Abstract
The 13-lined ground squirrel (Ictidomys tridecemlineatus), a hibernating species, is a natural model of physiological adaption to an extreme environment. During torpor, body temperature drops to 0–4°C, and the cortex is electrically silent, yet the brain stem continues to regulate cardiorespiratory function. The mechanisms underlying selective inhibition in the brain during torpor are not known. To test whether altered GABAergic function is involved in regional and seasonal differences in neuronal activity, cortical and medullary slices from summer-active (SA) and interbout aroused (IBA) squirrels were placed in a standard in vitro recording chamber. Silicon multichannel electrodes were placed in cortex, ventral respiratory column (VRC), and nucleus tractus solitarius (NTS) to record spontaneous neuronal activity. In slices from IBA squirrels, bath-applied pentobarbital sodium (300 μM) nearly abolished cortical neuronal activity, but VRC and NTS neuronal activity was unaltered. In contrast, pentobarbital sodium (300 μM) nearly abolished all spontaneous cortical, VRC, and NTS neuronal activity in slices from SA squirrels. Muscimol (20 μM; GABAA receptor agonist) abolished all neuronal activity in cortical and medullary slices from both IBA and SA squirrels, thereby demonstrating the presence of functional GABAA receptors. Pretreatment of cortical slices from IBA squirrels with bicuculline (100 μM; GABAA receptor antagonist) blocked pentobarbital-dependent inhibition of spontaneous neuronal activity. We hypothesize that GABAA receptors undergo a seasonal modification in subunit composition, such that cardiorespiratory neurons are uniquely unaffected by surges of an endogenous positive allosteric modulator.
Keywords: γ-aminobutyric acid receptors, ventral respiratory groups, nucleus tractus solitarius, respiratory control
during winter hibernating, mammals enter a state of torpor, defined by dramatically reduced metabolic and physical activity and low body temperature (Tb). The torpid state is interrupted periodically by interbout arousals to euthermia (Tb = 37°C) that generally last less than 24 h (3, 8). During torpor bouts, Tb drops to just above ambient temperature and can approach 0°C, and respiration and heart rate drop as low as 1% of euthermic values (39, 71). Neurons in the cortex, hippocampus, and thalamus are electrically silent in torpid hibernators (14, 67). Despite evidence for global forebrain depression, torpid hibernators maintain a robust, neuronally regulated cardiorespiratory output (37, 25, 41), suggesting that respiratory- and cardiovascular-related neurons in the brain stem remain active. The mechanisms that selectively depress the forebrain during hibernation are not well understood and may contribute to hibernators' unique ability to survive ischemia, hypovolemia, and hypothermia (5, 12, 31, 34, 66).
While euthanizing aroused hibernators for an unrelated study, we noted that pentobarbital sodium, an allosteric modulator of gamma aminobutyric acid type A (GABAA) receptors, when used at doses sufficient for euthanasia, rapidly immobilized animals yet had no observable effect on respiratory rhythm. Subsequently, we began to investigate the role of GABA and GABAergic synaptic transmission in respiratory control during hibernation. GABA is the primary inhibitory neurotransmitter in the brain, so increased GABA receptor activation is a logical candidate for forebrain neuronal depression during torpor. Activation of synaptic GABAA receptors produces acute, phasic inhibition, while extrasynaptic GABAA receptors modulate general network excitability via tonic inhibition through constant activation by ambient GABA (14, 43). Although GABAA receptors involved in phasic and tonic inhibition are activated by GABA at micromolar concentrations, tonic GABAA receptors may be particularly sensitive to both GABA and allosteric modulators of the receptor, such as barbiturates, benzodiazepines, ethanol, and neurosteroids (9, 43, 64). Allosteric modulators increase or decrease the flow of chloride ions through GABAA receptors via actions at sites distinct from the GABA binding site. Pentobarbital sodium, a positive allosteric modulator of GABAA receptors often used for euthanasia, rapidly induces respiratory depression and sensorimotor loss in mammals at high doses (69), yet the expression of a rare subunit yields a GABAA receptor insensitive to pentobarbital sodium (23). Allosteric modulation of GABAA receptors provides the capacity for selective activation of GABAA receptors in different brain regions. Accordingly, we hypothesized that the cortex of hibernating squirrels expresses GABAA receptors that are sensitive to positive allosteric modulators of GABAA receptors, such as pentobarbital, whereas respiratory- and cardiovascular-related neurons in the medulla increase expression of GABAA receptors that are insensitive to allosteric positive modulators.
To address these hypotheses, multichannel recordings of spontaneous neuronal activity were performed in vitro in medullary and cortical brain slices from 13-lined ground squirrels (Ictidomys tridecemlineatus). In medullary slices, neuronal activity in the ventral respiratory column (VRC) and the nucleus tractus solitarius (NTS) was measured before and after bath application of pentobarbital. The VRC is an elongated rostrocaudal column of respiratory-related neurons that contains the Bötzinger region, pre-Bötzinger complex (preBötC), and rostral and caudal ventral respiratory groups (rVRG and cVRG; 40). The NTS receives sensory afferent inputs from chemoreceptors and baroreceptors, as well as lung mechanoreceptors (2, 30). In our experiments, VRC neurons were sampled from a rostrocaudal area that contained all of these groups except the Bötzinger region, and NTS neurons were sampled from medial, ventromedial, and ventral NTS (Fig. 1). To test for regional differences in allosteric modulation of GABAA receptors, neuronal activity was also recorded in the primary motor and somatosensory regions of cortical slices (Fig. 1). To test for seasonal differences, recordings were performed in slices isolated from hibernating and summer-active (SA) ground squirrels. To rule out temperature-dependent effects, slices from hibernating squirrels were taken during interbout arousals (IBA), when squirrel Tb is similar to that of SA squirrels. Preliminary results were published in abstract form (17–19).
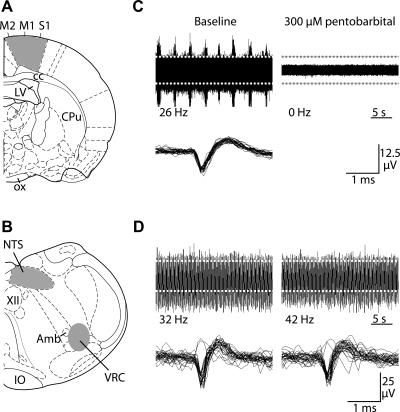
Fig. 1.
Multichannel recordings of spontaneous neuronal activity in cortical and medullary slices from interbout aroused (IBA) ground squirrels. A: cortical activity was recorded from neurons in the primary motor (M1), supplementary motor (M2), and primary somatosensory (S1) areas. Slices were cut approximately −1.30 mm caudal to bregma [anatomical images adapted from Paxinos and Watson (50)]. Shaded areas indicate electrode placement. B: medullary activity was recorded from the nucleus tractus solitarius (NTS) and ventral respiratory column (VRC). Slices were cut approximately −13.68 mm caudal to bregma. Shaded areas indicate electrode placement. C: representative cortical recordings are shown during baseline (top left) and after 60 min of pentobarbital sodium (300 μM) treatment (top right). Traces that cross the detection threshold (dashed white lines) are overlaid (bottom right). D: representative recordings from VRC neurons are shown during baseline (top left) and after 60 min of sodium pentobarbital (300 μM, top right). Traces that cross the detection threshold (dashed gray lines) are overlaid (bottom right).
METHODS
Experimental animals.
All experimental procedures were in accordance with the National Institutes of Health's guidelines and approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Thirteen-lined ground squirrels (I. tridecemlineatus) were trapped in and around Madison, WI, between May and September. Animals were housed individually with access to food and water ad libitum. From May through September, animals were maintained at an ambient temperature of 22°C with a 12:12-h light-dark cycle. In September through February, animals were housed in a dark room maintained at 4°C to facilitate hibernation. Food and water were removed after ∼2 wk of torpor/arousal cycles. All hibernating animals completed at least four full torpor bouts prior to being used in experiments. Torpor bouts were monitored daily by the sawdust method (4). Experiments were conducted during naturally occurring interbout arousals (n = 37 squirrels; November to February) or during the SA months (n = 15 animals; May to July). Tb of IBA hibernators was between 35°C and 38°C and SA animals between 36°C and 38°C.
Experimental preparation.
For electrophysiological recordings, squirrels were deeply anesthetized with 5% isoflurane and decapitated. Brains were removed and placed in cold artificial cerebrospinal fluid (aCSF). Medullary and cortical slices (350 μm thick) were cut with a vibrating microtome (Campden Instruments, Layfayette, IN). Cortical slices contained primary motor and primary somatosensory areas (Fig. 1A), while medullary slices contained the NTS and VRC (Fig. 1B). Slices were placed into an interface recording chamber (Warner Instruments, Hamden, CT) and subfused with warm aCSF at a rate of 8 ml/min. Slices were maintained at 37°C (Harvard Apparatus, Holliston, MA). Humidified gas (95% O2-5% CO O2) was blown across the top of the slices. The composition of the aCSF was (in mM) 120 NaCl, 26 NaHCO3, 20 glucose, 2 MgSO4, 1.0 CaCl2, and 1.25 Na2HPO4. To increase the yield of spontaneously active neurons, aCSF containing 9 mM KCl was used in most experiments.
Experimental protocol.
Recordings were made simultaneously from medullary and cortical slices taken from the same animal. One silicon 16-channel extracellular electrode (model a 4 × 4–3 mm 100–177, Neuronexus, Ann Arbor, MI) was placed in the VRC, one in the NTS, and two near the midline of the cortical slice, such that all six cortical layers were spanned (Fig. 1, A and B). Slices were allowed to equilibrate for 120 min. Thereafter, baseline activity was recorded for 60 min followed by application of pentobarbital sodium (150–300 μM), muscimol (20 μM, GABAA receptor agonist), or bicuculline (100 μM, GABAA receptor antagonist). Pentobarbital was applied to test properties of allosteric modulation of the GABAA receptor. Muscimol was applied to test for the presence and normal function of GABAA receptors. Bicuculline was applied to confirm that pentobarbital effects depended on GABAA receptors. Raw data signals were digitized at 25 kHz (Medusa PreAmp, Tucker-Davis Technologies, Alachua, FL) and sent by fiber-optic link to a digital signal processor (Pentusa Base Station, Tucker-Davis Technologies). Data were initially recorded and displayed by custom software programmed in MATLAB (v. 2006a, The MathWorks, Natick, MA).
Data analysis.
Individual neurons were identified and separated on the basis of their spike waveform shapes using principal component analysis (1). To group waveforms associated with an individual neuron, all waveforms were projected into the three-dimensional space spanned by the three eigenvectors with the largest associated eigenvalues. The KlustaKwik unsupervised clustering algorithm (16) was used to identify waveform clusters, assumed to correspond to individual neurons. Neuronal activity was averaged in 1.0-min bins throughout each experiment and normalized to the mean firing rate during a 60-min baseline recording prior to drug application. Individual neurons that were recorded on multiple, adjacent channels were counted only once. Neurons were discarded from analysis if their mean baseline firing rate were ≤0.01 Hz or if neurons were silent for >10 min preceding drug application. Individual bins were discarded if the absolute firing rate were >500 Hz or if the normalized firing rate increased and then decreased more than 100 standard deviations from the baseline mean in <3 min. By these criteria, only 4.7% of neurons and 0.0018% of data bins were discarded.
Statistics.
To compensate for possible artifacts due to drift, given the long duration of recordings, results from recordings of drug experiments were compared with equivalent time points in control experiments in which no drugs were applied (henceforth termed “time controls”). For statistical comparison, the last 5 min of neuronal activity during treatment were averaged across all neurons within a region and condition and compared with the last 5 min of neuronal activity in analogous time-control recordings. Group means were analyzed with a two-tailed Student's t-test, or ANOVA, where three or more groups were compared. Where appropriate, a post hoc Bonferroni analysis was used to identify differences between groups (Statistical Package for the Social Sciences, Chicago, IL). Differences were considered significant if P < 0.05. In statistical tests and calculation of SE, the total number of neurons per condition was used as the number of independent samples. All data reported are means ± SE.
RESULTS
Pentobarbital-dependent alterations in cortical and medullary neuronal firing rates.
In time-control experiments, neuronal activity in cortical and medullary slices was measured in normal aCSF. After 2 h, in slices from SA squirrels (n = 5), the mean normalized firing rate for cortical (n = 39), VRC (n = 20), and NTS (n = 37) neurons was 100 ± 26%, 137 ± 19%, and 120 ± 20% of baseline, respectively. Likewise, after 2 h, in slices from IBA squirrels (n = 4), the mean normalized firing rate for cortical (n = 22), VRC (n = 40), and NTS (n = 12) neurons was 107 ± 14%, 132 ± 15%, and 115 ± 14% of baseline, respectively. Thus, despite a trend for an increased firing rate in VRC neurons, there were no significant time-dependent changes in spontaneous firing. There were no significant differences between regions or seasons in time-control recordings (P > 0.05). Likewise, there were no significant differences between males (n = 3) and females (n = 4) in response to pentobarbital application (P > 0.05).
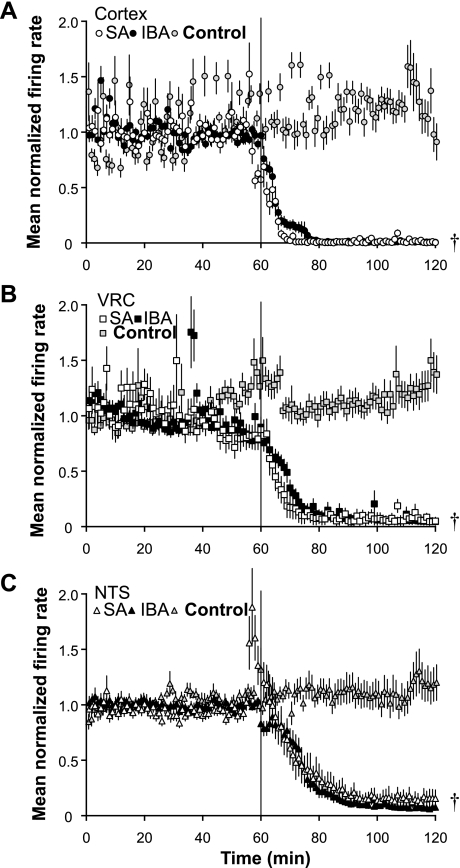
Changes in spontaneous neuronal firing rate induced by bath-applied pentobarbital (300 μM) differed by region (cortex vs. VRC vs. NTS) and by season (SA vs. IBA). In slices from SA animals (n = 6), pentobarbital reduced the firing rate of cortical (n = 77), VRC (n = 24), and NTS (n = 52) neurons to 18 ± 7%, 24 ± 9%, and 43 ± 7% of baseline, respectively, after a 60-min drug exposure (Fig. 2; P < 0.001 for all comparisons between SA time controls and treatment). In slices from IBA animals (n = 7), pentobarbital sodium reduced the firing rate of cortical (n = 79) neurons to 11 ± 1% after a 60-min drug exposure (P < 0.001 compared with time-controls; Fig. 2A). In contrast, the mean normalized firing rate of VRC (n = 54) and NTS (n = 37) neurons was 137 ± 20% (P > 0.05) and 70 ± 13% (P > 0.05) of baseline, respectively, after a 60-min drug exposure (Figs. 2, B and C).
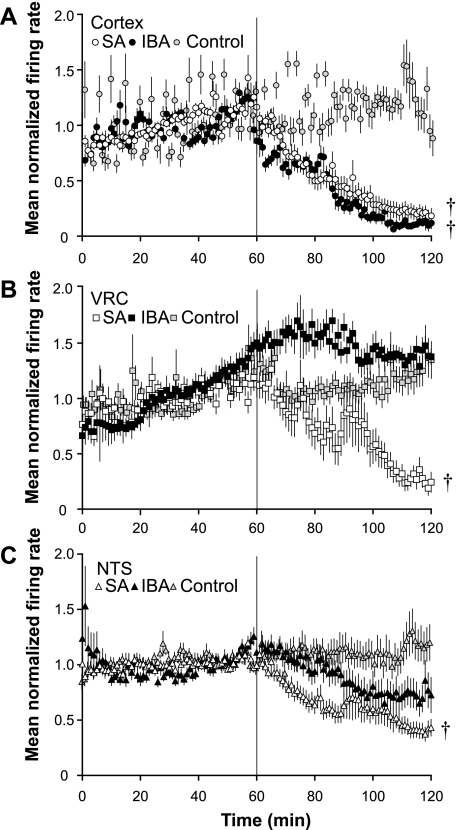
Fig. 2.
Pentobarbital sodium alters spontaneous activity of NTS, VRC, and cortical neurons. A: mean normalized firing rate is shown for cortical neurons in slices from summer-active (SA) squirrels (n = 6; n = 77 neurons; open circles) and IBA squirrels (n = 6; n = 79 neurons, solid circles) in response to 300 μM pentobarbital sodium (applied at vertical line). At 120 min, neuronal activity from both SA and IBA squirrels was decreased significantly compared with time control (P < 0.001). IBA time controls are shown (n = 4; n = 22 neurons; gray circles). B: mean normalized firing rate is shown for VRC neurons in slices from SA squirrels (n = 6; n = 21 neurons; open squares) and IBA squirrels (n = 7; n = 54 neurons; solid squares). At 120 min, SA neuron activity was decreased compared with time controls (P < 0.001), while IBA neuron activity was not different from time controls (P > 0.05) IBA time controls are shown (n = 4; n = 40 neurons; gray squares). C: mean normalized firing rate is shown for NTS neurons in slices from SA squirrels (n = 6; n = 50 neurons, open triangles) and IBA squirrels (n = 7; n = 37 neurons; solid triangles). At 120 min, SA neuron activity was decreased compared with time controls (P < 0.01), while IBA neuron activity did not differ from time controls (P = 0.091). IBA time controls are shown (n = 4; n = 12 neurons; gray triangles). Error bars indicate means ± SE. †P < 0.05 compared with time controls.
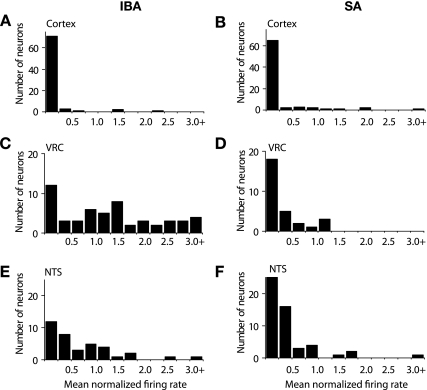
The distribution of mean normalized firing rates after the 60-min pentobarbital sodium exposure for cortical, VRC, and NTS neurons in slices from IBA and SA squirrels is shown in Fig. 3. Most mean normalized firing rates for cortical neurons were less than 0.25 of baseline in slices from IBA (71/78 neurons <0.25) and SA squirrels (65/77 neurons <0.25) (Fig. 3, A and B). For VRC neurons in slices from IBA squirrels, mean normalized firing rates were distributed throughout the range of 0.25 to >3.0 (Fig. 3C). For VRC neurons in slices from SA squirrels, mean normalized firing rates were mostly less than 0.25 (18/29 neurons <0.25) with 11/29 neurons distributed between 0.25 and 1.25; no neurons were found above 1.25 (Fig. 3D). For NTS neurons in slices from IBA squirrels, mean normalized firing rates in 23/37 neurons were distributed between 0.25 and 1.75 with one neuron >2.5 (Fig. 3E). For NTS neurons in slices from SA squirrels, mean normalized firings in 41/52 neurons were less than 0.5 with 10/52 neurons distributed between 0.5 and 1.75 (Fig. 3F).
Fig. 3.
Variability in neuronal activity at 60 min following bath-applied pentobarbital sodium. The activity of single neurons was normalized to the mean baseline activity for that neuron. Neurons were binned according to their normalized firing rates during the last 5 min of pentobarbital sodium (300 μM) application. A and B: number of neurons vs. mean normalized firing rate is shown for cortical neurons recorded in slices from IBA (n = 6; n = 79 neurons) and SA squirrels (n = 6; n = 77 neurons). C and D: number of neurons is shown for VRC neurons in brain stem slices from IBA (n = 7; n = 54 neurons) and SA squirrels (n = 6; n = 21 neurons). E and F: number of neurons is shown for NTS neurons in brain stem slices from IBA (n = 7; n = 37 neurons) and SA squirrels (n = 6; n = 21 neurons).
Pentobarbital-dependent alterations in neuronal firing rates are similar at lower [KCl].
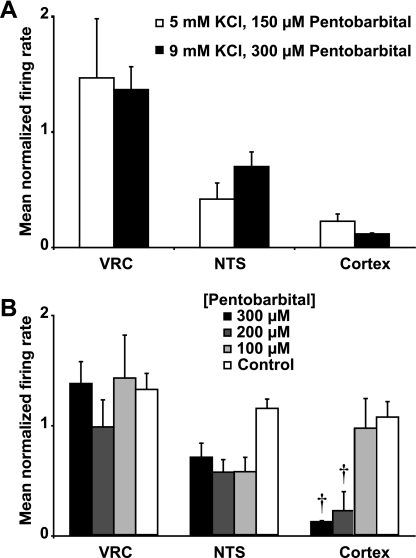
In the previous experiments, the [KCl] in the aCSF was 9.0 mM to increase the number of actively firing neurons and increase their spontaneous firing rate. To test whether similar results could be obtained at physiological [KCl], cortical and medullary slices from IBA squirrels (n = 4) were exposed to pentobarbital sodium (150 μM) for 60 min with aCSF [KCl] at 5.0 mM. The lower concentration of pentobarbital was used to compensate for the decreased level of excitation in the tissue associated with 5.0 mM KCl. Similar to the responses observed at 9.0 mM KCl, pentobarbital reduced the mean normalized firing rate of cortical neurons (n = 64) to 21 ± 6% after a 60-min drug exposure (P > 0.05 compared with firing rate at 9.0 mM [KCl]; Fig. 4A). In contrast, the mean normalized firing rate of VRC (n = 25) and NTS (n = 44) neurons was 147 ± 51% (P > 0.05) and 38 ± 13% (P > 0.05) of baseline, respectively, after a 60-min drug exposure (Fig. 4A). These results suggest that medullary insensitivity to pentobarbital during hibernation is observed at a more physiologically relevant [KCl].
Fig. 4.
Pentobarbital sodium produces similar effects in lower bath [KCl]. A: responses are shown for VRC, NTS, and cortical neurons in slices from IBA squirrels exposed to 5 mM KCl and 150 μM pentobarbital sodium (open bars) compared with 9 mM KCl and 300 μM pentobarbital sodium (solid bars). The data show the mean normalized firing rate during the last 5 min of a 60-min drug application. B: dose-dependent effects of sodium pentobarbital on spontaneous activity of VRC, NTS, and cortical neurons in slices from IBA squirrels are shown. The pentobarbital sodium concentrations that were tested include 300 μM (solid bars), 200 μM (dark gray bars), and 100 μM (light gray bars). IBA time controls (open bars) are shown. Spontaneous activity was unaltered at all three concentrations in VRC neurons (300 μM, n = 54; 200 μM, n = 48; 100 μM, n = 53; time control, n = 40) and NTS neurons (300 μM, n = 29; 200 μM, n = 14; 100 μM, n = 13; time control, n = 12). In contrast, spontaneous activity in cortical neurons was unaltered at 100 μM (n = 46), decreased by 78 ± 6% at 200 μM (n = 43; †P < 0.001) and decreased by 89 ± 1% at 300 μM (n = 79; †P < 0.001) compared with time controls (n = 22). The mean normalized firing rate represents the average during the last 5 min of a 60-min drug application. Error bars indicate means ± SE.
Pentobarbital-dependent alterations are not dose-dependent in VRC and NTS.
Because of pentobarbital's potential for non-GABAergic actions, we sought to determine whether the insensitivity of medullary neurons was dose dependent. To test whether VRC and NTS neuron resistance to pentobarbital was dose-dependent, pentobarbital (100 or 200 μM) was applied for 60-min to cortical and medullary slices from IBA squirrels (n = 3 squirrels for each concentration). The mean normalized firing rate for VRC and NTS neurons was unaltered, while pentobarbital-dependent depression of cortical neuron firing was dose dependent. For VRC neurons, the mean normalized firing rate was 98 ± 25% (n = 48; P > 0.05) and 142 ± 39% (n = 53; P > 0.05) of baseline after exposure to 200 and 100 μM pentobarbital, respectively (Fig. 4B). Likewise, for NTS neurons, the mean normalized firing rate was 57 ± 11% (n = 14; P > 0.05) and 57 ± 13% (n = 13; P > 0.05) of baseline, respectively (Fig. 4B). An ANOVA revealed a significant difference when the control traces and dose responses were compared (P < 0.05), yet the post hoc analysis indicated no significant individual differences. For cortical neurons, the mean normalized firing rate was 97 ± 20% (n = 46; P > 0.05) when exposed to 100 μM but reduced to 22 ± 6% (n = 43; P < 0.001) when exposed to 200 μM (Fig. 4B). Statistical comparisons are made between a given value and the corresponding time control. These results suggest that VRC and NTS neurons do not respond to any of the tested doses, while cortical neurons exhibited dose-dependent effects of pentobarbital.
GABAA receptor activation with muscimol depresses cortical, VRC, and NTS neurons.
To determine whether the inability of pentobarbital to affect neuronal activity in the VRC during hibernation could be explained by a lack of GABAA receptors, we applied muscimol, a direct agonist of the GABAA receptor. In cortical and medullary slices from IBA (n = 4) and SA squirrels (n = 4), bath application of muscimol (20 μM; 60-min exposure) nearly abolished spontaneous neuronal firing (Fig. 5). In slices from SA squirrels, muscimol reduced the mean normalized firing rate to 0.01 ± 0.2%, 6 ± 3%, and 14 ± 7% in cortical (n = 52), VRC (n = 12), and NTS (n = 48) neurons, respectively (P < 0.001 for all three compared with time-controls). Similarly, in slices from IBA squirrels, muscimol reduced the mean normalized firing rate to 0.01 ± 0.2%, 6 ± 3%, and 6 ± 2% in cortical (n = 73), VRC (n = 41), and NTS (n = 54) neurons, respectively. These data demonstrate that functional GABAA receptors are present in the VRC of ground squirrels during summer and hibernation.
Fig. 5.
Muscimol nearly abolishes spontaneous activity in cortical, VRC, and NTS neurons. A: mean normalized firing rate is shown for cortical neurons in slices from SA (n = 4; n = 52 neurons; open circles) and IBA squirrels (n = 4; n = 73 neurons, solid circles) in response to 20 μM muscimol (applied at vertical line). IBA time controls are shown (n = 4; n = 22 neurons, gray circles). B: mean normalized firing rate is shown for VRC neurons in slices from SA (n = 4; n = 12 neurons; open squares) and IBA squirrels (n = 4; n = 54 neurons; solid squares). IBA time controls are shown (n = 4; n = 40 neurons, gray squares). C: mean normalized firing rate is shown for NTS neurons in slices from SA (n = 4; n = 48 neurons, open triangles) and IBA squirrels (n = 4; n = 41 neurons; solid triangles). IBA time controls are shown (n = 4; n = 12 neurons, gray triangles). After a 60-min drug application, neuronal activity was nearly abolished in all neurons (†P < 0.05). Error bars indicate means ± SE.
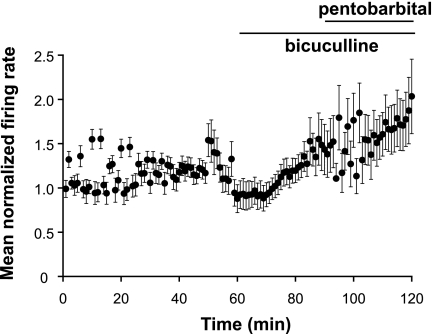
Because pentobarbital, like other barbiturates, is known to have non-GABAergic actions at high concentrations (70), we pretreated slices with bicuculline to determine the contribution of GABAA receptors in the cortical response to pentobarbital during hibernation. Bicuculline (100 μM; GABAA receptor antagonist) was bath applied for 30 min prior to simultaneous bath application of bicuculline (100 μM) and pentobarbital (300 μM) for 30 min to cortical slices from IBA squirrels (n = 4) (Fig. 6). The mean normalized firing rate in cortical neurons (n = 22) was not significantly increased at 145 ± 24% of baseline (P > 0.05) by bicuculline alone, and increased, not significantly, to 182 ± 33% of baseline (P > 0.05) with bicuculline and pentobarbital coapplication. Similarly, in slices from IBA squirrels (n = 2), the mean normalized firing rate of NTS neurons (n = 12) was 121 ± 23% of baseline (P > 0.05) with application of bicuculline alone, and 119 ± 25% of baseline (P > 0.05) with bicuculline and pentobarbital (data not shown). These data suggest that the observable actions of pentobarbital on the spontaneous neuronal activity in the cortex and NTS were exclusively at GABAA receptors.
Fig. 6.
Sodium pentobarbital effects in cortical neurons are blocked by bicuculline. In cortical slices from IBA squirrels (n = 4), mean normalized firing rate in 21 neurons was not altered by a 30-min exposure to bicuculline (100 μM). When bicuculline (100 μM) and pentobarbital sodium (300 μM) were bath applied simultaneously during the next 30 min, spontaneous activity in cortical neurons did not differ from time controls (P > 0.05) and was significantly greater than pentobarbital-treated cortical neurons without bicuculline pretreatment (P < 0.001; see Fig. 2A, solid circles). Error bars indicate means ± SE.
DISCUSSION
In this study, we found that during hibernation, aroused ground squirrels express pentobarbital-insensitive GABAA receptors on medullary neurons. In contrast, cortical neurons are highly sensitive to pentobarbital modulation throughout the year. Since medullary neurons in SA squirrels are pentobarbital sensitive, it appears that allosteric modulation of GABAA receptors is seasonally altered, while direct GABA-dependent activation is unchanged. To our knowledge, this represents the first demonstration of naturally occurring barbiturate-insensitive GABAA receptors in mammals. Our results suggest that medullary GABAA receptor plasticity may play a critical role in preserving cardiorespiratory function during the extreme changes in arousal state that define mammalian hibernation.
Relationship of GABAA receptor activation to neuronal firing.
In this study, we made extracellular multiunit recordings of neuronal activity in response to bath-applied pentobarbital. GABAA receptor-dependent currents in single neurons were not measured directly. Spontaneous neuronal activity is determined by factors such as membrane potential, intrinsic membrane properties, ionic conductances, and synaptic inputs. In addition, we cannot rule out that some of our results were due to complex network interactions (e.g., a neuron expressing GABAA receptors that inhibits a non-GABAergic neuron that inhibits a VRC neuron). However, GABAA receptors are expressed in VRC and NTS neurons (72, 73), and consistent effects with muscimol were observed in hundreds of neurons in thin slices that limit extensive synaptic connectivity. Thus, it is probable that most of the observed effects of GABAergic drugs on neuronal activity were due to interaction with GABAA receptors expressed on VRC, NTS, and cortical neurons.
High concentrations of pentobarbital (100–1,000 μM) can inhibit non-GABAergic receptors and block some ionic conductances in a manner that could reduce spontaneous neuronal activity (70). For example, pentobarbital inhibits AMPA receptors (27), suppresses sodium channels (68), and opens potassium channels (11). To rule out any non-GABAergic effects of pentobarbital in our studies, cortical slices were pretreated with bicuculline. In the presence of the antagonist, neurons were not responsive to subsequent bath-applied pentobarbital, suggesting that the inhibitory effects of sodium pentobarbital were due to GABAA receptor activation (Fig. 6).
GABAergic function during high vs. normal bath [KCl].
Most recordings of spontaneous neuronal activity in slices were performed with the aCSF potassium at 9 mM, while serum and CSF potassium levels in rodents are typically ∼3.0 mM (44). Although CSF potassium levels in hibernating and SA squirrels are not known, our experimental conditions at 9 mM KCl are likely hyperkalemic. Accordingly, intrinsic membrane properties, neuronal firing properties, and synaptic interactions were altered in our slices and may have affected GABAA receptor responses to drugs. To address this potential limitation of our experimental design, similar experiments were performed with aCSF potassium at 5 mM (Fig. 4). The main findings from experiments at 9 and 5 mM were essentially equivalent, suggesting that GABAA receptor responses to pentobarbital are not dependent on extracellular potassium levels. The advantage of increasing aCSF potassium to 9 mM is the large increase in viable recordings from cortical and medullary slices.
Effects of temperature on drug delivery.
The effect of in vivo pentobarbital in hamsters varies depending on activity state, Tb, and route of administration (42). Survival time following a lethal dose of pentobarbital delivered intraperitoneally to torpid hamsters is greater compared with active (euthermic) hamsters, and survival in torpid animals is greater when the drug is administered by intraperitoneal compared with intracerebroventricular injection. However, survival time after intracerebroventricular injection is similar in torpid and artificially hypothermic hamsters, suggesting no differences in pentobarbital sensitivity between the torpid and deeply hypothermic states (42). In our study, we eliminated the effect of Tb on central nervous system (CNS) activity by using IBA hibernators, whose Tb was similar to those of SA animals. Similarly, seasonal effects on peripheral drug metabolism and delivery to the brain are absent in slice recordings, as the flow rate and drug concentrations are held constant. Under these conditions, differences between SA and IBA squirrels in pentobarbital sensitivity of cortical and medullary neurons were readily apparent.
Identity of recorded neurons.
The cell types within the VRC, NTS, and the cortex are heterogeneous (29, 24, 40). Because our recordings were performed in slices, we cannot correlate neuronal recordings and drug responses with specific cell types. However, because of the nature of multichannel probes, we recorded from large numbers of neurons in each region. In the cortex, nearly all neurons were silenced by pentobarbital in slices from IBA and SA squirrels. In the VRC of slices from IBA squirrels, the spontaneous firing rate of the majority of neurons was not decreased by pentobarbital. Thus, this heterogeneous population of neurons behaved very similarly. In contrast, the spontaneous firing rate of some neurons in NTS in slices from IBA squirrels was decreased, whereas others were unaffected by pentobarbital. Thus, it is possible that different cell types in the NTS respond differently to pentobarbital (e.g., cardiovascular vs. respiratory).
The heterogeneous population of neurons within the NTS may have contributed to the trend toward significant differences between control and all doses of pentobarbital tested (Fig. 4B). Although the ANOVA was significant (P = 0.029), a Bonferroni post hoc test revealed individual differences that did not attain significance. Additionally, there were relatively few neurons recorded for this dose response compared with the number of cells analyzed for the principle findings, which may have contributed to this effect.
Role of GABAA receptors in respiratory control.
GABAergic synaptic transmission in the VRC and NTS plays a critical role in respiratory motor control with respect to rhythm generation, pattern formation, and chemosensory responses. For example, “network models” for adult mammals postulate that respiratory rhythm generation is due to GABAA-dependent reciprocal inhibition between specific groups of respiratory-related VRC neurons (54, 58, 59). Likewise, GABAA receptors modulate the magnitude and timing of bulbospinal premotoneuron activity in the rVRG and cVRG (73). Tonic GABAA receptor-dependent inhibition constrains bulbospinal VRG neurons to within 35–50% of their maximal discharge rate, which provides a wide dynamic range for neuronal discharge and allows the respiratory control system to adapt to different physiological conditions (73). Finally, the hypoxic ventilatory response in adult mammals is biphasic with ventilation initially increasing, and then decreasing (termed “hypoxic ventilatory decline”; 52). During hypoxia, GABA is released in both the NTS (65) and VRC (57), consistent with the hypothesis that GABAA receptor activation in NTS and VRC is involved in the hypoxic ventilatory decline (65). Also, GABAA receptor activation modulates excitatory synaptic inputs onto NTS neurons (6, 33, 72), suggesting that GABAA receptors are involved in processing respiratory-related sensory afferent information in NTS during normoxia. Because arterial blood gases are well regulated in ground squirrels during hibernation (46), we hypothesize that sufficient GABAergic function in the respiratory control system is maintained during torpor.
Role of GABAergic system during hibernation.
Ligand-driven mechanisms controlling hibernation have been investigated for more than 30 years (22, 38, 48). Some of the proposed molecules include circulating hormones such as vasopressin (21), and neurotransmitters, such as serotonin (45), histamine (61), and GABA (49). The GABAergic system is a logical candidate for the induction and maintenance of system-wide CNS inhibition during torpor. However, the role of GABA in hibernation is unclear because GABA levels in torpid animals were reported to either increase by 135% in cortex using proton nuclear magnetic resonance spectroscopy (20), or decrease by 50% in the striatum using microdialysis (49). Because there is no observable neuronal activity in the forebrain at low temperatures during torpor (67, 13), it is unlikely that synaptically released GABA is playing a prominent role in neuronal silencing during torpor.
GABAA receptors can actively elicit long-lasting, rapidly reversible, tonic inhibition of neuronal activity via positive allosteric modulation (32). Barbiturates, such as pentobarbital, can bind to GABAA receptors at a site distinct from the GABA binding site and potentiate the effects of GABA (47, 62). It can also directly activate GABAA receptors (28, 47). Positive allosteric modulators can achieve neuronal selectivity by binding to some, but not all, GABAA receptors. Binding selectivity of allosteric modulators is determined by the specific isoforms of GABAA receptors (15). Our data suggest that during hibernation, medullary GABAA receptors with specific properties are inserted into neurons such that normal, ligand-activated function is maintained, while the response to allosteric modulators is altered.
One potential mechanism for seasonal regional selectivity is a change in the pentameric combination of subunits in a GABAA receptor. The predominant synaptic GABAA receptor isoform is composed of two α1, two β2, and a γ2 subunits. Upon deletion of the γ2 subunit and the inclusion of α4, α5, δ, or ε subunits, GABAA receptors have an altered affinity for GABA and allosteric modulators, such as pentobarbital (7, 10, 23, 53). We speculate that altered subunit stoichiometry provides a plausible mechanism for the pentobarbital insensitivity described here. Different GABAA receptor isoforms could permit active and inactive zones in the brain during hibernation in the face of a seasonally produced endogenous modulator of GABAergic function.
We speculate that this sustained change in medullary GABAergic function during hibernation may allow intermittent surges (e.g., at the end of an IBA period) of an endogenous pentobarbital-like ligand to silence forebrain neurons yet permit continued medullary regulation of cardiorespiratory function. Neurosteroids are pentobarbital-like ligands that may fulfill such a role. Allopregnanolone, a neurosteroid, is a metabolite of progesterone, can bind to GABAA receptors at a site distinct from the GABA-binding site and, like pentobarbital, can potentiate the effects of GABA, as well as directly activate the receptor (36). Preliminary data suggest that allopregnanolone is more effective than pentobarbital at inhibiting spontaneous neuronal activity in the cortex of IBA squirrels, and actually increases spontaneous activity in NTS and VRG neurons at the same dose (Hengen KB, Behan M, Johnson SM, unpublished observations). While allosteric GABAA modulation is a plausible mechanism for arousal-state control, other neurotransmitter systems may be involved, and clearly many other physiological systems contribute to the regulation of hibernation.
Perspectives and Significance
Hibernation is a unique physiological state that allows mammals to survive extreme conditions of cold, decreased metabolism, and decreased blood flow to tissues, but the underlying mechanisms are not well understood. One explanation is that torpor may be regulated, in part, by endogenous ligands with actions similar to pentobarbital that silence neuronal activity in the forebrain yet leave cardiorespiratory neurons unaffected. We hypothesize that 13-lined ground squirrels selectively express ε subunit-containing GABAA receptors in cardiorespiratory brain stem neurons during hibernation. The gene for the ε subunit, whose protein is associated with pentobarbital insensitivity, appears to be highly conserved in all mammals, but there is no evidence for its natural expression and physiological function. Seasonal expression of ε subunit-containing GABAA receptors on cardiorespiratory neurons may be an elegant and simple mechanism for conferring resistance to increased central concentrations of endogenous ligands that act as positive allosteric modulators of GABAA receptors.
Determining the hibernator's specific manipulation of GABAA receptors on respiratory neurons will promote exploration of related protection in nonhibernating mammals. Although interesting and worthy of study in its own right, understanding the mechanisms that allow tissues to survive torpor can also lead to novel biomedical applications, such as improved methods for organ preservation (35) and protection of the brain during ischemia or cardiac arrest (8). In this study, we characterized a natural, novel GABAA receptor phenotype, in which the GABAA receptor is fully functional but resistant to allosteric modulation. Further understanding of the mechanisms by which pentobarbital-resistant GABAA receptors are induced and controlled in the CNS may allow for the development of anesthetics that reduce cortical activity yet allow normal cardiorespiratory homeostatic mechanisms to function. An anesthetic with these properties could eliminate some of the major deleterious side effects of anesthetics in critically ill or aged patients.
GRANTS
This work was funded by a National Institute of Aging Grant (AG-18760) and a National Institute of Neurological Diseases and Stroke Grant (NS-051580). K. B. Hengen was supported by an National Institutes of Health Institutional Training Grant (T32 HL07654-21).
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance of Dr. Justin Williams and Mark Chapman.
REFERENCES
- 1.Adamos DA, Kosmidis EK, Theophilidis G. Performance evaluation of PCA-based spike sorting algorithms. Comput Methods Programs Biomed 91: 232–244, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bonham AC, Chen CY, Sekizawa S, Joad JP. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol 101: 322–327, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Carey HV, Martin SL. Preservation of intestinal gene expression during hibernation. Am J Physiol Gastrointest Liver Physiol 271: G805–G813, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Carey HV, Potter KT, Peters TL, Epperson LE, Rose JC, Martin SL. Hibernating mammals have enhanced survival and reduced gut damage after hemorrhage. FASEB J 20: Abstract no. 903.1, 2006 [Google Scholar]
- 6.Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptors neurones in rats. J Physiol 562: 535–551, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies PA, Kirkness EF, Hales TG. Evidence for the formation of functionally distinct alphabetagammaepsilon GABA(A) receptors. J Physiol 537: 101–113, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem 102: 1713–1726, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrant M, Nusser Z. Variations on and inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Feng HJ, Bianchi MT, Macdonald RL. Pentobarbital differentially modulates α1β3δ and α1β3γ2L GABAA receptor currents. Mol Pharmacol 66: 988–1003, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci 25: 601–608, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab 14: 193–205, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Gabriel A, Klussmann FW, Igelmund P. Rapid temperature changes induce adenosine-mediated depression of synaptic transmission in hippocampal slices from rats (non-hibernators) but not in slices from golden hamsters (hibernators). Neuroscience 86: 67–77, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 28: 1421–1426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56: 763–770, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Harris KD, Henze DA, Csicsvari J, Hirase Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Hengen KB, Johnson SM, Carey HV, Behan M. Neural control of cardiorespiratory function in ground squirrels during hibernation. FASEB J 21: Abstract no. 965.15, 2007 [Google Scholar]
- 18.Hengen KB, Johnson SM, Carey HV, Behan M. Functional and molecular partitioning of the brain provides neuroprotection to cardiorespiratory nuclei in ground squirrels during hibernation. FASEB J 22: Abstract no. 757.2, 2008 [Google Scholar]
- 19.Hengen KB, Johnson SM, Carey HV, Behan M. Seasonally expressed remodeling of GABAA receptors in the hibernating brain confers viability in the face of anesthetic overdose. Soc Neurosci Abstr 531.22, 2008 [Google Scholar]
- 20.Henry PG, Russeth KP, Tkac I, Drewes LR, Andrews MT, Gruetter R. Brain energy metabolism and neurotransmission at near-freezing temperatures: in vivo (1)H MRS study of a hibernating mammal. J Neurochem 101: 1505–1515, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hermes ML, Kalsbeek A, Kirsch R, Buijs RM, Pevet P. Induction of arousal in hibernating European hamsters (Cricetus cricetus L.) by vasopressin infusion in the lateral septum. Brain Res 631: 313–316, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Hong J, Sigg DC, Coles JA, Jr, Oeltgen PR, Harlow HJ, Soule CL, Iaizzo PA. Hibernation induction trigger reduces hypoxic damage of swine skeletal muscle. Muscle Nerve 32: 200–207, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Irnaten M, Walwyn WM, Wang J, Venkatesan P, Evans C, Chang KS, Andresen MC, Hales TG, Mendelowitz D. Pentobarbital enhances GABAergic neurotransmission to cardiac parasympathetic neurons, which is prevented by expression of GABA(A) epsilon subunit. Anesthesiology 97: 717–724, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Jean A. The nucleus tractus solitarius: neuroanatomic, neurochemical and functional aspects. Arch Int Physiol Biochim Biophys 99: A3–A52, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Johansen K, Krog J, Reite O. Autonomic nervous influence on the hearth of the hypothermic hibernator. Annals Acad Sci Fenn 71: 243–255, 1964 [Google Scholar]
- 26.Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Bötzinger complex “island”. J Neurophysiol 85: 1772–1776, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Joo DT, Xiong Z, MacDonald JF, Jia Z, Roder J, Sonner J, Orser BA. Blockade of glutamate receptors and barbiturate anesthesia: increased sensitivity to pentobarbital-induced anesthesia despite reduced inhibition of AMPA receptors in GluR2 null mutant mice. Anesthesiology 91: 1329–1341, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Krampfl K, Wolfes H, Dengler R, Bufler J. Kinetic analysis of the agonistic and blocking properties of pentobarbital on recombinant rat alpha(1)beta(2)gamma(2S) GABA(A) receptor channels. Eur J Pharmacol 435: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Kriegstein AR, Dichter MA. Morphological classification of rat cortical neurons in cell culture. J Neurosci 3: 1634–1647, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurtz CC, Lindell SL, Mangino MJ, Carey HV. Hibernation confers resistance to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 291: G895–G901, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol 71: 67–80, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Len WB, Chan JY. GABAergic neurotransmission at the nucleus tractus solitarii in the suppression of reflex bradycardia by parabrachial nucleus. Synapse 42: 27–39, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Lindell SL, Klahn SL, Piazza TM, Mangino MJ, Torrealba JR, Southard JH, Carey HV. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am J Physiol Gastrointest Liver Physiol 288: G473–G480, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Lindell SL, Compagnon P, Mangino MJ, Southard JH. UW solution for hypothermic machine perfusion of warm ischemic kidneys. Transplantation 79: 1358–1361, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Liu QY, Chang YH, Schaffner AE, Smith SV, Barker JL. Allopregnanolone activates GABAA receptor/Cl− channels in a multiphasic manner in embryonic rat hippocampal neurons. J Neurophysiol 88: 1147–1158, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Lyman CP, O'Brien RC. Autonomic control of circulation during the hibernation cycle in ground squirrels. J Physiol 168: 477–499, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margules DL, Goldman B, Finck A. Hibernation: an opioid-dependent state? Brain Res Bull 4: 721–724, 1979 [DOI] [PubMed] [Google Scholar]
- 39.McArthur MD, Milsom WK. Changes in ventilation and respiratory sensitivity associated with hibernation in Columbian (Spermophilus columbianus) and golden-mantled (Spermophilus lateralis) ground squirrels. Physiol Zool 64: 940–959, 1991 [Google Scholar]
- 40.McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Milsom WK, Zimmer MB, Harris MB. Vagal control of cardiorespiratory function in hibernation. Exp Physiol 86: 791–796, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Miyazawa S, Shiina T, Takewaki T, Shimizu Y. Extension of time until cardiac arrest after injection of a lethal dose of pentobarbital in the hibernating syrian hamster. J Vet Med Sci 71: 383–385, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA (A) receptors. Trends Neurosci 27: 569–575, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Moghaddam B, Adams RN. Regional differences in resting extracellular potassium levels of rat brain. Brain Res 406: 337–340, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Murakami N, Kono R, Nakahara K, Ida T, Kuroda H. Induction of unseasonable hibernation and involvement of serotonin in entrance into and maintenance of its hibernation of chipmunks T. asiaticus. J Vet Med Sci 62: 763–766, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Musacchia XJ, Volkert WA. Blood gases in hibernating and active ground squirrels: HbO2 affinity at 6 and 38°C. Am J Physiol 221: 128–130, 1971 [DOI] [PubMed] [Google Scholar]
- 47.Nicoll RA, Wojtowicz JM. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res 191: 225–237, 1980 [DOI] [PubMed] [Google Scholar]
- 48.Oeltgen PR, Welborn JR, Nuchols PA, Spurrier WA, Bruce DS, Su TP. Opioids and hibernation. II. Effects of κ opioid U69593 on induction of hibernation in summer-active ground squirrels by “hibernation induction trigger” (HIT). Life Sci 41: 2115–2120, 1987 [DOI] [PubMed] [Google Scholar]
- 49.Osborne PG, Hu Y, Covey DN, Barnes BN, Katz Z, Drew KL. Determination of striatal extracellular γ-aminobutyric acid in non-hibernating and hibernating arctic ground squirrels using quantitative microdialysis. Brain Res 839: 1–6, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). San Diego, CA: Elsevier Academic, 2007 [Google Scholar]
- 51.Potts JT. Neural circuits controlling cardiorespiratory responses: baroreceptors and somatic afferents in the nucleus tractus solitarius. Clin Exp Pharmacol Physiol 29: 103–111, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Rahman M, Zhu D, Lindblad C, Johansson IM, Holmberg E, Isaksson M, Taube M, Backstrom T, Wang MD. GABA-site antagonism and pentobarbital actions do not depend on the alpha-subunit type in the recombinant rat GABA receptor. Acta Physiol (Oxf) 187: 479–488, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Curr Opin Neurobiol 6: 817–825, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60: 385–405, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol 2: 788–793, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol 514: 567–578, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24: 464–72, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res 165: 201–220, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. II. Network models of the central respiratory pattern generator. J Neurophysiol 77: 2007–2026, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Sallmen T, Lozada AF, Beckman AL, Panula P. Intrahippocampal histamine delays arousal from hibernation. Brain Res 966: 317–320, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Schulz DW, Macdonald RL. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: correlation with anticonvulsant and anesthetic actions. Brain Res 209: 177–188, 1981 [DOI] [PubMed] [Google Scholar]
- 63.Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31: 15–24, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther 116: 58–76, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tabata M, Kurosawa H, Kikuchi Y, Hida W, Ogawa H, Okabe S, Tun Y, Hattori T, Shirato K. Role of GABA within the nucleus tractus solitarii in the hypoxic ventilatory decline of awake rats. Am J Physiol Regul Integr Comp Physiol 281: R1411–R1419, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Uchida K, Shibuya I, Doi K. Difference in the mode of acute cold-induced hypothermia between rat and hamster. Jpn J Physiol 37: 207–222, 1987 [DOI] [PubMed] [Google Scholar]
- 67.Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol Regul Integr Comp Physiol 233: R213–R221, 1977 [DOI] [PubMed] [Google Scholar]
- 68.Wartenberg HC, Urban BW, Duch DS. Distinct molecular sites of anaesthetic action: pentobarbital block of human brain sodium channels is alleviated by removal of fast inactivation. Br J Anaesth 82: 74–80, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Yamada KA, Moerschbaecher JM, Hamosh P, Gillis RA. Pentobarbital causes cardiorespiratory depression by interacting with a GABAergic system at the ventral surface of the medulla. J Pharmacol Exp Ther 226: 349–355, 1983 [PubMed] [Google Scholar]
- 70.Yamakura T, Sakimura K, Mishina M, Shimoji K. The sensitivity of AMPA-selective glutamate receptor channels to pentobarbital is determined by a single amino acid residue of the alpha 2 subunit. FEBS Lett 374: 412–414, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Zatzman ML. Renal and cardiovascular effects of hibernation and hypothermia. Cryobiology 21: 593–614, 1984 [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, Mifflin SW. Receptor subtype specific effects of GABA agonists on neurons receiving aortic depressor nerve inputs within the nucleus of the solitary tract. J Auton Nerv Syst 73: 170–181, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Zuperku EJ, McCrimmon DR. Gain modulation of respiratory neurons. Respir Physiol Neurobiol 131: 121–133, 2002 [DOI] [PubMed] [Google Scholar]