activated through dietary sodium restriction and having an effect on sodium salt taste because of its regulation of ENaC and immune function (3, 5, 40, 45, 48, 49). However, the effects appear paradoxical because increased levels of aldosterone, as produced by sodium-restricted diets (3), usually increase the density and efficiency of ENaCs (2, 3, 19, 38). The current study examines whether aldosterone can substitute for a sodium-restricted diet to suppress taste and immune function following contralateral sectioning of the chorda tympani nerve. By administering aldosterone concomitantly with nerve transection, we hypothesized that both sodium responsiveness of the intact chorda tympani nerve and activated macrophages would decrease as seen in similar rats fed the sodium-restricted diet.
MATERIALS AND METHODS
Animals.
For neurophysiological experiments, 32 female Sprague-Dawley rats (Harlan, Indianapolis, IN) were 40–60 days of age at the time of experimental manipulations. Relatively young female rats were chosen because of the ease of exposing the chorda tympani nerve in the neck (see Chorda tympani nerve transection, aldosterone administration, and dietary sodium restriction) without damaging nearby muscles and nerves. Prior to surgery, rats were group housed with tap water, and Purina standard rat chow was freely available. Rats were kept on a 12:12-h light-dark cycle in a humidity- and temperature-controlled room. All protocols were approved by the University of Virginia Animal Care and Use committee and adhered to APS's Guiding Principles in the Care and Use of Animals.
For immunohistochemical experiments, 24 female, specified pathogen-free Sprague Dawley rats from Charles River Laboratories (Wilmington, MA) were 35–60 days old at the time of experimental manipulations. Rats were housed in cages with barrier tops, kept on a 12:12-h light-dark cycle and received autoclaved food, water, and bedding. These experiments were done in accordance with the Institutional Animal Care and Use Committee at the Medical College of Georgia and adhere to APS's Guiding Principles in the Care and Use of Animals.
Chorda tympani nerve transection, aldosterone administration, and dietary sodium restriction.
Each animal received either unilateral chorda tympani transection (CTX) or sham surgery (Sham), as described previously (22, 33, 39). Rats were given 0.1 ml atropine sulfate (0.54 mg ip) and anesthetized with brevital sodium (60 mg/kg body wt ip) or ketamine (40 mg/kg ip) mixed with xylazine (10 mg/kg ip). The right chorda tympani nerve was exposed by a ventral approach in the neck; blunt dissection techniques were used to prevent damage to nearby muscles. The chorda tympani nerve was transected at its junction with the lingual branch of the trigeminal nerve. Care was taken not to damage the trigeminal nerve throughout the surgery. Sham rats had the right chorda tympani exposed but not cut.
At the time of surgery (i.e., day 0), each rat was also implanted with a subcutaneous miniosmotic pump (Alzet, Cupertino, CA) containing either a 0.9% saline (Veh) or aldosterone (Aldo) solution after surgery. Miniosmotic pumps released aldosterone [Sigma-Aldrich, St. Louis, MO; polyethylene glycol (300 MW) as the vehicle] at a constant rate of 250 μg·kg−1·day−1 (3). This dose was chosen because of its demonstrated rapid (i.e., within 3 h) augmenting effects on ENaC subunit mRNA in kidney and colon and similar increase in circulating aldosterone levels to that found with dietary sodium restriction (3).
Following this procedure, rats were returned to their home cage and allowed to recover before nerve recordings or immunohistochemical experiments. There was no apparent effect of the surgery on mastication and feeding behavior; body weights returned to preoperative levels within 2 days of surgery. An additional group of rats received chorda tympani nerve sectioning, dietary sodium restriction, and saline vehicle administered through a miniosmotic pump (CTX + sodium restriction). To achieve rapid sodium restriction, rats in the CTX + Sodium Restriction group were given two injections of furosemide (10 mg ip each; Sigma-Aldrich, St. Louis, MO) within 24 h of the CTX; then, they were fed a sodium-restricted diet (0.03%; ICN Biomedicals; Solon, OH) and distilled water for the remainder of the experiment.
Neurophysiology.
The focus of the functional experiments was to test the hypothesis that the highly predictable, selective, and dramatic attenuation of sodium salt taste responses in sodium-restricted rats on the side of the tongue contralateral to CTX could be achieved by experimentally substituting aldosterone administration for the dietary manipulation. Neurophysiological taste responses were recorded in rats from five experimental groups: 1) CTX with aldosterone pumps (CTX + Aldo; n = 8), 2) sham with aldosterone pumps (Sham + Aldo; n = 7), 3) CTX with saline vehicle pumps (CTX + Veh; n = 6), 4) sham with saline vehicle pumps (Sham + Veh; n = 5), and 5) CTX with dietary sodium restriction (CTX + Sodium Restriction; n = 6). Whole nerve recordings were taken from the chorda tympani nerve contralateral to the sectioned nerve (i.e., from the intact CT) 4 to 6 days after nerve transection (27). This is the period in which taste responses from the contralateral chorda tympani nerve are very low compared with controls (∼25% of control responses) (27, 33). Briefly, rats were anesthetized with pentobarbital sodium (10 mg/kg body wt ip), with supplemental doses given as needed. A water-circulating heating pad was used to maintain the rat's body temperature at ∼37°C. The hypoglossal nerves were exposed and transected bilaterally to disrupt motor input to the tongue. The trachea was cannulated, and the rat was placed in a nontraumatic head holder (15). The left side of the head was dissected, and the chorda tympani nerve was exposed. The chorda tympani nerve was cut near its entrance into the tympanic bulla, teased away from the underlying connective tissues, desheathed, and placed on a platinum-recording electrode. The signal was amplified and integrated (time constant = 2.0 s). Data were recorded and analyzed using PowerLab (ADInstruments, Colorado Springs, CO).
Stimuli consisted of a concentration series of NaCl, sodium acetate (NaAc), NH4Cl, and KCl (50 mM, 100 mM, 250 mM, and 500 mM). Stimuli within each concentration series were presented in ascending order by applying ∼5 ml of solution slowly through a 10-ml syringe, and remained on the tongue for 40 s followed by at least 40 s of rinse. In addition, a series of NaCl was mixed with 50 μM amiloride hydrochloride, an epithelial sodium channel blocker (ENaC) (13, 21). Amiloride (50 μM) was also used as the rinse in these series. The height of the integrated response was measured from the period 5 to 20 s after stimulus onset to eliminate temperature and tactile responses, while including only the steady-state responses for analysis. Responses to 500 mM NH4Cl were taken before and after a stimulus series to assess the stability of the nerve; data were eliminated if the difference were greater than 10%. The average relative response of each stimulus was calculated by standardizing it to the average 500 mM NH4Cl response before and after the stimulus series.
ED1 immunohistochemistry and image analysis.
Activated macrophages were analyzed in groups given the same treatment as in neurophysiological experiments: 1) CTX with aldosterone pumps (CTX + Aldo; n = 6), 2) sham with aldosterone pumps (Sham + Aldo; n = 5), 3) CTX with saline vehicle pumps (CTX + Veh; n = 4), 4) sham with saline vehicle pumps (Sham + Veh; n = 5), and 5) CTX with dietary sodium restriction (CTX + Sodium Restriction; n = 4). Rats were euthanized with pentobarbital sodium (80 mg/kg ip) at day 2 following surgery, at the peak of the macrophage response to CTX (32). The anterior tongue was removed rapidly, frozen and cryosectioned at 8 μm, as described previously (6, 32). Approximately 300 sections were collected from the fungiform papillae field, including ∼150 sections from the anterior tongue, ∼75 sections located 2 mm caudal to the anterior sections and another 75 sections after 2 mm of the tongue was skipped.
Macrophages were identified with the widely used and well-characterized ED1 antibody (Serotec, Raleigh, NC), which detects a lysosomal antigen in activated macrophages (14). The expression patterns and dynamics of ED1 immunoreactivity in the injured peripheral taste system have been determined in previous work (32). Slides were fixed with 0.2% glutaraldehyde in PBS (pH 7.5), and nonspecific staining was blocked using 3.0% H2O2 and 2.0% goat serum for 30 min. Sections were incubated in primary antibody (1:400) for 2 h at room temperature, followed by biotinylated goat anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA) and avidin-biotin complex (Vector Laboratories, Burlingame, CA) with diaminobenzidine as the chromogen, and lightly counterstained with hematoxylin.
Immunolabeled leukocytes were analyzed with a computer imaging system equipped with a digital color camera (Cool Snap; Roper Scientific, Tucson, AZ) and MetaMorph software (Universal Imaging, Sunnyvale, CA), as described previously (32). Images were captured at ×50 and were used to quantify leukocytes in four regions/coronal section: 1) the denervated epithelium and lamina propria, 2) the denervated submucosa and muscle, 3) the intact, contralateral epithelium and lamina propria, and 4) the intact submucosa and muscle. Immunopositive leukocytes inside vessels were not counted because fresh-frozen tissue may contain variable numbers of intravascular leukocytes (32). For each region described, a standard-sized region of interest was placed to encompass the most immunopositive label. As described previously, this region of interest was 206.4 mm2/section (32). To quantify macrophages, four images were acquired in the submucosa/muscle and the epithelium/lamina propria regions on the cut (right) or intact (left) sides of the tongue. The process used to select sections for imaging was also standardized (32). Because macrophages are difficult to count because of their wispy processes, stained pixels were digitally marked on each image, and the percentage of stained pixels/standard area were determined to quantify macrophages.
Data analysis.
For nerve recordings, the standardized data were pooled for each concentration of each stimulus, and group means and standard errors were calculated. Means of a given stimulus were compared among groups using one-way ANOVAs with a α level of 0.05. If a significant difference was detected by an ANOVA, post hoc analysis using Newman-Keuls multiple comparison tests was used to determine group mean differences (α of P ≤ 0.05). Mean levels of ED1-positive staining were compared among treatment groups using one-way ANOVAs, followed by Newman-Keuls multiple-comparison tests where appropriate (α of P ≤ 0.05).
RESULTS
Neurophysiology.
Taste responses from the intact chorda tympani nerve revealed significant effects of combined aldosterone administration and CTX 4 to 6 days after transection (Figs. 1 and 2), and the effects were similar to those observed in sodium-restricted rats after CTX (27, 32, 33).
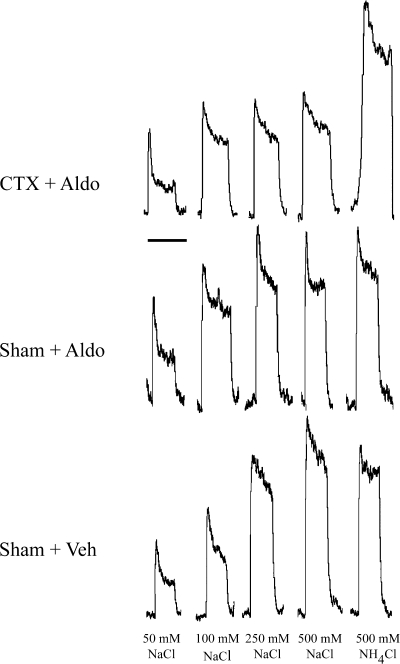
Fig. 1.
Integrated responses from the chorda tympani nerve to a concentration series of NaCl and to 500 mM NH4Cl in a rat receiving a chorda tympani nerve section on the contralateral side and supplemental aldosterone (CTX + Aldo; top), sham surgery and aldosterone (Sham + Aldo; middle), and sham with a saline vehicle (Sham + Veh; bottom). Increasing NaCl concentration produces an increase in chorda tympani nerve response in Sham + Aldo and Sham + Veh rats, so that the steady-state response to 500 mM NaCl is similar to the response to 500 mM NH4Cl. Increasing concentration of NaCl in CTX + Aldo rats saturates at ∼100 mM NaCl, which is about 50% of the steady-state response to 500 mM NH4Cl. Scale bar = 1 min.
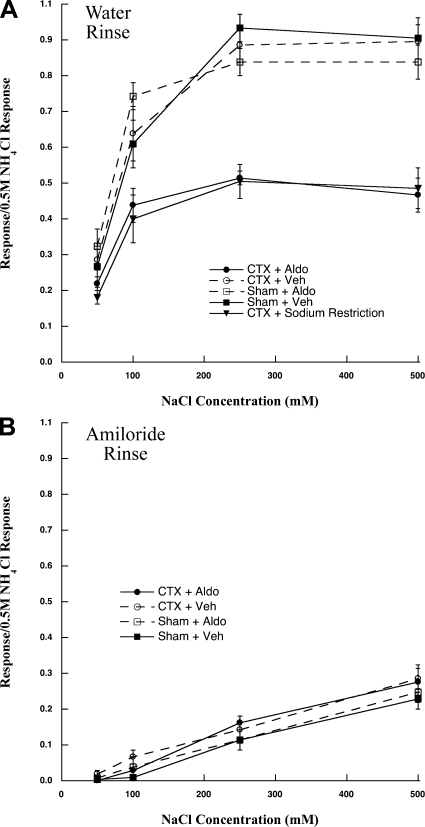
Fig. 2.
A: mean ± SE responses of the chorda tympani nerve to a concentration series of NaCl in rats receiving a chorda tympani nerve section on the contralateral side and given aldosterone (CTX + Aldo) or a saline vehicle (CTX + Veh), or rats with a sham surgery and given aldosterone (Sham + Aldo) or a saline vehicle (Sham + Veh). Water was used as the solvent for the stimuli and the rinse. These data are compared with data in which rats sustained a chorda tympani nerve section on the contralateral side and then fed a low-NaCl diet (CTX + Sodium Restriction). Means from CTX + Veh, Sham + Aldo, and Sham + Veh were similar to each other (P > 0.05), but all were significantly greater than those in CTX + Aldo and CTX + Sodium Restriction rats (P < 0.05). B: mean ± SE responses of the chorda tympani nerve to a concentration series of NaCl mixed in amiloride in the same rats shown as in A. Amiloride was used as the solvent for the stimuli and the rinse. None of the means differed from each other when stimuli were mixed in amiloride (P > 0.05).
In Sham + Veh rats, the relative responses to NaCl increased as stimulus concentrations increased up to 250 mM and remained constant through 500 mM (Figs. 1 and 2A). Responses to NaCl in Sham + Aldo and CTX + Veh rats were similar to Sham + Veh rats at all stimulus concentrations (P > 0.10; Figs. 1 and 2A). Therefore, neither aldosterone alone (Sham + Aldo) nor CTX alone (CTX + Veh) produced attenuated responses to NaCl (Fig. 2A).
In contrast, CTX + Aldo rats had significantly reduced relative responses to 100 mM (P < 0.03), 250 mM (P < 0.001), and 500 mM (P < 0.001) compared with the three control groups (Sham + Veh, Sham + Aldo, CTX + Veh). For example, the response to 500 mM NaCl in CTX + Aldo rats was reduced by ∼50% compared with Sham + Veh rats. The response reduction in CTX + Aldo rats were similar to CTX + Sodium Restriction rats (P > 0.05; Fig. 2A). Therefore, both CTX and aldosterone were necessary to produce the attenuated response in the chorda tympani nerve similar to that found in rats in which CTX was coupled with dietary sodium restriction (Fig. 2A). These group-related differences in chorda tympani nerve responses to NaCl were eliminated with the lingual application of 50 μM amiloride (P > 0.10; Fig. 2B), indicating that the response attenuation involves alterations in ENaC function.
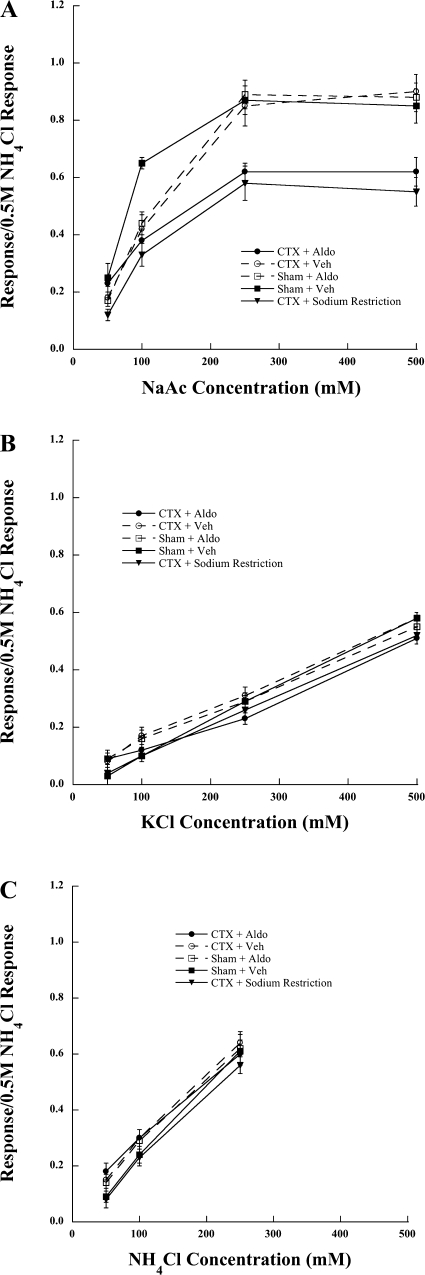
The group-related differences attributed to NaCl were also expressed in responses to sodium acetate (NaAc), but not for responses to KCl and NH4Cl. Relative responses to 250 mM and 500 mM NaAc in CTX + Aldo rats and CTX + Sodium Restriction rats were significantly lower than responses in Sham + Veh, Sham + Aldo, and CTX + Veh rats (all P < 0.01; Fig. 3A). Finally, the experimental effects of CTX coupled with aldosterone were limited to sodium salts. No significant differences occurred among the groups, including CTX + Sodium Restriction) in response to the nonsodium salts, NH4Cl and KCl (P > 0.10, Figs. 3, B and C).
Fig. 3.
Means ± SE responses of the chorda tympani nerve to a concentration series of sodium acetate (NaAc; A), KCl (B), or NH4Cl (C) in rats receiving a chorda tympani nerve section on the contralateral side and given aldosterone (CTX + Aldo) or a saline vehicle (CTX + Veh), or rats with a sham surgery and given aldosterone (Sham + Aldo) or a saline vehicle (Sham + Veh). These data are compared with data in which rats had a chorda tympani nerve section on the contralateral side and then fed a low-NaCl diet (CTX + Sodium Restriction).
Analysis of ED1+ activated macrophages.
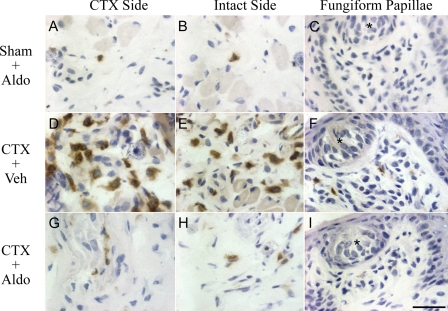
Few macrophages were observed on either side of the tongue in Sham + Aldo rats (Fig. 4, A–C). Macrophages were also rare in control groups receiving sham sectioning and vehicle (Sham + Veh) or receiving nerve section, sodium restriction, and vehicle (CTX + Sodium Restriction; not shown). In contrast, there was a robust macrophage response on both the sectioned (Fig. 4D) and intact (Fig. 4E) sides of the tongue after CT sectioning and administration of vehicle (CTX + Veh). This increase occurred in the submucosa (Fig. 4, G and H), lamina propria, and within fungiform papillae (Fig. 4F). After nerve sectioning and treatment with aldosterone, however, few macrophages were found in any of these regions (Fig. 4, G–I). Thus, aldosterone suppressed the bilateral macrophage response to neural injury. Control staining, in which the primary antibody was omitted or an isotype-matched irrelevant antibody was substituted (not shown), showed minimal nonspecific immunoreactivity (6, 32).
Fig. 4.
ED1+-activated macrophages (brown immunoreactivity) at day 2 postsectioning. There were few macrophages on the CTX (A) or intact (B) sides of the tongue or within fungiform papillae (C) after sham sectioning and aldosterone (Sham + Aldo). Following CTX and vehicle (CTX + Veh), activated macrophages were abundant in the submucosa of the CTX (D) and intact (E) sides of the tongue and in intact fungiform papillae (F). Aldosterone prevented the macrophage response to nerve injury on the sectioned side (CTX + Aldo; G), the intact side (H), and in intact fungiform papillae (I). Stars in C, F, and I indicate taste buds. Scale bar = 30 μm.
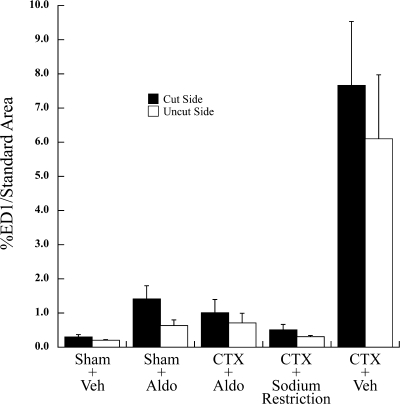
Postinjury effects of aldosterone were further examined by image analysis, as shown as Fig. 5. Our findings confirmed the qualitative analysis, in that macrophage levels were significantly reduced by nerve section and aldosterone (CTX + Aldo) compared with sectioning alone (CTX + Veh; P < 0.001). The inhibitory effect of aldosterone on activated macrophages was bilateral: the response to sectioning was reduced by almost 90% on both the injured and intact sides of the tongue (P < 0.001). In fact, aldosterone reduced the macrophage response to nerve injury to the same level found in sodium-restricted rats (CTX sodium restriction; P > 0.05; Fig. 5). Sham-sectioned control groups exhibited low levels of macrophages whether treated with vehicle (Sham + Veh) or aldosterone (Sham + Aldo) (P > 0.05 vs. CTX + Aldo and CTX + Sodium Restriction; Fig. 5). These effects were consistent across the rostral-caudal axis of the fungiform papillae field, although they were more dramatic in the anterior region, where both the number of taste buds and macrophages were proportionally increased (32). In summary, macrophage levels paralleled neural responses. Aldosterone, like dietary sodium restriction, inhibited both the immune response to nerve injury and sodium taste function in the intact nerve.
Fig. 5.
Analysis of activated macrophages (percentage of ED1/standard area). Solid bars represent macrophage levels on the CTX side of the tongue, or the right side in sham-sectioned groups. Hatched bars show levels measured on the contralateral, uninjured side. Macrophages remained low in groups receiving sham CTX, whether pumps administered vehicle (Sham + Veh) or aldosterone (Sham + Aldo). The macrophage response to nerve section also remained at baseline levels on both sides of the tongue in sodium-restricted rats (CTX + Na+ Restriction), as expected. CTX dramatically increased activated macrophages on both sides of the tongue (CTX + Veh) (P < 0.001 vs. values from each of the other groups), but aldosterone prevented this response (CTX + Aldo).
DISCUSSION
We sought to test the hypothesis that CTX coupled with aldosterone administration at concentrations comparable to that produced by dietary sodium restriction would produce neurophysiological and immunological findings similar to that seen in rats in which CTX was coupled with dietary sodium restriction. The main finding of this study is that there was a suppression of the immune response to CTX and an attenuation of sodium salt responses in the intact, contralateral chorda tympani nerve when aldosterone was continuously infused after CTX. After unilateral CTX, the number of activated macrophages in the anterior tongue was significantly lower in rats given continual, subcutaneous aldosterone, similar to that found in rats placed on a sodium-restricted diet. Concomitantly, rats administered aldosterone after CTX showed a decrease in the relative whole nerve response to sodium salts in the intact chorda tympani nerve but not to nonsodium salts, similar to that seen in sodium-restricted rats. Also similar to data from rats fed a sodium-restricted diet, the application of amiloride, a selective epithelial sodium channel (ENaC) blocker, eliminated differences between experimental groups, suggesting changes in electrophysiological responses to sodium are due to a change in this sodium transduction pathway. Taken together, these data strongly support our hypothesis that CTX coupled with increased levels of aldosterone mimic the effects found when CTX is coupled with the dietary manipulation.
Previous research demonstrated that sodium restriction leads to an attenuation of chorda tympani nerve responses to sodium salts when combined with nerve or lingual epithelial injury, also resulting in an attenuated lingual macrophage response to injury (6, 7, 22, 32, 33, 39). Stimulating the immune response with LPS injections eliminates these differences (6, 39). Thus, alterations in sodium taste function following CTX are accompanied by corresponding changes in immune function. It is not clear if these concomitant changes are correlational or causative. That is, parallel pathways affected by CTX coupled with dietary sodium restriction could influence taste responses and immune function independently or the immune response could directly impact downstream peripheral taste responses. We favor the latter because experimental alterations of immune function also impact taste function. Data consistent with this view include 1) experimental upregulation of the immune system with LPS, even in uncut rats, increases taste responses to sodium salts (39); 2) an attenuation of immune function by dietary sodium restriction after CTX leads to altered taste function in the contralateral, intact nerve (6, 7, 32, 33); 3) the timecourse of functional changes following CTX can be predicted by the timecourse of immune alterations (46); and 4) the magnitude of functional effect on the intact chorda tympani nerve is generally related to the amount of injury and the distance from the nerve's receptive field (22). All of these taste response effects appear to involve ENaC function, thereby leading to the specificity of altering sodium salt taste responses.
Of particular relevance for this work is a related question that involves our control groups: does dietary sodium restriction alone directly influence taste responses? In contrast to data obtained from our control groups (see Figs. 2 and 3), other laboratories found decreased sodium taste responses in the chorda tympani nerve following dietary sodium restriction and a lack of amiloride sensitivity following furosemide injections, all without a nerve cut (4, 10, 11, 17). These data suggest that dietary sodium restriction alone has an effect on taste responses. However, the short-term effects on taste responses are not of the magnitude that we show here and elsewhere when CTX is combined with dietary sodium restriction. The results from control groups here are consistent with findings from our other studies (22, 24, 26, 27, 33, 39), with the exception of one. The study that is the exception showed that a transient, short-term chorda tympani response change occurs with sodium restriction alone (days 2 and 3, but not thereafter) (46). This effect may be due to the short-term effects of furosemide injections. Although it is not clear why these discrepancies exist, the use of different experimental protocols, including various furosemide dosages, periods postdietary manipulation, and different standard responses used for normalization, may lead to different interpretations. Nonetheless, it is clear from the current data and our other studies that CTX combined with the sodium-restricted diet produces profound alterations in taste responses in the intact nerve compared with groups that only have a CTX or the dietary manipulation.
The current study also provides another dimension to how nerve injury, immune function, and peripheral gustatory functions interrelate by showing that the immune and neurophysiological effects of combining CTX with dietary sodium restriction can be mimicked if supplemental aldosterone is substituted for the diet. Aldosterone was chosen here as a first step in uncovering the underlying cellular/molecular mechanism because of its upregulation during dietary sodium restriction and its role in regulating epithelial sodium channels (2, 3, 19, 38). Paradoxically, increased aldosterone often increases ENaC function in other epithelial cells, either by affecting the affinity of the channel to sodium (via subunit composition) or by increasing the number of functional channels in the cell membrane (2, 3, 19, 38). Importantly, aldosterone also increased amiloride sensitivity in isolated taste receptor cells in vitro (29) and in vivo (23). Unfortunately, comparisons of taste responses in the absence of amiloride are not available from these two studies; therefore, direct comparisons with results shown here cannot be made. However, we found no change in amiloride sensitivities compared with controls (Sham + Veh) in groups other than rats with CTX and aldosterone treatment (CTX + Aldo) in which amiloride sensitivity was attenuated instead of enhanced (Figs. 2 and 3). In both the previous in vitro (29) and in vivo (23) study, aldosterone was injected once (23) or twice (29) within 48 h or 6 h, respectively, compared with the current study, in which aldosterone was continuously infused up to 6 days. These methodological differences may account for differences in results among the studies, but again, it is clear that CTX coupled with aldosterone has a greater effect on taste responses from the intact nerve than if CTX or aldosterone administration were presented alone.
Importantly, these data along with previous work from our laboratories (6, 22, 32, 39) show that there are key interactions between the immune system and peripheral gustatory function. Namely, an appropriate macrophage response induced through axotomy and/or cellular damage is necessary for normal ENaC function in taste buds beyond the site of injury, yielding a stable and normal taste response to sodium salts. Upregulation or downregulation of the immune system by LPS (6, 39) or by aldosterone, respectively, upregulates or downregulates channel function distant to the site of tissue damage.
We show that aldosterone has a potent immunosuppressive effect on the macrophage response to neural injury. Likewise, restriction of dietary sodium (upstream from elevated aldosterone levels), depresses both macrophage levels and taste function soon after neighboring axotomy (22, 32). Aldosterone is considered proinflammatory since it can stimulate leukocyte infiltration, proinflammatory cytokine release, and markers of oxidative stress during cardiovascular and renal injury (5, 40). The length of exposure may be critical in resolving the difference between neural and cardiovascular injury models. We demonstrate that 2 days of aldosterone blocks the activated macrophage response to neural injury. The mineralocorticoid is typically given for weeks in models of artherosclerosis (7, 31, 44). It appears that long-term administration of aldosterone along with ongoing vascular injury in hypertensive rats potentiates inflammation. For example, 4 wk of aldosterone infusion were required to induce macrophage infiltration in a cardiac injury model (uninephrectomy plus 1% NaCl) (43). In contrast, axotomy stimulates an acute macrophage response in the taste system in parallel with degeneration of taste receptor cells and CT neurons (32). The effects of aldosterone may, therefore, diverge from anti-inflammatory to proinflammatory if injury persists. Interestingly, sodium taste function in the intact CT also changes from deficient to hypersensitive with time (27).
We propose that aldosterone links the immune response to injury and functional plasticity in the peripheral taste system. In a normal dietary environment, CTX stimulates a bilateral macrophage response to injury. Normal taste function is maintained in the intact taste receptor field. When either dietary sodium restriction or aldosterone is administered, levels of activated macrophages remain at low baseline levels. This suggests that aldosterone prevents macrophage recruitment to the injured taste system, despite its proinflammatory reputation.
In previous work, dietary sodium restriction inhibited the expression of vascular cell adhesion molecule (VCAM)-1 on lingual vessels after CTX (7). This adhesion molecule stimulates monocyte extravasation to injured tissues where they become macrophages. Aldosterone may mimic the effect of the diet on VCAM-1, reducing macrophage infiltration. The hormone had varying effects on VCAM-1 in studies using cardiac injury models. Infusion of aldosterone combined with 1% dietary NaCl had no effect on VCAM-1 mRNA in rat heart, even after 30 days (43). In contrast, 4 wk of aldosterone in combination with uninephrectomy and oral NaCl increased VCAM-1 staining in cardiac vessels, although gene expression was not significantly elevated (41). Aldosterone increases VCAM-1 mRNA levels in vitro (20), indicating that the complex interactions between the hormone, monocytes/macrophages, and vessels impose additional rules. In summary, aldosterone appears to have diverse influences on immune function depending on the tissue, type and length of injury, and duration of treatment. These factors might be important considerations as aldosterone antagonists are considered as anti-inflammatory therapies (31).
Perspective and Significance
These results show that, when coupled with a unilateral section of the chorda tympani nerve, aldosterone mimics the effects of a sodium-restricted diet. Aldosterone decreases both taste responses to sodium salts in the intact chorda tympani nerve and immune responses to injury in the tongue. These data are opposite of what is predicted from data collected from nonlingual epithelia concerning the influence of aldosterone on sodium transport and on immune function. They also raise further questions about the mechanisms by which aldosterone acts on gustatory tissue. Finally, the findings from this study are the first to indicate interrelationships among the peripheral gustatory system, hormonal systems, and the immune system.
GRANTS
This work was supported by National Institutes of Health Grants DC006938 to D.L.H. and DC005811 to L.P.M.
REFERENCES
- 1.Aguilera G, Hauger RL, Catt KJ. Control of aldosterone secretion during sodium restriction: adrenal receptor regulation and increased adrenal sensitivity to angiotensin II. Proc Natl Acad Sci USA 75: 975–979, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher C, Eren R, Kahn L, Yeger O, Garty H. Expression of the amiloride-blockable Na+ channel by RNA from control versus aldosterone-stimulated tissue. J Biol Chem 267: 16061–16065, 1992 [PubMed] [Google Scholar]
- 3.Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol Cell Physiol 271: C605–C611, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Bernstein IL, Taylor EM. Amiloride sensitivity of the chorda tympani response to sodium chloride in sodium-depleted Wistar rats. Behav Neurosci 106: 722–725, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Brown NJ. Aldosterone and vascular inflammation. Hypertension 51: 161–167, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cavallin MA, McCluskey LP. Lipopolysaccharide-induced up-regulation of activated macrophages in the degenerating taste system. J Neurosci Res 80: 75–84, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cavallin MA, McCluskey LP. Upregulation of intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 after unilateral nerve injury in the peripheral taste system. J Neurosci Res 85: 364–372, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cheal M, Dickey WP, Jones LB, Oakley B. Taste fiber responses during reinnervation of fungiform papillae. J Comp Neurol 172: 627–646, 1977 [DOI] [PubMed] [Google Scholar]
- 9.Cheal M, Oakley B. Regeneration of fungiform taste buds: temporal and spatial characteristics. J Comp Neurol 172: 609–626, 1977 [DOI] [PubMed] [Google Scholar]
- 10.Contreras RJ, Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol 73: 569–594, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis KS, Krause EG, Contreras RJ. Altered NaCl taste responses precede increased NaCl ingestion during Na+ deprivation. Physiol Behav 72: 743–749, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Denton DA. The hunger for salt : an anthropological, physiological, and medical analysis. New York: Springer-Verlag, 1982 [Google Scholar]
- 13.DeSimone JA, Ferrell F. Analysis of amiloride inhibition of chorda tympani taste response of rat to NaCl. Am J Physiol Regul Integr Comp Physiol 249: R52–R61, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54: 589–599, 1985 [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson RP. Nontraumatic headholder for rats. Physiol Behav 1: 97–98, 1966 [Google Scholar]
- 16.Fregly MJ, Rowland NE. Role of renin-angiotensin-aldosterone system in NaCl appetite of rats. Am J Physiol Regul Integr Comp Physiol 248: R1–R11, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Garcia JM, Curtis KS, Contreras RJ. Behavioral and electrophysiological taste responses change after brief or prolonged dietary sodium deprivation. Am J Physiol Regul Integr Comp Physiol 295: R1754–R1761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Sanchez C, Holland OB, Higgins JR, Mathieu R, Gruber GM, Milewich L, Kaplan NM. Mineralocorticoid activity of 16β-hydroxydehydroepiandrosterone and related steroids. J Lab Clin Med 88: 571–577, 1976 [PubMed] [Google Scholar]
- 19.Greig ER, Baker EH, Mathialahan T, Boot-Handford RP, Sandle GI. Segmental variability of ENaC subunit expression in rat colon during dietary sodium depletion. Pflügers Arch 444: 476–483, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Hashikabe Y, Suzuki K, Jojima T, Uchida K, Hattori Y. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol 47: 609–613, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223: 403–405, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Hendricks SJ, Sollars SI, Hill DL. Injury-induced functional plasticity in the peripheral gustatory system. J Neurosci 22: 8607–8613, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herness MS. Aldosterone increases the amiloride-sensitivity of the rat gustatory neural response to NaCl. Comp Biochem Physiol Comp Physiol 103: 269–273, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Hill DL. Susceptibility of the developing rat gustatory system to the physiological effects of dietary sodium deprivation. J Physiol (Lond) 393: 413–424, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill DL, Mistretta CM. Developmental neurobiology of salt taste sensation. Trends Neurosci 13: 188–195, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Hill DL, Mistretta CM, Bradley RM. Effects of dietary NaCl deprivation during early development on behavioral and neurophysiological taste responses. Behav Neurosci 100: 390–398, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Hill DL, Phillips LM. Functional plasticity of regenerated and intact taste receptors in adult rats unmasked by dietary sodium restriction. J Neurosci 14: 2904–2910, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollenberg NK, Chenitz WR, Adams DF, Williams GH. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin II in normal man. J Clin Invest 54: 34–42, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405: 406–420, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Mangold JE, Hill DL. Postnatal reorganization of primary afferent terminal fields in the rat gustatory brainstem is determined by prenatal dietary history. J Comp Neurol 509: 594–607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29: 367–374, 2008 [DOI] [PubMed] [Google Scholar]
- 32.McCluskey LP. Up-regulation of activated macrophages in response to degeneration in the taste system: effects of dietary sodium restriction. J Comp Neurol 479: 43–55, 2004 [DOI] [PubMed] [Google Scholar]
- 33.McCluskey LP, Hill DL. Sensitive periods for the effect of dietary sodium restriction on intact and denervated taste receptor cells. Am J Physiol Regul Integr Comp Physiol 283: R1275–R1284, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Mistretta CM, Hill DL. Development of the taste system: basic neurobiology. In: Handbook of Clinical Olfaction and Gustation, edited by Doty RL. New York: Marcel Dekker, 1995, p. 635–668 [Google Scholar]
- 35.Mistretta CM, Hill DL. Development of the taste system: basic neurobiology. In: Handbook of Olfaction and Gustation, edited by Doty RL. New York: Marcel Dekker, 2003, p. 759–782 [Google Scholar]
- 36.Oakley B. Altered temperature and taste responses from cross-regenerated sensory nerves in the rat's tongue. J Physiol 188: 353–371, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakley B. Reformation of taste buds by crossed sensory nerves in the rat's tongue. Acta Physiol Scand 79: 88–94, 1970 [DOI] [PubMed] [Google Scholar]
- 38.Palmer LG, Li JH, Lindemann B, Edelman IS. Aldosterone control of the density of sodium channels in the toad urinary bladder. J Membr Biol 64: 91–102, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Phillips LM, Hill DL. Novel regulation of peripheral gustatory function by the immune system. Am J Physiol Regul Integr Comp Physiol 271: R857–R862, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Rickard A, Young M. Corticosteroid receptors, macrophages and cardiovascular disease. J Mol Endocrinol 42: 449–459, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 283: H1802–H1810, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Schulkin J. Sodium Hunger: The Search for a Salty Taste New York: Cambridge University Press, 1991 [Google Scholar]
- 43.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol 161: 1773–1781, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tostes RC, Touyz RM, He G, Chen X, Schiffrin EL. Contribution of endothelin-1 to renal activator protein-1 activation and macrophage infiltration in aldosterone-induced hypertension. Clin Sci (Lond) 103Suppl 48: 25S–30S, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Van Driessche W, Zeiske W. Ionic channels in epithelial cell membranes. Physiol Rev 65: 833–903, 1985 [DOI] [PubMed] [Google Scholar]
- 46.Wall PL, McCluskey LP. Rapid changes in gustatory function induced by contralateral nerve injury and sodium depletion. Chem Senses 33: 125–135, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Whitehead MC, Frank ME, Hettinger TP, Hou LT, Nah HD. Persistence of taste buds in denervated fungiform papillae. Brain Res 405: 192–195, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Will PC, DeLisle RC, Cortright RN, Hopfer U. Induction of amiloride-sensitive sodium transport in the intestines by adrenal steroids. Ann NY Acad Sci 372: 64–78, 1981 [DOI] [PubMed] [Google Scholar]
- 49.Will PC, Lebowitz JL, Hopfer U. Induction of amiloride-sensitive sodium transport in the rat colon by mineralocorticoids. Am J Physiol Renal Fluid Electrolyte Physiol 238: F261–F268, 1980 [DOI] [PubMed] [Google Scholar]
- 50.Ye Q, Heck GL, DeSimone JA. Voltage dependence of the rat chorda tympani response to Na+ salts: implications for the functional organization of taste receptor cells. J Neurophysiol 70: 167–178, 1993 [DOI] [PubMed] [Google Scholar]