Abstract
Sepsis elicits severe alterations in cardiac function, impairing cardiac mitochondrial and pressure-generating capacity. Currently, there are no therapies to prevent sepsis-induced cardiac dysfunction. We tested the hypothesis that administration of a mitochondrially targeted antioxidant, 10-(6′-ubiquinonyl)-decyltriphenylphosphonium (MitoQ), would prevent endotoxin-induced reductions in cardiac mitochondrial and contractile function. Studies were performed on adult rodents (n = 52) given either saline, endotoxin (8 mg·kg−1·day−1), saline + MitoQ (500 μM), or both endotoxin and MitoQ. At 48 h animals were killed and hearts were removed for determination of either cardiac mitochondrial function (using polarography) or cardiac pressure generation (using the Langendorf technique). We found that endotoxin induced reductions in mitochondrial state 3 respiration rates, the respiratory control ratio, and ATP generation. Moreover, MitoQ administration prevented each of these endotoxin-induced abnormalities, P < 0.001. We also found that endotoxin produced reductions in cardiac pressure-generating capacity, reducing the systolic pressure-diastolic relationship. MitoQ also prevented endotoxin-induced reductions in cardiac pressure generation, P < 0.01. One potential link between mitochondrial and contractile dysfunction is caspase activation; we found that endotoxin increased cardiac levels of active caspases 9 and 3 (P < 0.001), while MitoQ prevented this increase (P < 0.01). These data demonstrate that MitoQ is a potent inhibitor of endotoxin-induced mitochondrial and cardiac abnormalities. We speculate that this agent may prove a novel therapy for sepsis-induced cardiac dysfunction.
Keywords: caspase, proteolysis
recent data demonstrate a strong association between the severity of alterations in mitochondrial function and an increased mortality in patients with sepsis (3). There are several potential mechanisms by which alterations in mitochondrial function could influence the development of organ dysfunction and death. First, reductions in mitochondrial function act to alter cellular energy metabolism, reducing the availability of high-energy phosphates and increasing the reliance upon nonoxidative mechanisms of high-energy phosphate generation (10). Theoretically, such alterations could lead to intracellular metabolic alterations that may compromise cellular function. Second, some forms of mitochondrial dysfunction can lead to generation of excess production of toxic reaction products (e.g., hydrogen peroxide, lipid peroxides) with the potential to produce widespread alterations in organelle function (11, 30). Finally, mitochondria play a major role in the induction of caspase activation via release of cytochrome c (21). Caspase activation, in turn, is now recognized as an important mediator of sepsis-induced cellular injury in a number of organs, including the heart, kidney, liver, and diaphragm (18, 27, 31, 32).
Taken together, these three processes (alterations in mitochondrial energy metabolism, mitochondrial release of toxins, mitochondrially triggered caspase activation) may interact to induce organ dysfunction, progressive multisystem failure, and ultimately, death. In theory, free radicals may play a pivotal role in the induction of all three of these mitochondrially linked processes. Free radicals can damage mitochondria directly (2), reducing ATP generation; can generate byproducts that leave mitochondria and produce toxic effects on other organelles (17); and are a known trigger of caspase activation and apop tosis (21). As a result, agents that reduce mitochondrial free radical generation may theoretically prevent organ failure.
On the basis of this logic, the present study was performed to determine whether administration of 10-(6′-ubiquinonyl)-decyltriphenylphosphonium (MitoQ), a powerful mitochondrially targeted antioxidant (29), could reduce the evolution of mitochondrial dysfunction and organ failure in sepsis. We focused this study on the effects of sepsis and MitoQ on cardiac function because this particular organ is known to fail with severe sepsis (23), to have significant sepsis-induced mitochondrial abnormalities (28), and to develop contractile dysfunction, partly because of caspase-mediated contractile protein cleavage (18). These experiments tested the specific hypotheses that: 1) endotoxin administration would elicit significant reductions in cardiac mitochondrial function, caspase activation, and reductions in cardiac pressure-generating capacity; 2) administration of MitoQ would largely prevent cardiac mitochondrial dysfunction and reductions in cardiac pressure-generating capacity; and 3) MitoQ would also ablate sepsis-induced increases in myocardial caspase activation.
METHODS
Experimental protocol.
Experiments were performed using rats (200–250 g body wt, n = 34 total) and mice (25–35 g body wt, n = 18). Both species were used because our techniques to assess mitochondrial performance are optimized for assessment of mouse hearts, while our Langendorf technique is optimized for measurements using rats. Approval for this work was granted by the Institutional Animal Care and Use Committee. Animals were given food and water ad libitum and housed in university facilities. Saline (60 mg·kg−1·day−1) was administered subcutaneously to maintain fluid volume status. Animals were sedated with pentobarbital (50 mg/kg ip) before euthanasia.
Four experimental groups were examined: 1) a control group given water orally and injected intraperitoneally with saline (0.3 ml) for 2 days, 2) animals injected intraperitoneally with endotoxin (Escherichia coli endotoxin from Sigma, St. Louis, MO) 8 mg·kg−1·day−1 for 2 days and given water orally, 3) a group given water containing 500 μM MitoQ and injected intraperitoneally with endotoxin 8 mg·kg−1·day−1 for 2 days, and 4) a group given water containing 500 μM MitoQ and injected intraperitoneally with saline for 2 days. MitoQ administration was begun on the day of endotoxin injection, so this experiment was designed to determine whether this drug could “prevent” cardiac dysfunction in response to endotoxin administration. The dosage of MitoQ was chosen based on preliminary experiments by M. P. Murphy demonstrating physiologic effects at this concentration in the study of nonmuscular tissues. At the end of 48 h after initial injections, animals were killed and hearts were removed. Excised hearts from mice (n = 4–5/group) were used to assess cardiac mitochondrial function, caspase activity, and OxyBlot determinations. Cardiac mitochondrial assessment consisted of determination of state 3/state 4 oxygen consumption polarographically, measurement of FCCP-coupled respiration, and measurement of NADH oxidase activity. Excised hearts from rats (n = 8–9/group) were used to either assess cardiac function via the Langendorf technique or were frozen immediately after excision, stored at −80°C, and later assayed for caspase activity and OxyBlot determinations.
Several experimental animal models can be used to examine the effects of sepsis on organ function. Overall, the animal model that is thought to provide the best representation of the human response to infectious agents is the cecal ligation perforation model of sepsis. For studying sepsis-induced cardiac dysfunction, however, many investigators employ the injection of endotoxin (LPS) since Natanson et al. (19) have shown that this latter model induces alterations in cardiac function that closely simulates the pattern observed in human patients with septic shock. For this reason, we chose to use endotoxin injection as a model of sepsis in the present study. It will be important, however, to test this agent in other animal models of sepsis to determine whether it also has beneficial effects on other organs and does not interfere with host defenses.
Assessment of states 3 and 4 mitochondrial respiration.
Mitochondria oxygen consumption was assessed using a Clark-type electrode (4). Hearts were first placed in cold isolation buffer (180 mM KCL, 5 mM MOPS, 2 mM EGTA, pH 7.25 at 4°C). After mincing finely with scissors, muscle pieces were homogenized for two, 7-s periods using a Polytron homogenizer set at one-half speed. A portion of this homogenate was assayed for protein content; the remaining homogenate was centrifuged at 600 g for 7.5 min at 4°C, and the pellet discarded. The supernatant was centrifuged at 5,000 g for 10 min at 4°C, and the resulting mitochondrial pellet was resuspended in isolation buffer. State 3 and state 4 mitochondrial oxygen respiration rates were then measured according to established procedures (4) using a Clark-type oxygen electrode (Instech, Plymouth Meeting, PA). For these assays, mitochondria were diluted to a protein concentration of 0.5 mg/ml in buffer (120 mM KCl, 5 mM KH2PO4, 5 mM MOPS, 1 mM EDTA, pH 7.25). State 2 respiration was initiated by adding pyruvate (10 mM)/malate (2.5 mM), and ADP (0.5 mM) was added to initiate state 3. The ADP-to-O ratio was determined from the total oxygen consumption elicited during state 3 respiration after administration of a known amount of ADP. The ATP production rate was calculated as the product of state 3 respiration rate times the ADP-to-O ratio.
FCCP respiration rates.
For this assay, an aliquot of mitochondria, isolated as described in the preceding paragraph was added to buffer (120 mM KCl, 5 mM KH2PO4, 5 mM MOPS, 1 mM EDTA, pH 7.25) in an Instech chamber containing a Clark electrode. Oxygen consumption rates were then determined after the addition of FCCP (10 nM).
Electron transport chain NADH oxidase assay.
The electron transport chain NADH oxidase assay was used to assess the respiration rate of isolated, uncoupled mitochondria in response to the addition of exogenous NADH; a reduction in this rate indicates inhibition of electron transport by the mitochondrial proteins (5). Mitochondrial isolates were placed in hypotonic respiration solution (20 mM KH2PO4, 0.1 mM EDTA, pH 7.25) to induce mitochondrial swelling (permitting access of exogenous NADH to the electron transport chain). We then added cytochrome c to a final concentration of 700 μM to compensate for any potential loss of cytochrome c from the intermembrane space. NADH (20 mM) was added to initiate respiration, and we measured NADH-stimulated oxygen consumption rates.
Assessment of cardiac pressure-generating capacity.
Pressure-generating capacity of hearts was assessed using the Langendorf technique (6). In brief, after pentobarbital anesthesia (50 mg/kg), the chest was opened and the heart removed with associated ascending aorta. The aortic root was quickly cannulated and the cannulated heart attached to a constant pressure temperature-controlled (37°C) Langendorf perfusion circuit (Radnoti Glass, Monrovia, CA). Aortic root pressure was adjusted to 80 mmHg and perfused with Krebs-Henselheit solution (pH 7.40, 135 mM NaCl, 5 mM KCl , 11.1 mM dextrose, 2.5 mM CaCl2, 1 mM MgSO4, 14.9 mM NaHCO3, 1 mM NaHPO4, 50 U/l insulin, gassed with 95% O2-5% CO2). A thin-walled balloon was inserted into the left ventricle via the left atrium and attached to a pressure transducer. Pacer wires were inserted into the right atrium. After a 15-min equilibration period, stepwise increments and decrements in left ventricular balloon diastolic pressure were made by infusing small amounts of saline into the balloon circuit while the heart was paced at 300 beats/min. Developed systolic pressures were recorded over a range of diastolic pressure from 10 to 30 mmHg. using a Gould strip chart recorder (Gould Electronics, Cleveland, OH).
Determination of caspase activity.
A modified BIOMOL assay (BIOMOL, Plymouth Meeting, PA) was employed to determine caspase activity for muscle homogenates (14). For this assay, muscle homogenate (100 μg protein) was added to assay buffer and a caspase-specific fluorogenic substrate. For caspase 3 activity determination we used a caspase 3-specific fluorogenic substrate, 30 μM N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC). To determine caspase 8 activity we used 30 μM Ac-IETD-AMC, and to determine caspase 9 activity we used 30 μM Ac-LEHD-AMC. Duplicate determinations were made with muscle homogenate, assay buffer, substrates, and specific caspase inhibitors for each sample. For inhibitors, we used 20 nM DEVD-CHO to inhibit caspase 3, 20 nM IETD-CHO to inhibit caspase 8, and 20 nM LEHD-CHO to inhibit caspase 9. For each set of measurements, immediately after substrate was added, a baseline fluorescent measurement of AMC was performed using a Molecular Devices spectrofluorophotometer (excitation frequency of 360 nm and emission frequency of 460 nm). This measurement was then repeated after 0.5 h of incubation at 30°C. AMC and caspase standards were used to quantitate activity levels.
Determination of OxyBlot density.
Prior to SDS-PAGE gel analysis, samples used for OxyBlot determination were prepared as per the manufacturers instructions (OxyBlot Kit; Chemicon, Temecula, CA). Derived protein samples for OxyBlot determinations were then diluted with an equal volume of a loading buffer (126 mM Tris·HCL, 20% glycerol, 4% SDS, 1.0% 2-mercaptoethanol, 0.005% bromphenyl blue, pH 6.8). Samples were then loaded onto 12% Tris glycine polyacrylamide gels, and proteins were separated by electrophoresis (Novex Minicell II). Standard molecular weight markers were also loaded onto gels to permit approximation of protein band molecular weights. After electrophoresis, proteins were transferred to polyvinylidene fluoride membranes and incubated overnight at 4°C with antibodies to targeted proteins. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies, and antibody binding was detected using enhanced chemiluminescence (NEN Life Science Products, Boston, MA). Gel densitometry was performed using a Microtek (Carson, CA) scanner and UN-SCAN-IT software (Silk Scientific, Orem, UT).
Cardiac TNF-α concentrations.
Tissue TNF-α levels were measured on cardiac homogenates using the BIOMOL TNF-α ELISA assay kit. The manufacturer's instructions were followed when performing these assays.
Statistical analysis.
ANOVA was used for comparison of caspase levels and diaphragm forces across experimental groups. Tukeys test was used to determine differences between individual groups following ANOVA. A P value of < 0.05 was taken as indicating statistical significance. Data are presented as means ± SE.
RESULTS
Protein carbonyl formation and cardiac TNF-α levels.
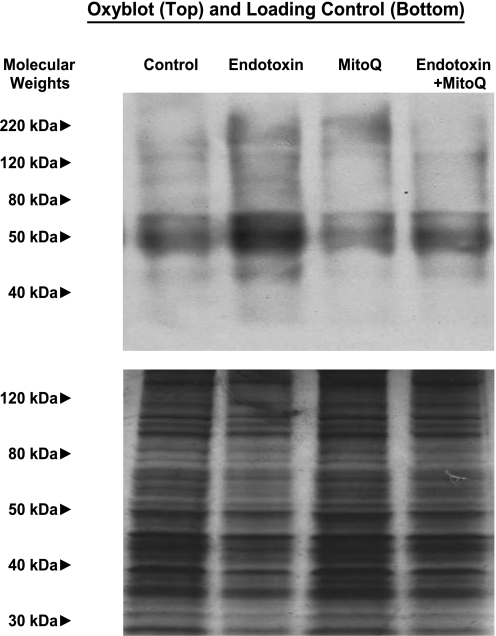
We assessed oxidative stress for cardiac samples from the four experimental groups using the OxyBlot technique, which determines protein carbonyl formation, as shown in Fig. 1. For rats, we found that hearts from endotoxin (LPS)-treated animals manifested substantially denser protein carbonyl bands than observed for samples from control animals (P < 0.001). Moreover, administration of MitoQ in concert with endotoxin prevented this increase in protein carbonyl formation, with OxyBlot band density for this group of experiments similar to that observed for control animals given saline or animals given MitoQ alone (P < 0.01). Similar responses were noted for OxyBlot assessments made on samples from mice, with total OxyBlot band density averaging 11.6 ± 0.4 densitometry units (DU) for control, 14.5 ± 0.1 DU for LPS, 10.9 ± 0.5 DU for LPS + MitoQ, and 11.7 ± 0.4 DU for MitoQ alone groups (P < 0.01 for comparison of control to LPS and control to LPS + MitoQ groups). As a control, we also performed blots in which 2,4-dinitrophenylhydrazine was not included in the sample incubation mixture; these blots were completely blank, indicating that the OxyBlot staining represented in Fig. 1 specifically assesses protein carbonyl side group reaction with the OxyBlot reagent.
Fig. 1.
Cardiac OxyBlots. Top: OxyBlot determinations for cardiac samples from representative control, endotoxin, 10-(6′-ubiquinonyl)-decyltriphenylphosphonium (MitoQ), and endotoxin + MitoQ-treated animals. OxyBlot protein staining was more prominent for the endotoxin sample compared with the other groups. Bottom: a duplicate gel examining silver staining for all proteins contained in these samples; this serves as a loading control.
Cardiac TNF-α levels were increased in the LPS-treated animals and were also elevated above control levels in the LPS + MitoQ group. Mean mouse cardiac TNF-α levels averaged 52 ± 3, 183 ± 24, 148 ± 39, and 29 ± 6 pg/mg, respectively, for control, LPS, LPS + MitoQ, and MitoQ groups (P < 0.01 for comparison of LPS and LPS + MitoQ to the control group).
Parameters of mitochondrial respiration.
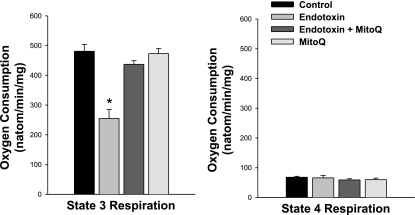
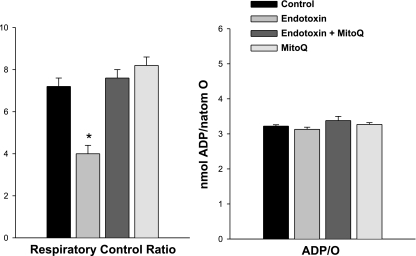
As expected, endotoxin administration had significant deleterious effects on mitochondrial function, with mouse heart mitochondrial isolates from endotoxin-treated animals demonstrating lower state 3 respiration rates (Fig. 2, P < 0.001), lower respiratory control ratios (Fig. 3, P < 0.01) and lower levels of calculated ATP production (P < 0.01) than observed for mitochondria isolated from the hearts of control animals. Endotoxin administration also reduced mitochondrial NADH utilization (P < 0.01) and reduced maximal uncoupled respiration rates following FCCP administration (P < 0.01, Table 1). These changes are consistent with an effect of endotoxin to inhibit electron flow through the electron transport chain. Administration of MitoQ prevented these endotoxin-induced changes in mitochondrial function, with state 3 respiration rates, and respiratory control ratios for cardiac mitochondrial isolates for animals given both endotoxin and MitoQ significantly higher than values for samples taken from animals given endotoxin alone (P < 0.01 for each of these comparisons, Figs. 2 and 3). Values for animals given MitoQ alone were similar to those obtained from saline-treated control animals. Calculated ATP production was also maintained by MitoQ administration with ATP production rates of 1,537 ± 71, 807 ± 103, 1,473 ± 60, and 1,544 ± 49 nmol·min−1·mg−1, respectively, for control, endotoxin, endotoxin + MitoQ, and MitoQ alone groups (P < 0.01 for comparison of endotoxin to the other experimental groups).
Fig. 2.
State 3 and state 4 respiration rates. State 3 respiration rate is a measure of maximum ADP-stimulated oxygen consumption, and state 4 respiration rate is a measure of basal oxygen consumption in the absence of ADP. Left: endotoxin administration elicited a large reduction in cardiac mitochondria state 3 rate (P < 0.001 for comparison of controls and to the endotoxin group), and administration of MitoQ prevented this endotoxin-induced reduction (P < 0.01 for comparison of the endotoxin group to the endotoxin + MitoQ group). Right: state 4 rates were similar for all experimental groups. *Statistically significant difference from the other groups.
Fig. 3.
Respiratory control and ADP-to-O ratios. The ADP-to-O ratio is an index of the coupling of oxygen consumption to oxidative phosphorylation. Endotoxin reduced the respiratory control ratio (RCR) (P < 0.001, for comparison of the control to the endotoxin group) but did not change the ADP-to-O ratio. Administration of MitoQ prevented this endotoxin-induced reduction in RCR (P < 0.01 for comparison of endotoxin to the endotoxin + MitoQ group). *Statistically significant difference from the other groups.
Table 1.
NADH utilization and FCCP-stimulated respiration for mitochondrial isolates
| Control | Endotoxin | Endotoxin + MitoQ | MitoQ | |
|---|---|---|---|---|
| NADH utilization rate, nmol·min−1·mg−1 | 5,585±221 | 2,242±339* | 4,172±131 | 4,802±216 |
| FCCP-stimulated respiration, natom·min−1·mg−1 | 561±39 | 253±55* | 442±10 | 479±17 |
Endotoxin administration elicited a large reduction in NADH utilization (P < 0.001 for comparison of control to endotoxin groups), and FCCP-stimulated respiration (P < 0.001 for comparison of control to endotoxin). Administration of 10-(6'-ubiquinonyl)-decyltriphenylphosphonium (MitoQ), prevented these endotoxin-induced alterations (P < 0.01 for comparison of NADH utilization between endotoxin and endotoxin + MitoQ groups; P < 0.01 for comparison of FCCP-stimulated respiration between these two groups).
Statistically significant difference between the endotoxin alone treated group and the other experimental groups.
We also found that the dose of endotoxin used in the present study did not induce uncoupling of oxidative phosphorylation or a rise in resting oxygen consumption, since ADP-to-O ratios and state 4 respiration rates for cardiac mitochondrial isolates from endotoxin-treated animals were similar to those for saline-treated controls (Figs. 2 and 3).
Effects on caspase activity levels.
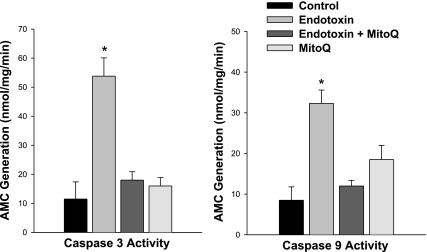
Endotoxin administration elicited a significant increase in rat cardiac caspase 3 and caspase 9 activity levels as shown in Fig. 4 (P < 0.001 and P < 0.001, respectively, for comparison of caspase 3 and caspase 9 levels between control and endotoxin-treated groups). In parallel with the effects of this agent to attenuate oxidative stress and preserve mitochondrial function, this agent also prevented these endotoxin induced increases in caspase 3 and 9 activity (P < 0.01 for comparison of endotoxin to endotoxin + MitoQ groups). Similar responses were observed for samples from mouse experiments, with caspase 3 activity averaging 6.2 ± 1.1, 59.0 ± 7.6, 13.1 ± 8.0, and 9.8 ± 6.7 nM·min−1·mg−1, respectively, for control, endotoxin, endotoxin + MitoQ. and MitoQ groups (P < 0.01 for comparison of control to endotoxin and for endotoxin to endotoxin + MitoQ groups).
Fig. 4.
Cardiac caspase 3 (left) and caspase 9 (right) activity. Endotoxin induced a large increase in both cardiac caspase 3 and caspase 9 activities (P < 0.001 for comparison of control to endotoxin-treated groups). MitoQ administration blocked this endotoxin effect on caspase activities (P < 0.01 for comparison of caspase 3 between endotoxin and endotoxin + MitoQ groups; P < 0.01 for comparison of caspase 9 between these 2 groups). *Statistically significant difference from the other groups. AMC, amino-4-methylcoumarin.
Effects on cardiac pressure generation.
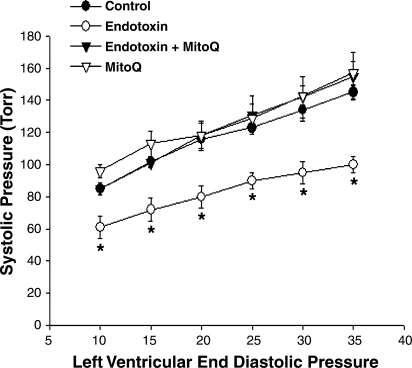
Endotoxin elicited a significant downward shift in the rat cardiac systolic pressure-diastolic pressure relationship, as shown for pressure tracings from representative experiments in Fig. 5 and for group mean data in Fig. 6. These reductions in pressure-generating capacity occurred over the entire range of diastolic pressures examined (10 to 35 mmHg) with, on average 29%, 31%, 31%, 27%, 29%, and 31% reductions in systolic pressure for hearts from endotoxin-treated animals at diastolic pressures of 10, 15, 20, 25, 30, and 35 mmHg, respectively (P < 0.01 for comparison of control to endotoxin groups for all diastolic pressures). MitoQ administration prevented this endotoxin-induced reduction in cardiac pressure generation, with systolic pressures for hearts from animals given both endotoxin and MitoQ similar to values for saline-treated control hearts at all tested levels of diastolic pressure. Hearts from animals given MitoQ alone had pressure-generating levels similar to those obtained for control animals.
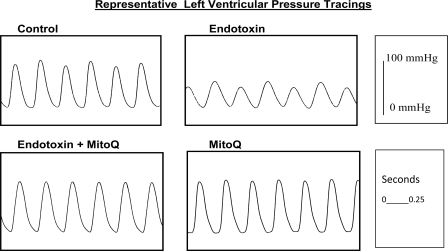
Fig. 5.
Representative left ventricular pressure tracings. The heart from an animal given endotoxin administration developed a much lower left ventricular pressure (right top tracing) compared with the pressure generated by a heart from a control animal (left top tracing). Developed left ventricular pressure for a heart from an animal given both endotoxin and MitoQ (left bottom tracing) was similar to the pressure in a control heart. Pressures for animals given MitoQ alone (right bottom tracing) were similar to control.
Fig. 6.
Systolic pressure-to-diastolic pressure relationship. Endotoxin-induced a significant reduction in developed left ventricular pressure for cardiac Langendorf preparations over the entire range of diastolic pressures assessed [P < 0.01 for comparison of control (•) to endotoxin (○)]. Hearts from animals given both endotoxin and MitoQ (▴) developed systolic pressures higher than those for the endotoxin group (P < 0.02). Systolic pressures for the hearts from animals given MitoQ alone (▵) were similar to controls. *Statistically significant difference from the other groups.
Alterations in rat ventricular +dP/dt as the result of endotoxin treatment and administration of MitoQ were similar to the pattern of changes observed in developed systolic pressures (see Table 2), with endotoxin reducing this index of cardiac performance and MitoQ preventing these endotoxin-induced changes. The relationship between diastolic pressure and the volume added to the left ventricular balloon circuit was similar for the four experimental conditions (see Table 3), suggesting that precontraction left ventricular compliance was not significantly altered by the various treatments.
Table 2.
Rate of pressure development (+dp/dt) and rate of pressure reduction (−dp/dt) for rat hearts
| Control | Endotoxin | Endotoxin + MitoQ | MitoQ | |
|---|---|---|---|---|
| 10 mmHg | ||||
| +dp/dt | 2,100±113 | 1,531±189 | 2,050±159 | 2,420±121 |
| −dp/dt | 1,026±93 | 614±108* | 1,003±114 | 1,240±68 |
| 15 mmHg | ||||
| +dp/dt | 2,490±105 | 1,762±90* | 2,500±159 | 2,830±220 |
| −dp/dt | 1,286±64 | 770±97* | 1,135±127 | 1,444±141 |
| 20 mmHg | ||||
| +dp/dt | 2,896±68 | 1,920±165* | 2,751±268 | 2,886±223 |
| −dp/dt | 1,501±52 | 886±106* | 1,578±195 | 1,442±121 |
| 25 mmHg | ||||
| +dp/dt | 2,940±111 | 2,101±124* | 2,950±318 | 3,106±185 |
| −dp/dt | 1,476±74 | 1,050±105 | 1,613±190 | 1,596±100 |
| 30 mmHg | ||||
| +dp/dt | 3,130±167 | 2,240±201* | 3,062±258 | 3,460±89 |
| −dp/dt | 1,632±99 | 1,143±131 | 1,825±295 | 1,780±64 |
| 35 mmHg | ||||
| +dp/dt | 3,450±50 | 2,370±196* | 3,438±329 | 3,670±107 |
| −dp/dt | 1,836±45 | 1,199±128* | 1,825±175 | 2,016±127 |
Values are means ± SE (in mmHg/s). Endotoxin-induced reductions in +dp/dt for contractions assessed at diastolic pressures between 15 and 35 mmHg and concomitant administration of MitoQ prevented these endotoxin induced changes
Statistically significant difference between the endotoxin-alone treated group and the other experimental groups (control, endotoxin plus MitoQ, MitoQ alone).
Table 3.
Diastolic pressure-volume relationships for rat hearts
| Control | Endotoxin | Endotoxin + MitoQ | MitoQ | |
|---|---|---|---|---|
| 10 mmHg | 394±12 | 388±15 | 398±9 | 394±19 |
| 15 mmHg | 446±13 | 438±14 | 447±12 | 443±20 |
| 20 mmHg | 480±14 | 468±13 | 482±14 | 477±23 |
| 25 mmHg | 509±15 | 493±15 | 509±15 | 505±24 |
| 30 mmHg | 530±17 | 513±15 | 531±15 | 526±25 |
| 35 mmHg | 546±18 | 526±17 | 546±15 | 542±27 |
Values are means ± SE (in μl). Diastolic pressure-volume relationships were similar for the four experimental groups.
DISCUSSION
This study found that endotoxin-induced sepsis causes 1) an increase in cardiac levels of a marker of oxidative stress, 2) a profound reduction in cardiac mitochondrial function, 3) reductions in cardiac pressure-generating capacity, and 4) activation of the intrinsic caspase pathway within the heart. Moreover, we found that administration of the potent, mitochondrially targeted antioxidant MitoQ abrogated each of these endotoxin-induced alterations in cardiac physiology and biochemistry. Mitochondrial assays were performed in mice, while assessment of cardiac contractile function was made for rats because of methodological requirements. Two key indices, namely, caspase 3 and protein carbonyl levels, were performed in both rats and mice, with similar results obtained in both species.
Sepsis and cardiac dysfunction.
While many septic patients have an increased cardiac output related to reductions in cardiac afterload secondary to peripheral vasodilatation, it is thought that most septic patients have some degree of cardiac dysfunction that limits cardiac output below the levels one would expect in a patient with a normal cardiovascular system subjected to the same afterload reduction (14). As a result, cardiac dysfunction in sepsis may contribute to reductions in tissue oxygen delivery in most patients with sepsis. Moreover, in an important subset of septic patients, reductions in cardiac performance are so severe that cardiac output is substantially reduced, even to the level observed after massive myocardial infarction. In these particular patients, cardiac dysfunction is often the cause of death (22).
Over the past 20 years substantial progress has been made in identifying the mechanism by which sepsis elicits cardiac dysfunction. In early work, Snell and Parrillo (24) identified a circulating substance termed myocardial depressant factor that could be isolated from animals and humans with septic shock, that was found to induce cardiovascular dysfunction when given to donor animals, and also, that induced cardiac dysfunction in isolated cells when incubated with cardiomyocytes in vitro. This work also identified myocardial depressant factor as a combination of TNF-α and IL-1β (15). In more recent experimentation, several groups have shown that sepsis elicits significant activation of caspase pathways in the heart and have suggested that caspase-mediated contractile protein cleavage may be a major mechanism by which cytokines, i.e., TNF-α and IL-1β, induce cardiac dysfunction (18). This work has shown that cardiac caspase activation occurs primarily via the intrinsic caspase pathway following endotoxin administration, resulting in an increase in cardiac caspase 9 activity (16). Endotoxin-induced cardiac caspase 9 activation, in turn, triggers caspase 3 activation and is associated with cleavage of several cardiac contractile proteins, including troponin T (19). In keeping with this theory, Communal et al. (7) found that incubation of isolated cardiac contractile elements with activated caspase 3 results in a marked depression of cardiac contractile protein force-generating capacity and cleavage of several important elements of the cardiac contractile protein matrix (including troponin T, actin, and actinin).
Several of the findings in the present study are consistent with these previous reports. We found that endotoxin administration was associated with severe alterations in cardiac function, with systolic pressure generation depressed significantly (%, on average) over a wide range of cardiac diastolic pressures (10–35 mmHg). Also in keeping with previous reports, we found that endotoxin elicited significant increases in cardiac caspase 3 and caspase 9 activation.
Sepsis and mitochondrial dysfunction.
While a role for caspase activation in the development of cardiac dysfunction resulting from sepsis seems reasonably plausible based on recent reports, there is far more controversy regarding the role that mitochondrial dysfunction plays in the induction of failure of the heart and other organs in sepsis. Supporting the potential importance of mitochondrial abnormalities in sepsis, a number of previous studies have shown that this condition is associated with the acute development of fairly severe cardiac mitochondrial dysfunction, producing reductions in function that are comparable in magnitude to those observed in patients with congenital mitochondrial disorders associated with the development of cardiomyopathy (28). In addition, Brealey et al. (3) have shown that the existence of mitochondrial dysfunction in tissue samples taken from septic ICU patients is associated with a poor prognosis, and is a strong predictor of death. Certainly, the findings of the present study are consistent with these previous reports. We found that endotoxin administration was associated with severe reductions in multiple indices of cardiac mitochondrial function, reducing state 3 respiration rates, maximal ATP production rates, and the respiratory control ratio.
While we found that sepsis reduced state 3 respiration rates in the present study, others have suggested that sepsis and endotoxemia can also induce reductions in the total mitochondrial volume content of tissues. These data are complementary, i.e., the total capacity of the mitochondrion to generate ATP is the combined product of its state 3 rate/milligram mitochondrial protein, its ADP-to-O ratio, and the total mitochondrial protein or volume content. As a result, total mitochondrial energy-generating capacity may be even more depressed by sepsis than is evident from the state 3 data presented in the present experiment.
Whether or not the energetic alterations engendered by the development of mitochondrial dysfunction during sepsis are directly responsible for poor clinical outcomes is controversial. On the one hand, Solomon et al. (25) have suggested that it is unlikely that cardiac mitochondrial derangements engendered by sepsis produce organ failure by altering cardiac ATP levels because they found that cardiac energy stores were only mildly reduced in septic animals with substantial cardiac dysfunction. On the other hand, the presence of normal ATP levels does not necessarily mean that mitochondrial function is normal. Theoretically, compensatory measures that limit cardiac work to match reduced energy supply levels due to mitochondrial dysfunction may preserve ATP levels, but at the expense of reduced cardiac performance.
In addition, there are other mechanisms by which alterations in mitochondrial function can elicit organ dysfunction that are unrelated to changes in energy metabolism. We have previously shown that sepsis-induced cardiac mitochondrial dysfunction is associated with a significant increase in the generation of reactive oxygen species (i.e., superoxide, hydrogen peroxide) by cardiac mitochondria (26). Reactive oxygen species generated by mitochondria can alter organelle function directly and can also generate byproducts, such as hydroxynonenal, that can react with and alter protein function (9). In addition, some work suggests that mitochondrially generated free radicals can react with mitochondrial porin, opening this pore and facilitating release of cytochrome c from the intermembrane mitochondrial space (13). Cytochrome c, in turn, triggers formation of the apoptosome, leading to caspase 9 activation (5).
The findings of the present study are consistent with the possibility that cardiac free radical generation is linked to caspase activation and thence to reductions in cardiac pressure-generating capacity in sepsis. Specifically, we found that MitoQ, a potent free radical scavenger, prevented cardiac mitochondrial dysfunction, caspase 9 activation, caspase 3 activation, and reductions in cardiac pressure-generating capacity after endotoxin administration. In addition, MitoQ also prevented endotoxin-induced increases in cardiac levels of protein carbonyls, an index of tissue free radical generation. These findings are consistent with the possibility that reactive oxygen species derived from mitochondria play an important role in triggering caspase-mediated cardiac contractile protein damage in endotoxin-induced sepsis.
The present study is the first, of which we are aware, to examine the effects of a mitochondrially targeted antioxidant, MitoQ, on cardiac mitochondrial and pressure-generating capacity in an animal model of sepsis. MitoQ is a ubiquinone derivative specifically targeted to mitochondria via covalent attachment to a lipophilic triphenylphosphonium cation through an aliphatic carbon chain (1, 8, 12). Because mitochondria have a large membrane potential gradient, with a highly negative internal charge, the phosphonium cation on this molecule is strongly attracted to the mitochondrial interior, while the aliphatic side chain incorporated into this agent facilitates insertion in the lipid bilayer of the mitochondrial membrane. As a result, this agent preferentially localizes to mitochondria where it is concentrated at levels 500- to 1,000-fold those in the extracellular space. Previous in vitro work has suggested that this results in MitoQ performing as an extremely potent mitochondrial-targeted antioxidant (8, 12). Moreover, in vitro work has also shown this agent potently blocks oxidative stress-induced cellular apoptosis, while nonmitochondrially targeted ubiquinone analogs have no such effect (12). The present work extends these previous in vitro studies by demonstrating this agent is also capable of blocking caspase activation in the intact heart following endotoxin administration. Our finding that this agent preserves mitochondrial state 3 rates may be due to its antioxidant effects, preventing oxidative modification and loss of function of key mitochondrial proteins. Alternatively, this agent may directly participate in maintaining electron flux through the proximal portion of the electron transport chain since it directly associates with the inner mitochondrial membrane.
Perspectives and Significance
The current work focused on an examination of the effects of MitoQ on cardiac caspase activation and cardiac dysfunction, demonstrating that this agent prevents reductions in cardiac mitochondrial and contractile function when given to animals with endotoxin-induced sepsis. Recent studies indicate that sepsis is associated with mitochondrial dysfunction and caspase activation in numerous organs, including the liver, kidney, skeletal muscle, intestine, and lymphoid tissues (20, 27, 31, 32). It is important to note that one recent study found that MitoQ also preserved renal and liver function in an animal model of sepsis (18). Additional work will be needed to determine whether MitoQ is also capable of preventing mitochondrial dysfunction and caspase activation in other tissues and organs and using other animal models of sepsis (e.g., cecal ligation perforation, pneumonia, bacterial infusion). If so, this agent may prove useful as a therapeutic means of preventing organ failure in sepsis.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-80429, HL-81525, HL-63698, HL-80609, and HL-69821.
DISCLOSURE
M. P. Murphy is a consultant for Antipodean Pharmaceuticals, Inc., San Francisco, CA.
ACKNOWLEDGMENTS
We would like to thank Antipodean Pharmaceuticals, Inc., San Francisco, CA, for providing the MitoQ used in these studies.
REFERENCES
- 1.Adam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Atamna H, Frey WH., II Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion 7: 297–310, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Callahan LA, Stofan D, Szweda L, Nethery D, Supinski GS. Free radicals alter maximal diaphragmatic oxygen consumption in endotoxin-induced sepsis. Free Radic Biol Med 30: 129–138, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Respir Crit Care Med 172: 861–868, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Callahan LA, Supinski GS. Diaphragm and cardiac mitochondrial creatine kinases are impaired in sepsis. J Appl Physiol 102: 44–53, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A 99: 6252–6256, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Targeting mitochondria. Acc Chem Res 41: 87–97, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Humphries KM, Yoo Y, Szweda LI. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry 37: 552–557, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Hüttemann M, Lee I, Samavati L, Yu H, Doan JW. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta 1773: 1701–1720, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Jiang J, Huang Z, Zhao Q, Feng W, Belikova NA, Kagan VE. Interplay between bax, reactive oxygen species production, and cardiolipin oxidation during apoptosis. Biochem Biophys Res Commun 368: 145–150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant, and antiapoptotic properties. J Biol Chem 276: 4588–4596, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kim TS, Yun BY, Kim IY. Induction of the mitochondrial permeability transition by selenium compounds mediated by oxidation of the protein thiol groups and generation of the superoxide. Biochem Pharmacol 66: 2301–2311, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care 8: 376–388, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Krieger A, Symeoneides S, Kumar A, Parrillo JE. Myocardial dysfunction in septic shock: Part II. Role of cytokines and nitric oxide. J Cardiothorac Vasc Anesth 15: 485–511, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Lancel S, Joulin O, Favory R, Goossens JF, Kluza J, Chopin C, Formstecher P, Marchetti P, Neviere R. Ventricular myocyte caspases are directly responsible for endotoxin-induced cardiac dysfunction. Circulation 111: 2596–2604, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Leverve XM. Mitochondrial function and substrate availability. Crit Care Med 35, Suppl 9: S454–S460, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med 45: 1559–1565 2008 [DOI] [PubMed] [Google Scholar]
- 19.Natanson C, Eichenholz PW, Danner RL. Endotoxin, and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med 169: 823–832, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neviere R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med 163: 218–225, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev 39: 443–455, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35: 1599–1608, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Schremmer B, Dhainaut JF. Heart failure in septic shock: effects of inotropic support. Crit Care Med 18, Suppl 1: S49–S55, 1990 [PubMed] [Google Scholar]
- 24.Snell RJ, Parrillo JE. Cardiovascular dysfunction in septic shock. Chest 99: 1000–1009, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Solomon MA, Correa R, Alexander HR, Koev LA, Cobb JP, Kim DK, Roberts WC, Quezado ZMN, Scholz TD, Cunnion RE, Hoffman WD, Bather J, Yatsiv I, Danner RL, Banks SM, Ferrans VJ, Balaban RS, Natanson C. Myocardial energy metabolism and morphology in a canine model of sepsis. Am J Physiol Heart Circ Physiol 266: H757–H768, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Supinski GS, Callahan LA. Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction. Am J Respir Crit Care Med 173: 1240–1247, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol 100: 1770–1777, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Supinski GS, Callahan LA. Hemin prevents cardiac and diaphragm mitochondrial dysfunction in sepsis. Free Radic Biol Med 40: 127–137, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Tauskela JS. MitoQ–a mitochondria-targeted antioxidant. Drugs 10: 399–412, 2007 [PubMed] [Google Scholar]
- 30.Taylor DE, Ghio AJ, Piantadosi CA. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch Biochem Biophys 316: 70–76, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Wan L, Bellomo R, Di Giantomasso D, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care 9: 496–502, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Wesche-Soldato DE, Chung CS, Gregory SH, Salazar-Mather TP, Ayala CA, Ayala A. CD8+ T Cells promote inflammation and apoptosis in the liver after sepsis. Role of Fas-FasL. Am J Pathol 171: 87–96, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]