Abstract
Paradoxically, bilateral transection of the chorda tympani nerve (CTX) raises the taste discrimination threshold for the free fatty acid, linoleic acid (LA), yet the chorda tympani nerve (CT) is unresponsive to lingual application of LA alone. LA may require a background of saliva to activate taste cells, since CTX decreases saliva production through denervation of the submaxillary and sublingual salivary glands. To assess the role of saliva, we measured LA taste discrimination thresholds for animals whose submaxillary and sublingual salivary glands were removed and also recorded CT responses to LA mixed in artificial saliva. Partial desalivation shifted LA discrimination thresholds from between 5.5 and 11 μM to between 11 and 22 μM. However, this effect was not as pronounced as previously seen with CTX animals. Surprisingly, the CT was unresponsive to LA mixed with artificial saliva, suggesting that artificial saliva may lack components necessary for LA taste. Additionally, fats may primarily enhance other tastes. We previously reported that LA increases CT responses to monosodium glutamate (MSG). Thus we also recorded CT whole nerve responses to taste mixtures of LA and sodium chloride (NaCl), sucrose (SUC), citric acid (CA), or quinine hydrochloride (QHCl) in anesthetized rats. We found that LA increased CT responses to NaCl but did not alter CT responses to SUC, CA, and QHCl. Thus CT recordings either lack the sensitivity to detect small changes to SUC, CA, and QHCl or LA may affect CT responses to MSG and NaCl only, perhaps by specifically modulating gustatory processing of Na+.
Keywords: fat taste, free fatty acids, electrophysiology, electrolytes
although vilified in public media as the cause of the obesity epidemic currently affecting much of the industrialized world, fats are important for many biological processes. In particular, essential free fatty acids (FFAs), such as the polyunsaturated FFA linoleic acid (LA), are crucial for healthy brain and heart development and function (39, 47). However, since essential FFAs cannot be synthesized by the body (10), they must be obtained from foods consumed. Thus dietary detection of essential FFAs is important for life.
Despite their importance for normal biological functioning, there is ongoing debate regarding how essential FFAs are orally detected. In the past, it was thought that fats were detected solely by their olfaction and textural attributes (19, 29, 46). However, recent evidence suggests that fats and FFAs (such as LA) possess gustatory attributes as well. Rats strongly prefer FFA solutions even when olfaction and texture are minimized (9) and FFAs have minimal texture (e.g., the viscosity of 88 μM LA is only ∼1.5% greater than that of water; see Ref. 28), unlike other fats. Moreover, rats do not rely on olfaction to discriminate LA, since rats can distinguish between LA and water at low (10 μM) concentrations even with ablation of the olfactory bulb (37). Thus FFAs are ideal for examining the role of taste in the perception of fats.
However, fats are consumed in the form of triglycerides that then are subsequently broken down into FFAs by lingual lipase (lipolysis). Thus, in order for FFAs to activate the gustatory system, lipolysis in the oral cavity must occur rapidly (i.e., before fats are swallowed). In this regard, ingested fats are broken down quickly into FFAs within 1–5 s (18) and, may be detected in the mouth well before subsequently activating receptors downstream in the gastrointestinal tract in rats. Even more convincing, the prevention of the breakdown of fats into FFAs by the addition of a lingual lipase inhibitor greatly reduces rats' preference for fat solutions (18). Together, these diverse results suggest that ingested fats, once broken down into FFAs, may activate taste receptor cells (TRCs) located in the mouth, although the mechanism by which this occurs remains unknown.
Recently, the fatty acid translocase/transporter CD36 has received considerable attention for its role in FFA preferences (8, 20, 35). Moreover, FFA stimulation of circumvallate papillae, located in the posterior oral cavity, increases intracellular Ca2+, resulting in release of neurotransmitters, such as 5-hydroxytryptamine and norepinephrine (6). However, CD36 is not present in fungiform papillae, located in the anterior two-thirds of the tongue (20). Thus whether a similar FFA transporter/translocase is present in fungiform papillae in the anterior part of the tongue remains unknown. In this regard, it is likely that FFAs directly act upon membrane-bound receptors in fungiform papillae, since intracellular application of LA has no effect on isolated fungiform papillae activity, whereas extracellular application of LA does (13). Unfortunately, no such membrane-bound FFA receptor has been indentified in fungiform papillae. However, the resulting FFA transduction cascade may include FFA G protein-coupled receptors, such as GPR40 and GPR120 as well as the TrpM5 ion channel (4, 36).
Even more importantly, little is known about the peripheral neural pathways that carry FFA taste information to the brain. The chorda tympani nerve (CT), a gustatory nerve that innervates fungiform papillae on the anterior of the tongue, appears to carry FFA taste information, since bilateral transaction of the CT (CTX) raises the taste discrimination threshold for LA (31, 43). Surprisingly, the CT is unresponsive to lingual stimulation with LA (44), and individual neurons in the geniculate ganglion (the location of the cell bodies of CT gustatory sensory neurons) are also unresponsive to lingual LA stimulation (1).
Although perplexing, there are several explanations for this lack of neural responsiveness to LA, such as LA could require a background of saliva to activate taste cells, i.e., taste cells are incapable of responding to LA unless there is saliva present. In support of this, CTX results in denervation of the submaxillary and sublingual salivary glands (38). Although this partial desalivation has no effect on lingual lipase activity (42), it does result in a decrease in saliva. Thus LA interacting with the saliva would explain, in part, the discrepancy between behavioral data (in which saliva is present) and CT electrophysiological data (in which saliva is rinsed off during water rinses).
Moreover, although FFAs may be effective taste stimuli by themselves, fat rarely is consumed alone. Thus fats may exert a powerful influence by modulating or enhancing other tastes. In this regard, essential FFAs, but not saturated FFAs, inhibit delayed-rectifying potassium channels in isolated TRCs from fungiform papillae (12–14). This inhibition leads, presumably, to a broadening of action potentials and prolonging the release of neurotransmitters. Thus LA may augment taste responses to other taste stimuli.
In support of this, LA enhances behavioral taste responses to sucrose (SUC; see Refs. 33 and 43) and also increases CT responses to monosodium glutamate (MSG) in rats (44). However, LA decreases behavioral responses to sodium chloride (NaCl), citric acid (CA), and quinine hydrochloride (QHCl) solutions (33). One explanation for these diverse findings is that LA increases the intensity of other tastes, making preferred tastes more palatable and nonpreferred tastes more aversive. However, whether changes in behavioral taste preferences for SUC, NaCl, CA, and QHCl when LA is added are mediated by increased CT responses remains unknown.
Accordingly, the following experiments have the following two primary goals: 1) to examine the importance of saliva in LA taste using a conditioned taste aversion (CTA) protocol in conjunction with a parallel electrophysiological study and 2) to further clarify the role of the CT in LA taste using LA-taste mixtures. To explore the role of saliva in LA taste, experiment 1 determined whether removal of the submaxillary and sublingual salivary glands (which are denervated following CTX) affects LA taste discrimination. Experiment 2 examined CT responses to LA mixed with artificial saliva (AS), and experiment 3 measured CT responses to LA-taste mixtures of “classic” taste stimuli (i.e., salty, NaCl; sweet, SUC; sour, CA; and bitter, QHCl) to determine whether the influence is broad or, rather, limited to only MSG.
METHODS
Subjects
Adult male rats (Sprague-Dawley; Charles River Breeding Laboratories) were housed individually in transparent plastic cages in a temperature-controlled colony room (72°F) and maintained on a 12:12-h light-dark cycle with lights on at 0700. Unless specified otherwise, rats had free access to Purina Rat Chow (no. 5001) and deionized water ad libitum. The Institutional Animal Care and Use Committee at Florida State University approved all procedures.
Chemicals
Because of its lipophilic nature, LA (99% pure; Sigma) was dissolved in 5 mM ethanol (Et). All other reagent-grade chemicals were mixed in deionized water, except where noted.
Statistical Analyses
Data are presented as group means ± SE. Data were analyzed using appropriate one-, two- or three-way ANOVA (Statistica; StatSoft, Tulsa, OK), with repeated measures for within- subjects effects where applicable. Tukey's honest significant difference tests were used to assess statistically significant (P < 0.05) main effects or interactions.
Electrophysiological Recordings
CT whole nerve electrophysiological recordings were obtained in urethane-anesthetized (1.5 g/kg body wt) rats using methods described previously (22, 32). Briefly, the trachea was cannulated, and rats were placed in a nontraumatic head holder. With the use of a mandibular approach, the right CT branch of the facial nerve then was exposed and transected where it enters the tympanic bulla. The perineurium was removed to the point where the lingual nerve joins the CTm and the distal portion of the cut nerve was placed on a tungsten wire electrode. A silver indifferent electrode placed in the muscle near the nerve allowed differential amplification (×10,000) of nerve activity.
The tongue was slightly extended and held in place with a small suture attached to the ventral surface. Taste stimuli were applied across the tongue at a constant flow rate of 50 μl/s for 10 s, which approximates the fluid volume consumed by a rat licking from a drinking spout obtaining ∼5–7 μl/lick at a rate of 6–7 licks/s (21). A custom computer program controlled input to a mixing platform, allowing rapid (∼2 s) switching and/or mixing while maintaining continuous solution flow of 50 μl/s. Each taste stimulus was followed by a 60- to 90-s rinse to ensure that nerve activity returned to stable, baseline levels.
Sensory nerve activity was recorded and stored on videotape for off-line analysis using a GW Instrument 15-s data acquisition board and custom software. Amplified nerve activity was integrated using a root mean square calculation and a 150-ms time constant. Baseline neural activity was recorded during rinses for ≥30 s preceding each stimulus. Average baseline activity (in μV) for the 15-s period immediately before each taste stimulus was used to calculate the area under the curve, expressed as response above baseline, for the integrated response during each stimulus. NaCl (600 mM) was applied for 10 s at the beginning and at the end of the recording protocol, which typically was ∼40 min, to evaluate the viability of the nerve. If the response to NaCl at the end of the protocol varied by >15% of the initial NaCl response, the data from the recording were not included in the analysis. Each response was then normalized to the average 300 mM ammonium chloride standard stimulus, which was applied at the beginning and end of the recording protocol, to control for individual differences in signal strength between preparations.
Experiment 1: Role of the Submaxillary and Sublingual Salivary Glands in LA Taste Discrimination
Presurgery water restriction and desalivation surgery.
Male rats were placed on a water restriction schedule that gave animals access to 10 min of water in the morning (training) and 30 min of water in the afternoon daily. Once they drank reliable quantities of deionized water (≥7 ml) in the 10-min training sessions, rats were anesthetized with pentobarbital sodium (50 mg/kg ip; Abbot Laboratories), and a topical analgesic (bupivacaine) was applied to the surgical site. Access to the submaxillary and sublingual salivary glands was made via a single midline incision in the ventral neck. Following blunt dissection of muscle tissues at the level of the carotid notch, the salivary glands, enclosed in a common connective tissue sheath, were tied off, cauterized, and removed bilaterally (DESAL; n = 20). In a separate group of sham-operated controls (SHAM; n = 22), the salivary glands were exposed, but not removed. Following surgery, wounds were closed with sutures, and rats were allowed to recover for 7 days before behavioral testing.
CTA.
After desalivation surgery (7 days), rats were placed once again on the same water restriction regimen. Once all animals reliably drank volumes of water ≥7 ml, rats received 88 μM LA in a graduated drinking tube [conditioning day, day (D) 1; ∼9 days after surgery]. After 10 min, fluid intake was recorded, and rats were injected intraperitoneally with 3 meq/kg body wt of 0.15 M LiCl or 0.15 M NaCl (control).
Rats were given water for 10 min on D2, and the LiCl-induced CTA to 88 μM LA was verified on D3 in 10-min, 2-bottle (LA and water) tests. Rats were tested for the generalization of the CTA to less concentrated LA solutions (44, 22, 11, and 5.5 μM) in additional two-bottle (LA and water) tests conducted on D4–7. During these generalization tests, one LA concentration was given each day with presentation in descending order of concentration. To ensure that the results obtained were not a result of an extinction of the conditioned aversion, the CTA to 88 μM LA again was assessed after the final day of generalization testing (D8).
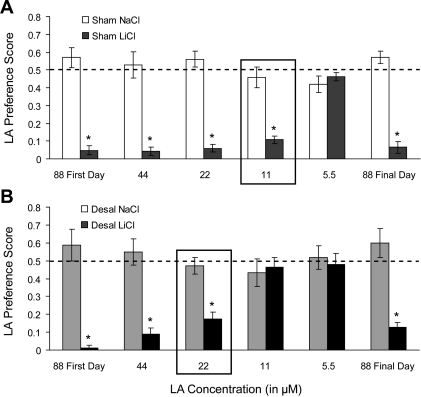
To minimize the possibility that the effects were attributable to position preference related to training and/or conditioning, the position of the drinking tubes was randomized so that, for each rat, tubes containing the LA solutions were equally likely to be in the “conditioning” position or in the “nonconditioning” position on any test day. To further ensure that there was no bottle preference, rats had to sample each bottle before the subsequent bottle was put on the cage during all preferences tests. Preference scores were calculated as intake of LA (ml) per total fluid intake (ml). A preference score of ∼0.5 indicates that animals consumed equal amounts of test solution and dH2O. Preference scores >0.5 indicate that animals consumed more LA than water (i.e., preference for LA), and, conversely, preference scores <0.5 indicate that animals consumed less LA than water (i.e., aversion to LA). The point at which LiCl-treated animals do not show an aversion to LA, as indicated by increased preference scores (that are similar to their corresponding NaCl counterparts), indicates the approximate LA discrimination threshold. Additionally, decreased saliva after removal of the submaxillary and sublingual salivary glands decreases efficiency of food intake by increasing the length of a bout in a meal (38). Therefore, we compared the averaged total fluid intake for each test day for each animal across experimental groups to determine whether partial desalivation affected fluid consumption.
Taste cell viability.
To determine whether partial desalivation impacts the viability of taste cells, animals were killed with an overdose of urethane, and then the tongues were removed following the last day of testing (∼2 wk after surgery). After 2–4 days postfixation in 4% paraformaldehyde, the anterior portion of the tongue was isolated, placed in distilled water, and then dipped in 0.5% methylene blue for ∼1 min. The epithelium then was separated from the underlying connective tissue and muscle and flattened between two glass slides. The number of fungiform papillae with visible taste pores was then counted under a light microscope. The percentage of fungiform papillae with taste pores was then calculated as follows: [(no. of taste pores/no. of fungiform papillae) × 100].
Experiment 2: CT Responses to Lingual Application of LA Mixed in AS
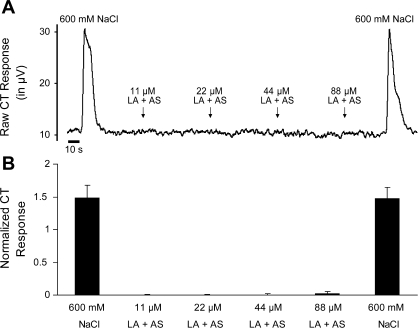
Whole nerve recordings (n = 7) were obtained from the CT as described above. LA (11, 22, 44, and 88 μM) mixed with AS (15 mM NaCl, 22 mM KCl, 3 mM CaCl2, and 0.6 mM MgCl2; see Ref. 17) and 5 mM Et was applied in ascending order of concentration across the tongue for 10 s. Between stimuli, AS + 5 mM Et flowed uninterruptedly over the tongue to minimize transient thermal or tactile responses.
Experiment 3a: CT Responses to LA and NaCl
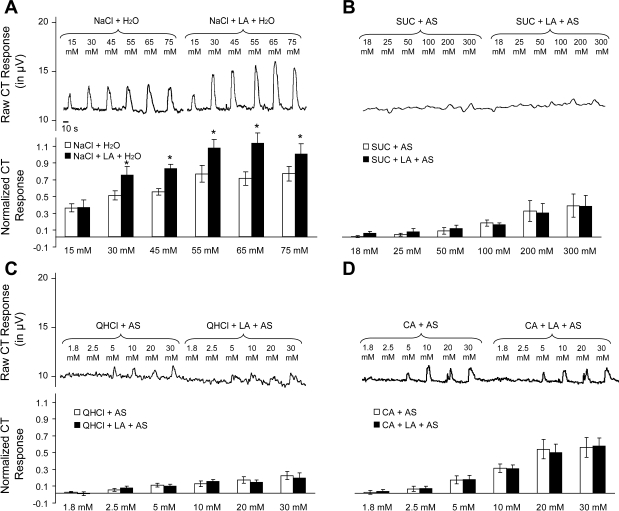
Whole nerve electrophysiological recordings were obtained from the CT as described above. The stimulation protocol consisted of a series of three concentrations of NaCl (either 15, 30, and 44 mM or 55, 65, and 75 mM; both n = 6) presented three times: once mixed with deionized water, once mixed with 5 mM Et, and once mixed with 88 μM LA + Et. This is in contrast to experiment 3b in which taste stimuli were mixed with AS (see below). Because AS decreases CT responses to Na+, through salivary Na+ adaptation of TRCs (26), we chose to minimize Na+ adaptation, which could affect the results obtained. Between stimuli, deionized water (for stimuli mixed in water), Et (for stimuli + Et solutions), or 88 μM LA + Et (for stimuli + LA + Et solutions) flowed uninterruptedly over the tongue. Preliminary analysis revealed that CT responses to NaCl mixed in water and mixed in Et were not different [F(1,14) = 0.09, P = 0.77 and F(1, 10) = 1.14, P = 0.31, respectively]. Therefore, a single NaCl value for each stimulus concentration was calculated for each rat by averaging the responses to NaCl mixed in water and mixed in Et.
Experiment 3b: CT Responses to LA and SUC, CA, or QHCl
Whole nerve electrophysiological recordings were obtained from the CT as described above using a protocol similar to the one used in experiments 2 and 3a, with two exceptions. First, all taste solutions were mixed with AS [which does not produce an electrophysiological response when presented with LA alone (experiment 2)] to more closely mimic the chemical environment found in the mouth. Second, our results from experiments 2 and 3a (as well as our previous study; see Ref. 44) found that CT responses to taste stimuli are not affected by the addition of Et (i.e., responses to taste solutions mixed in water were not different from responses to taste solutions mixed in Et). Therefore, we eliminated presentation of taste stimuli mixed in water. This allowed us to test a greater range of taste stimuli concentrations (i.e., 6 rather than 3) during a protocol. Specifically, the stimulation protocol consisted of a series of six concentrations of SUC (18, 25, 50, 100, 200, and 300 mM; n = 5), CA (1.8, 2.5, 5, 10, 20, and 30 mM; n = 5), and QHCl (1.8, 2.5, 5, 10, 20, and 30 mM; n = 5) presented two times: once mixed with Et + AS and once mixed with 88 μM LA + Et + AS. Between stimuli, Et + AS (for stimuli + Et + AS solutions) or 88 μM LA + Et + AS (for stimuli + LA + Et + AS solutions) flowed uninterruptedly over the tongue to minimize transient thermal or tactile responses.
RESULTS
Experiment 1: LA Taste Discrimination in Partially Desalivated Animals
Total fluid intake did not differ between experimental groups [F(3,76) = 1.18, P = 0.35], suggesting that removal of the submaxillary and sublingual salivary glands did not impair the ability of DESAL animals to consume test solutions (Table 1). Moreover, 10 min intake of test solutions closely resembled the values calculated for preference scores (Table 1). Thus we compared LA preference scores across experimental groups and test days. Figure 1 shows LA preference scores for 5.5, 11, 22, 44, and 88 μM LA by control (SHAM; A) and partially desalivated (DESAL; B) animals. As expected, LiCl treatment resulted in a robust aversion to 88 μM LA [F(1,38) = 206.17, P < 0.001] that remained unchanged from the first to final day of testing [F(1,38) = 3.21, P = 0.81], indicating that the aversion to LA was not extinguished during the course of testing. Moreover, LA preference scores by LiCl-treated animals were significantly less than NaCl-treated animals [F(1,38) = 81.35, P < 0.001], and LA preference scores were different between SHAM and DESAL animals [F(1,38) = 4.95, P < 0.05]. Post hoc analyses of the significant interaction between group, treatment, and concentration [F(3,114) = 3.90, P < 0.05] revealed that preference scores for LA were significantly lower for SHAM LiCl- treated animals (n = 11) than for SHAM NaCl-treated animals (n = 11) at 11, 22, and 44 μM LA (all P < 0.001). However, the preference score for LA between SHAM NaCl- and LiCl-treated animals was not different at 5.5 μM. Thus the LA taste discrimination threshold for SHAM animals is between 5.5 and 11 μM LA. In contrast, LA preference scores by LiCl-treated DESAL animals (n = 11) were significantly lower than for NaCl-treated DESAL animals (n = 9) at 22 and 44 μM LA only (P < 0.01). Preference scores for LA were not different between NaCl-treated and LiCl-treated DESAL animals at 5.5 and 11 μM LA (P = 0.99 and 0.98, respectively). In other words, the LA taste discrimination threshold is between 11 and 22 μM LA for desalivate animals. Together, these results suggest that removal of the submaxillary and sublingual salivary glands raises the LA taste discrimination threshold from between 5.5 and 11 μM to between 11 and 22 μM.
Table 1.
Total fluid intake and two-bottle fluid intake for each test day for all treatment groups
| Test Day | Fluid | SHAM NaCl | SHAM LiCl | DESAL NaCl | DESAL LiCl |
|---|---|---|---|---|---|
| Total Fluid Intake | |||||
| 1 | 88 μM LA | 13.5±0.7 | 9.9±0.8 | 11.8±1.3 | 9.6±0.7 |
| 2 | 44 μM LA | 12.8±0.8 | 11.2±0.6 | 12.4±1.1 | 11.1±0.6 |
| 3 | 22 μM LA | 15.2±0.6 | 13.2±0.9 | 11.4±0.9 | 12.4±0.5 |
| 4 | 11 μM LA | 14.9±0.9 | 14.6±1.5 | 14.2±1.8 | 12.4±0.9 |
| 5 | 5.5 μM LA | 13.3±1.0 | 13.3±0.9 | 14.1±1.1 | 14.0±0.7 |
| 6 | 88 μM LA | 13.8±1.7 | 11.4±1.1 | 13.1±0.8 | 12.6±0.8 |
| Two-Bottle Fluid Intake | |||||
| 1 | Water | 6.0±0.9 | 9.5±0.8 | 4.7±0.9 | 9.5±0.6 |
| 88 μM LA | 7.5±0.7 | 0.5±0.2 | 7.1±1.2 | 0.2±0.2 | |
| 2 | Water | 6.2±1.0 | 10.6±0.5 | 5.9±1.3 | 10.1±0.6 |
| 44 μM LA | 6.6±0.9 | 0.5±0.3 | 6.6±0.9 | 1.0±0.4 | |
| 3 | Water | 6.7±1.0 | 12.4±1.0 | 6.2±1.8 | 10.3±0.6 |
| 22 μM LA | 8.5±1.1 | 0.8±0.5 | 5.2±1.5 | 2.1±0.7 | |
| 4 | Water | 7.9±1.0 | 12.5±1.0 | 8.0±1.8 | 6.8±0.6 |
| 11 μM LA | 7.0±1.1 | 2.1±0.5 | 6.2±1.5 | 6.1±0.7 | |
| 5 | Water | 7.8±1.0 | 7.2±0.6 | 6.6±0.9 | 7.5±0.9 |
| 5.5 μM LA | 5.5±0.6 | 6.1±0.4 | 7.6±1.4 | 6.5±0.8 | |
| 6 | Water | 5.4±0.6 | 11.6±0.8 | 5.0±0.9 | 11.1±0.6 |
| 88 μM LA | 9.0±1.0 | 0.7±0.3 | 8.1±1.4 | 1.5±0.3 | |
Values are presented as means ± SE. DESAL, desalivation; LA, linoleic acid.
Fig. 1.
Linoleic acid (LA) taste discrimination threshold by sham (SHAM) and desalivate (DESAL) animals. Conditioned taste aversion to LA by SHAM (A) and DESAL (B) NaCl (white or light gray bars, respectively)- and LiCl (dark gray and black bars, respectively)-treated animals. The black box indicates the approximate LA taste discrimination threshold. *NaCl-treated significantly different from LiCl-treated, P < 0.05. Values are presented as means ± SE.
There was no difference in the percentage of CT-innervated fungiform papillae with intact taste pores between the left and right side of the tongue [F(1,40) = 0.05, P = 0.83]. Thus the pore counts from each side were totaled in a single value for each animal. Similar to the results observed between each side of the tongue, there were no significant differences in the percentage of fungiform papillae with intact taste pores between any of the groups [F(1,38) = 0.19, P = 0.66] (data not shown).
Experiment 2: CT Responses to LA and AS
The CT was not responsive to LA at any concentration [F(3,24) = 0.58, P = 0.63] (Fig. 2). Furthermore, the lack of CT response to LA was not attributable to decreased nerve viability, since CT responses to 600 mM NaCl were robust and did not change over the course of the electrophysiological recording [F(1,12) = 0.01, P = 0.98].
Fig. 2.
Chorda tympanic nerve (CT) whole nerve activity in response to lingual application of NaCl (600 mM) and LA (11, 22, 44, and 88 μM) mixed with artificial saliva (AS). A: representative trace of CT whole nerve integrated, rectified activity (μV) from a male rat. B: mean ± SE normalized CT responses to lingual application of NaCl and LA mixed with AS.
Experiment 3a: CT Responses to LA and NaCl
The CT was responsive to 15, 30, and 45 mM NaCl in a concentration-dependent manner [F(2,20) = 22.12, P < 0.001] and CT responses to NaCl + LA were significantly greater than to NaCl alone [F(1,10) = 6.20, P < 0.05] overall. Post hoc analysis of the significant interaction between solution and concentration [F(2,20) = 3.93, P < 0.05] indicated that the addition of LA increased CT responses to 30 and 45 mM NaCl (P < 0.05), but not to 15 mM (P = 0.99). Similarly, CT responses were not different between 55, 65, or 75 mM NaCl [F(2,20) = 0.85, P = 0.44]. However, the addition of LA did increase CT responses to NaCl [F(2,20) = 4.77, P < 0.05] at all three concentrations (all P < 0.01; Fig. 3A).
Fig. 3.
CT whole nerve activity in response to lingual application of NaCl (A), sucrose (SUC, B), quinine hydrochloride (QHCl, C), and citric acid (CA, D) mixed with LA. A–D, top: representative trace of CT whole nerve integrated, rectified activity (μV) from a male rat; bottom: mean ± SE normalized CT responses to lingual application of taste stimulus and LA. *Taste stimulus + LA significantly greater than taste stimulus alone, P < 0.05.
Experiment 3b: CT Responses to LA and SUC, CA, and QHCl
As shown in Fig. 3B, the CT was largely unresponsive to the lowest three SUC concentrations and responded only weakly to the more concentrated SUC solutions, although CT responses to SUC increased in a concentration-dependent manner [F(5,40) = 12.80, P < 0.001] overall. In particular, post hoc analysis of the concentration main effect found that CT responses to 18, 25, 50, and 100 mM SUC were not different from each other (all P > 0.19), but these responses were significantly less than CT responses to 200 and 300 mM (P < 0.01). CT responses to 200 and 300 mM SUC were not different from each other (both P = 0.81). Moreover, the addition of LA did not alter CT responses to SUC [F(1,8) = 0.01, P = 0.93].
Similar to the results seen in regard to SUC, CT responses increased with increasing CA concentration [F(5,40) = 36.92, P < 0.001]. In particular, post hoc analysis of the concentration main effect found that CT responses to 1.8, 2.5, and 5 mM CA were not different from each other (all P > 0.08), but these responses were significantly less than CT responses to 10, 20, and 30 mM (all P < 0.01). CT responses to 30 mM CA were greater than CT responses to 20 and 10 mM (all P > 0.01), and CT responses to 20 mM CA were greater than responses to 10 mM (P < 0.01). Moreover, CT responses to 20 and 30 mM CA were not different from each other (both P = 0.93). Finally, the addition of LA did not change CT responses to CA [F(1, 8) = 0.01, P = 0.98] (Fig. 3C).
The CT was responsive to a range of QHCl concentrations [F(5,40) = 15.17, P < 0.001]. In particular, post hoc analysis of the concentration main effect found that CT responses to 1.8 mM were significantly less than responses to 5, 10, 20, and 30 mM QHCl (all P < 0.05), but CT responses to 1.8 and 2.5 mM QHCl were not different from each other (P = 0.44). CT responses to 2.5 mM QHCl were significantly less than responses to 10, 20, and 30 mM QHCl (all P < 0.05), and CT responses to 5, 10, 20, and 30 mM QHCl were not different from each other (all P > 0.20). Finally, CT responses to QHCl were not different between QHCl and QHCl + LA [F(1,8) = 0.02, P = 0.89] (Fig. 3D).
DISCUSSION
Despite compelling evidence that fats (and, specifically, FFAs) have a taste, comparatively less in known about the peripheral neural pathways that carry fat taste information from the tongue to the brain. In this regard, the CT is important for FFA detection, since CTX raises the taste threshold for the FFA LA (31, 43). However, the exact role of the CT in LA taste is unclear, since lingual application of LA produces no CT electrophysiological response (44). In this regard, CTX results in decreased saliva production, through denervation of the submaxillary and sublingual salivary glands (3, 38), and saliva is present in LA behavioral studies but is washed off during CT electrophysiological recordings. Thus LA may require saliva, or a component thereof, to elicit both a behavioral and an electrophysiological CT response.
Saliva and LA
To test this idea, we determined whether removal of the submaxillary and sublingual salivary glands, which are denervated by CTX, impairs LA taste discrimination. We found that decreased saliva production through removal of these salivary glands shifts the LA taste discrimination threshold from between 5.5 and 11 μM (as measured in SHAM animals) to between 11 and 22 μM (DESAL; Fig. 1). Importantly, the magnitude of this shift in LA discrimination is not as large as seen after CTX, which shifts the LA taste discrimination threshold to between 22 and 44 μM (43). Moreover, DESAL animals still can detect 88 and 44 μM LA (as indicated by a decreased LA preference score by LiCl-treated DESAL animals), which may be attributable to saliva produced by the remaining salivary glands and ducts (5, 11) and/or CT sensory information. Finally, these results are not attributable to anatomic changes in the peripheral gustatory system, such as increased papillae ketatosis and shrinkage that is associated with complete elimination of saliva production (30), since there were no differences in the percentage of fungiform papillae with intact taste pores between SHAM and DESAL groups (data not shown; see also Ref. 41). Thus saliva appears to be important for LA taste discrimination, and the effect of CTX in LA taste discrimination is attributable to both destruction of CT sensory input and decreased saliva production.
Given these data, we also recorded CT responses to lingual application of LA mixed with artificial salivary electrolytes. Similar to the results previously obtained with LA mixed with water (44), LA mixed in AS did not produce a detectable CT response at any concentration (Fig. 2). Although these results are puzzling, there are several explanations. First, LA may be a weak stimulus for CT electrophysiological responses although clearly it is an effective stimulus for behavioral responses. Moreover, AS may produce a subtle inhibition of CT activity that whole nerve recording techniques lack the sensitivity to detect. However, this is unlikely, since rinsing with AS increases the baseline spontaneous firing over that seen when rinsing with water (data not shown). Moreover, although rinsing with AS does decrease CT response amplitude to NaCl (26), this is attributable the increased baseline firing when AS is added, rather than a decreased responsiveness of the CT to NaCl.
Furthermore, there are other issues perhaps worth exploring. CT whole nerve recordings reflect the combined activity of many individual CT fibers. Therefore, LA + AS may activate individual CT gustatory neurons whose responses are obscured in whole nerve summation. Extracellular single-cell recordings from geniculate ganglion neurons, which innervate CT TRCs on the anterior tongue, would be able to detect subtle changes in gustatory responses to LA + AS. However, this issue has not been investigated to date using single cell electrophysiological recordings. Second, and more importantly, the AS used contains only the major ions found in saliva. AS does not contain proteins, bicarbonates, enzymes, and mucous naturally found in saliva (11). Moreover, the concentrations of ions found in saliva can vary depending on numerous factors, including dietary state, sex, species, whether a drug (such as pilocarpine) is used to stimulate saliva release, and even time of day (5, 7, 11).
In this regard, one of the primary functions of saliva is to transport gustatory stimuli to taste receptors on the tongue. Therefore, LA may require the assistance of an unknown FFA transporter found in saliva to reach and subsequently activate gustatory receptors. For instance, an 18,000 molecular mass secretory protein (unnamed) found in the von Ebner's glands may carry lipophilic compounds to taste receptors (34). Moreover, the FFA translocase, CD36, which is highly expressed in circumvallate papillae in the back part of the tongue, may be important for FFA taste in the posterior tongue, since inactivation of the CD36 gene abolishes the preference for FFA in mice (20). However, CD36 is not present in CT-innervated fungiform papillae in the anterior tongue (20) and has not been found in saliva.
Moreover, one important candidate molecule for LA taste processing is Na+ because salivary Na+ can modulate taste sensitivity and is also important for nerve cell function. In particular, Na+ taste thresholds are directly related to salivary Na+ concentration, since Na+ detection decreases with decreased salivary Na+ concentration and, conversely, increases as salivary Na+ concentration increases (2, 27). This effect, presumably, results from adaptation of taste cells to constant weak Na+ stimulation. Thus salivary Na+ could be important for LA taste transduction as well. In this regard, although AS contains Na+ (i.e., 15 mM), salivary Na+ concentrations fluctuate from ∼15 to 40 mM NaCl (7, 40), suggesting salivary Na+ concentrations greater than the 15 mM NaCl found in AS may be important for LA taste. In fact, we found in experiment 3a that LA increases CT responses to 30 and 45 mM NaCl, Na+ concentrations that are in the physiological range of those found naturally in saliva, providing support for the importance of salivary Na+ in LA taste.
Given these ideas, one future approach to access the contribution of natural saliva would be to simply collect saliva from rat sublingual and submaxillary salivary glands (possibly via either electrical or drug-induced gland stimulation). However, it is unknown whether an adequate volume of saliva (i.e., ∼100 ml) could be collected for use in an electrophysiological experiment, unless stimulated saliva from many animals was pooled. Nevertheless, such an experiment would mimic the natural milieu normally bathing taste receptors of the tongue, which would definitively determine whether components of saliva are critical for LA taste. Also, future studies may directly test the role of Na+ in LA taste. Using dilute NaCl (30 or 45 mM) as a rinse in whole nerve recordings of CT responses to LA or measuring the LA discrimination threshold of partially desalivated animals that are given LA mixed with 30 or 45 mM NaCl would determine whether salivary Na+ is important for LA taste.
NaCl, SUC, CA, and QHCl + LA
To continue our exploration of fat as a potent taste stimulus, we measured the integrated responses of the CT nerve to coapplication of LA and NaCl, SUC, CA, and QHCl. The addition of LA increased CT responses to all three concentrations of NaCl (i.e., 55, 65, and 75 mM; Fig. 3A). Thus LA modulates CT responses to NaCl. These results differ slightly from the effects we previously observed in CT responses to LA-MSG mixtures (44), in which the addition of LA increased CT responses to only 40 and 100 mM MSG. It is likely that the lack of enhancement to 300 mM MSG is attributable to either LA insensitivity or a saturation of MSG electrophysiological activity at this MSG concentration. Therefore, if CT responses to 40, 60, and 100 mM MSG were recorded, it is likely that LA would enhance CT responses to all three MSG concentrations, which is similar to the effects observed with 55, 65, and 75 mM NaCl.
Unlike the effects observed with LA + NaCl (as well as the aforementioned LA + MSG) taste mixtures, the addition of LA did not alter CT responses to SUC, CA, and QHCl (Fig. 3, B–D). These results are surprising, but suggest at least two possibilities. LA may enhance CT responses to SUC, CA, and QHCl, but, because the CT is weakly responsive to these taste stimuli in rats, CT whole nerve recordings may lack the sensitivity to detect these subtle changes. In this regard, LA enhancement of both NaCl and MSG CT responses occurs only at relatively dilute concentrations, since unpublished observations by Pittman et al. found that the addition of LA does not alter CT responses to more concentrated (i.e., 250 and 500 mM) NaCl solutions nor does the addition of LA increase CT responses to 300 mM MSG. Therefore, LA may only enhance CT responses to comparatively dilute taste stimuli. Measurement of electrophysiological responses of other gustatory nerves that are more responsive to SUC, CA, and QHCl, such as the greater superficial petrosal and the glossopharyngeal nerves (15, 45), or single cell extracellular recordings from the geniculate ganglion (the location of CT fiber cell bodies) to LA-taste mixtures would test this idea.
It is more probable, however, that LA may enhance CT responses to NaCl (and previously with MSG) only, perhaps by specifically modulating gustatory processing of Na+, which is found in both MSG and NaCl. This possibility may be explored in future studies using a pharmacological antagonist of epithelial Na+ channels or a nonsodium salt, such as potassium chloride.
It should be noted that both NaCl taste stimuli used in our current study and MSG stimuli used in our previous study (44) were mixed with water (to minimize decreased CT responsiveness from the adaptation of TRCs to Na+; see Ref. 26), whereas SUC, CA, and QHCl were mixed with AS. Thus one concern is that AS may prevent LA from enhancing other taste stimuli. However, it is unlikely that the lack of LA modulation of SUC, CA, and QHCl is attributable to a suppressive effect of AS for several reasons. First, AS increases baseline whole nerve CT activity (through addition of ions found in AS; see Ref. 26). Second, although AS slightly decreases CT responses to hydrochloric acid and QHCl, the addition of AS increases CT responses to SUC (23–26). Third, unpublished observations from our laboratory found that LA does not increase CT responses to SUC even when SUC is mixed in water. Thus the lack of LA modulation of taste stimuli, especially in regard to SUC, is not attributable to suppressive effects of AS. Nevertheless, comparison of CT response to LA-taste stimuli solutions mixed in both water and AS would differentiate between the effect of AS on MSG and NaCl and other taste stimuli.
In summary, these experiments explored the role of the CT and saliva in LA gustatory processing. We found that saliva is important for LA taste discrimination, since removal of the submaxillary and sublingual salivary glands (which are denervated following CTX) raises the LA taste discrimination threshold. Thus impairment of LA taste detection following CTX is attributable to removal of CT afferent (sensory information) as well as decreased saliva. Surprisingly, the CT is unresponsive to lingual application of LA, even when mixed with AS. However, saliva is a watery solution made of a large number of proteins, ions (e.g., K+ and Cl−), enzymes, and even mucous (16), any of which may be important for fat taste and that are not found in the AS used in the current study.
Furthermore, the addition of LA increased CT responses to NaCl, but not to SUC, CA, and QHCl. One explanation for differing LA effects is that the CT is weakly responsive to SUC, CA, and QHCl and much more responsive to NaCl (and MSG). Because the effects of LA on CT responses to taste stimuli appear to be subtle, CT whole nerve recordings lack the resolution to detect small changes in CT responses to LA-SUC, -CA, and -QHCl mixtures. Alternatively, LA may only modulate taste responses to MSG and NaCl (or the common component of both taste stimuli, Na+). Future electrophysiological recordings from CT-innervated geniculate ganglion sensory neurons and other gustatory nerves will further elucidate the role of LA, as well as other FFAs, in the peripheral gustatory system.
Perspectives and Significance
There is compelling behavioral evidence that dietary fat's allure in motivating consumption comes in part from its sensory attributes, particularly taste. In particular, there are many converging studies in rats and mice utilizing a range of methods demonstrating the potency of fat taste in consumption. However, it has been extremely difficult to discern how fat is having such powerful behavioral effect in physiological studies of the peripheral gustatory system. Fat, in the form of FFA, drives intake, but fails to activate taste afferent neurons in the way or degree seen in behavioral studies. This disconnect reminds us of the challenges inherent in conducting parallel behavioral and physiological studies to gain an understanding of the physiological process underlying function. The approach may seem straightforward, but the riddle may not be easily solved without consideration of unique factors. Fat taste poses unique problems not evident in physiological studies of sweet, salt, sour, bitter, and amino acid taste. One unique factor demonstrated here is the necessity of saliva. But this is only one factor that partially explains the riddle; there are many other factors to examine to fully explain how the peripheral gustatory system detects and codes information about fat taste.
GRANTS
This research was supported by NIH grants from the National Institute on Deafness and Communication Disorders (DC-04785: R. J. Contreras; DC-008934-02: J. M. Stratford).
ACKNOWLEDGMENTS
Portions of these data were presented in preliminary form at the 29th annual meeting of the Association for Chemoreception Sciences in Sarasota, FL, April 25–27, 2007; the 15th annual meeting of the Society for the Study of Ingestive Behavior in Steamboat, CO, July 24–29, 2007; the 37th annual meeting of the Society for Neuroscience in San Diego, CA, November 3–7, 2007; and the 15th International Symposium on Olfaction and Taste in San Francisco, CA, July 21–26, 2008.
REFERENCES
- 1.Breza JM, Curtis KS, Contreras RJ. Monosodium glutamate but not linoleic acid differentially activates gustatory neurons in the rat geniculate ganglion. Chem Senses 32: 833–846, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Contreras RJ, Catalanotto FA. Sodium deprivation in rats: salt thresholds are related to salivary sodium concentrations. Behav Neural Biol 29: 303–314, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Contreras RJ, Gomez MM, Norgren R. Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol 190: 373–394, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Damak S, Cartoni C, Yasumatsu K, le Coutre J, Ninomiya Y. GPR40 knockout mice have diminished taste responses to fatty acids. Chem Senses (Abstr) In press [Google Scholar]
- 5.Doty RL. Handbook of Olfaction and Gustation New York: Dekker, 2003 [Google Scholar]
- 6.El-Yassimi A, Hichami A, Besnard P, Akhtar Khan N. Linoleic acid induces calcium signaling, SRC-kinase phosphorylation and neurotransmitters release in mouse CD36-positive gustatory cells. J Biol Chem 283: 12949–12959, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Field J, Magoun HW, Hall WE. The sense of taste. In: Handbook of Physiology, Neurophysiology Washington, DC: Am Physiol Soc, 1959, p. 507 [Google Scholar]
- 8.Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett 414: 461–464, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Fukuwatari T, Shibata K, Iguchi K, Saeki T, Iwata A, Tani K, Sugimoto E, Fushiki T. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol Behav 78: 579–583, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Galli C, Rise P. Origin of fatty acids in the body: endogenous synthesis versus dietary intakes. Eur J Lipid Sci Technol 108: 521–525, 2006 [Google Scholar]
- 11.Getchell TV. Smell and Taste in Health and Disease New York: Raven, 1991 [Google Scholar]
- 12.Gilbertson TA. Gustatory mechanisms for the detection of fat. Curr Opin Neurobiol 8: 447–452, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol Cell Physiol 272: C1203–C1210, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann NY Acad Sci 855: 165–168, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Harada S, Yamamoto T, Yamaguchi K, Kasahara Y. Different characteristics of gustatory responses between the greater superficial petrosal and chorda tympani nerves in the rat. Chem Senses 22: 133–140, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Hart PS. Salivary abnormalities in Prader-Willi syndrome. Ann NY Acad Sci 842: 125–131, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hirata S, Nakamura T, Ifuku H, Ogawa H. Gustatory coding in the precentral extension of area 3 in Japanese macaque monkeys; comparison with area G. Exp Brain Res 165: 435–446, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol 285: R447–R454, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kinney NE, Antill RW. Role of olfaction in the formation of preference for high-fat foods in mice. Physiol Behav 59: 475–478, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177–3184, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundy RF, Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol 82: 2970–2988, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Lundy RF, Jr, Contreras RJ. Tongue adaptation temperature influences lingual nerve responses to thermal and menthol stimulation. Brain Res 676: 169–177, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med 11: 216–229, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Matsuo R, Yamamoto T. Effects of inorganic constituents of saliva on taste responses of the rat chorda tympani nerve. Brain Res 583: 71–80, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Matsuo R, Yamamoto T. Taste nerve responses during licking behavior in rats: importance of saliva in responses to sweeteners. Neurosci Lett 108: 121–126, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Matsuo R, Yamauchi Y, Morimoto T. Role of submandibular and sublingual saliva in maintenance of taste sensitivity recorded in the chorda tympani of rats. J Physiol 498: 797–807, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBurney DH, Pfaffmann C. Gustatory adaptation to saliva and sodium chloride. J Exp Psychol 65: 523–529, 1963 [Google Scholar]
- 28.McCormack DN, Clyburn VL, Pittman DW. Detection of free fatty acids following a conditioned taste aversion in rats. Physiol Behav 87: 582–594, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mela DJ. Sensory assessment of fat content in fluid dairy products. Appetite 10: 37–44, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Nanda R, Catalanotto FA. Long-term effects of surgical desalivation upon taste acuity, fluid intake, and taste buds in the rat. J Dent Res 60: 69–76, 1981 [DOI] [PubMed] [Google Scholar]
- 31.Pittman D, Crawley ME, Corbin CH, Smith KR. Chorda tympani nerve transection impairs the gustatory detection of free fatty acids in male and female rats. Brain Res 1151: 74–83, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Pittman DW, Contreras RJ. Rearing on basal or high dietary NaCl modifies chorda tympani nerve responses in rats. Physiol Behav 77: 277–289, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Pittman DW, Labban CE, Anderson AA, O'Connor HE. Linoleic and oleic acids alter the licking responses to sweet, salt, sour, and bitter tastants in rats. Chem Senses 31: 835–843, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Schmale H, Holtgreve-Grez H, Christiansen H. Possible role for salivary gland protein in taste reception indicated by homology to lipophilic-ligand carrier proteins. Nature 343: 366–369, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol 293: R1823–R1832, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 tastesignaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504–R1513, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JC. Gustation as a factor in the ingestion of sweet and fat emulsions by the rat. Physiol Behav 82: 181–185, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Smith JC, Miller IJ, Jr, Krimm RF, Nejad MS, Beidler LM. A comparison of the effects of bilateral sections of the chorda tympani nerve and extirpation of the submaxillary and sublingual salivary glands on the eating and drinking patterns of the rat. Physiol Behav 44: 435–444, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci 16: 159–221, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Spielman AI. Interaction of saliva and taste. J Dent Res 69: 838–843, 1990 [DOI] [PubMed] [Google Scholar]
- 41.St. John SJ, Markison S, Guagliardo NA, Hackenberg TD, Spector AC. Chorda tympani transection and selective desalivation differentially disrupt two-lever salt discrimination performance in rats. Behav Neurosci 111: 450–459, 1997 [PubMed] [Google Scholar]
- 42.St. John SJ, Spector AC. Combined glossopharyngeal and chorda tympani nerve transection elevates quinine detection thresholds in rats (Rattus norvegicus). Behav Neurosci 110: 1456–1468, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Stratford JM, Curtis KS, Contreras RJ. Chorda tympani nerve transaction alters linoleic acid taste discrimination by male and female rats. Physiol Behav 89: 311–319, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Stratford JM, Curtis KS, Contreras RJ. Linoleic acid increases chorda tympani nerve responses to and behavioral preferences for monosodium glutamate by male and female rats. Am J Physiol Regul Integr Comp Physiol 295: R764–R772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanimura S, Shibuya T, Ishibashi T. Neural responses of the glossopharyngeal nerve to several bitter stimuli in mice. Comp Biochem Physiol Comp Physiol 108: 189–194, 1994 [PubMed] [Google Scholar]
- 46.Verhagen JV, Rolls ET, Kadohisa M. Neurons in the primate orbitofrontal cortex respond to fat texture independently of viscosity. J Neurophysiol 90: 1514–1525, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Wong C, Marwick TH. Obesity cardiomyopathy: diagnosis and therapeutic implications. Nat Clin Pract Cardiovasc Med 4: 480–490, 2007 [DOI] [PubMed] [Google Scholar]