Abstract
By sensing intracellular energy levels, ATP-sensitive potassium (KATP) channels help regulate vascular tone, glucose metabolism, and cardioprotection. SUR2 mutant mice lack full-length KATP channels in striated and smooth muscle and display a complex phenotype of hypertension and coronary vasospasm. SUR2 mutant mice also display baseline cardioprotection and can withstand acute sympathetic stress better than normal mice. We now studied response to a form of chronic stress, namely that induced by 4 wk of daily exercise on SUR2 mutant mice. Control mice increased exercise capacity by 400% over the training period, while SUR2 mutant mice showed little increase in exercise capacity. Unexercised SUR2 mutant showed necrotic and regenerating fibers in multiple muscle skeletal muscles, including quadriceps, tibialis anterior, and diaphragm muscles. Unlike exercised control animals, SUR2 mutant mice did not lose weight, presumably due to less overall exertion. Unexercised SUR2 mutant mice showed a trend of mildly reduced cardiac function, measured by fractional shortening, (46 ± 4% vs. 57 ± 7% for SUR2 mutant and control, respectively), and this decrease was not exacerbated by chronic exercise exposure. Despite an improved response to acute sympathetic stress and baseline cardioprotection, exercise intolerance results from lack of SUR2 KATP channels in mice.
Keywords: KATP channel, sulfonylurea receptor, SUR2, skeletal myopathy, exercise intolerance
the mechanisms that underlie the beneficial effects of regular exercise on metabolism and endurance are incompletely understood. ATP-sensitive potassium channels (KATP channels) are found in muscle and the cardiovascular system and serve as cellular energy sensors. KATP channels include a regulatory subunit, sulfonylurea receptor (SUR) 1 or 2, and a pore-forming potassium channel, Kir6.1 or 6.2. Genetic deletion of the gene encoding Kir6.2 affects KATP channels in the pancreas, heart, and skeletal muscle due to the lack of the pore-forming subunit of KATP channels (18). Two previous studies have investigated the role of KATP channels in adaptation to chronic stress and exercise, and both demonstrated an impaired response in Kir6.2-null mice (15, 24). Although defects in skeletal muscle and cardiovascular function were reported, the role of KATP channel involvement is complicated by the ability of Kir6.2 to couple with either sulfonylurea receptor 1 (SUR1) or 2 (SUR2).
In the heart, KATP channels play critical roles during periods of sympathetic stress and protect the heart against ischemia (16, 23, 27). Kir6.2-null mice stressed with 21 days of experimental hypertension had significantly increased mortality compared with control mice (14). Cardiac function was impaired at baseline and after dobutamine exposure, and hypertensive Kir6.2-null mice developed cardiac hypertrophy with fibrosis (14). Similar results were observed in Kir6.2-null mice when hypertension was induced by aortic banding (26). These Kir6.2-null mice developed congestive heart failure with impaired left ventricular function and cardiac hypertrophy with fibrosis, ultimately resulting in impaired exercise tolerance and increased mortality (26). In both studies, administration of verapamil, a calcium channel antagonist, improved phenotype in hypertensive Kir6.2-null mice, implicating aberrant cardiomyocyte calcium handling as an underlying mechanism of disease (14, 26).
KATP channels are also known to play important roles in skeletal muscle contraction and fatigue. Activation of KATP channels by the KATP channel opener pinacidil during fatigue limits action potential amplitude, thereby reducing the amount of calcium that enters the cell and force generation (8). While the fiber's overall ability to produce force during fatigue is compromised, energy stores are preserved for later utilization. The absence of KATP channels results in contractile dysfunction secondary to multiple mechanisms, including direct fiber damage, increased levels of unstimulated intracellular calcium during fatigue, and excessive depolarization (5, 6, 9). As a result, muscles isolated from Kir6.2-null mice develop increased resting tension and generate less force following fatigue compared with isolated control muscles (6, 9). Because of these deficiencies, skeletal muscle lacking KATP channels exhibits a faster rate of fatigue (5, 6). KATP channels are also directly involved with glucose uptake by skeletal muscle, and in both Kir6.2 and SUR2 mutant mice, glucose uptake by skeletal muscle is enhanced (4, 17).
Kir6.2-null mice have an impaired response to chronic exercise (15). In normal mice, 4 wk of daily swimming was associated with a marked increase in exercise capacity and weight loss, whereas similarly exercised Kir6.2-null mice did not increase exercise capacity or lose weight (15). Only 70% of Kir6.2-null mice survived the 4-wk exercise protocol, while all control mice survived. This increased mortality presumably resulted from cardiovascular deficits, as Kir6.2-null mice developed cardiac hypertrophy with cardiomyocyte damage resulting in significantly reduced cardiac output (15). Treadmill running elicited a similar response in Kir6.2-null mice (24). Kir6.2-null mice were able to increase exercise capacity but significantly less than control cohorts. Skeletal muscle from exercised Kir6.2-null mice demonstrated a significant increase in centrally nucleated fibers, indicating enhanced muscle damage/regeneration. Diaphragm muscle from exercised Kir6.2-null mice contained the most severe damage with increased fibrosis and the presence of inflammatory cells (24). No significant muscle damage was present in exercised control animals or sedentary Kir6.2-null mice.
In the current investigation, we examined the effects of exercise training on SUR2 mutant mice. SUR2 mutant mice were unable to increase exercise capacity. However, unlike Kir6.2-null mice, we found an underlying skeletal muscle myopathy in nonexercised SUR2 mutant mice. Unlike exercised controls, SUR2 mutant mice did not lose weight with exercise, but these animals also did not exercise as much as controls. Although baseline fasting serum glucose levels are lower in unexercised SUR2 mutant mice, this difference was not evident in exercised SUR2 mutants, suggesting that serum glucose levels are unlikely to account for impaired exercise tolerance. A modest reduction in cardiac fractional shortening was observed in SUR2 mutant mice, and this decrease was not worsened by exercise training. Therefore, an underlying skeletal muscle myopathy due to lack of sarcolemmal KATP channels in skeletal muscle likely accounts for the impaired response to chronic exercise.
METHODS
Animals.
SUR2 mutant mice were previously generated by targeted disruption of exons 14–18 encoding the nucleotide binding fold 1, as described previously (4). Heterozygous SUR2 mutant mice were bred onto the FVB mouse substrain for more than five generations, as described previously (13). Heterozygous mutant mice were interbred to generate homozygous mutant SUR2 animals. Female SUR2 mutant mice were used for experimental studies since these mice have less vascular spasm and a better survival rate than male mice (3). Age-matched female wild-type FVB mice (n = 19, were purchased from Harlan Sprague Dawley, (Indianapolis, IN) for control cohorts. Animals were housed, treated, and handled in accordance with guidelines set forth by the University of Chicago's Institutional Animal Care and Use Committee, the Animal Welfare Act regulations, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Exercise protocol.
Mice were randomly assigned into four cohorts: sedentary control, sedentary SUR2 mutant, exercised control, and exercised SUR2 mutant (all n = 5, n = 20 total). Exercised cohorts underwent 4 wk of daily exercise consisting of swimming or treadmill running. Mice from the exercise cohorts were subjected to swimming to exhaustion in warm water (∼35°C) under the careful watch of an observer daily except on days exercise capacity was measured. To measure exercise capacity, mice underwent treadmill running (Columbus Instruments, Columbus, OH) at 20 m/min at a 20° incline at timepoints throughout the 4-wk exercise period [week 0 (baseline), week 1, week 2, and week 4). Mice ran until they were consistently unable to maintain pace with the treadmill, despite physical prodding. Exercise capacity was defined as the total distance run by the mouse. Body mass for all mice was measured on a weekly basis. Experiments on the exercised cohorts were conducted and tissues harvested within 4 days after completion of the chronic exercise period.
Blood glucose measurements.
Blood glucose was measured using a HemoCue B Glucose Analyzer (HemoCue, Lake Forest, CA) on blood collected from tail vein pricks. We recorded glucose measurements from fed mice (∼8:00 AM) following the 4-wk exercise period, and fasting blood glucose measurements were collected after a 16-h overnight fast. Glucose tolerance tests were then administered (2 mg/g ip glucose) with subsequent blood glucose measurements at 30, 60, and 120 min after glucose injection.
Echocardiography.
All mice underwent echocardiography to measure cardiac function (VisualSonics Model 660; VisualSonics, Toronto, Ontario, Canada). Isoflurane anesthesia was delivered at 3% during induction and at 0.5% per gram body wt for maintenance via nose cone. Images were captured using a 40-mHz mechanical transducer. Left ventricular fractional shortening was calculated from images taken via two-dimensional M mode from the parasternal short-axis view. Percent left ventricular fractional shortening was defined as [(diastolic left ventricular internal diameter − systolic left ventricular internal diameter)/diastolic left ventricular internal diameter] × 100.
ECG telemetry.
Continuous ambulatory ECG recordings were obtained from exercised (n = 2) and sedentary (n = 2) SUR2 mutant mice using PhysioTel Implants (Model TA10EA-F20; Data Sciences International, St. Paul, MN) (3). Under isoflurane anesthesia, monitors were surgically implanted subcutaneously on the dorsum and ECG leads tunneled subcutaneously into a Lead II configuration and sutured into place using 5/0 monofilament suture. ECG data were then analyzed by manual screening for evidence of electrocardiographic changes. The incidence and duration of each episode of coronary vasospasm during the screening periods were documented and reported as seconds of ST segment elevation per hour of screened ECG data.
Histopathology.
Heart and skeletal muscle, including quadriceps, tibialis, diaphragm, triceps, extensor digitorum longus, and gastrocnemius/soleus were harvested for histological analysis. Hearts were weighed, and then fixed in 10 × buffered formalin. Skeletal muscle was harvested and fixed in 10 × buffered formalin or snap-frozen in liquid nitrogen-cooled isopentane. Formalin-fixed sections of heart, quadriceps, diaphragm, and tibialis were paraffin-embedded, sectioned at a thickness of 8 μm, and then stained with hematoxylin and eosin. Masson trichrome staining was conducted for visualization of collagen. The hematoxylin-and-eosin-stained tissue sections were then blinded and screened for evidence of damage, including myopathic fibers, fibrosis, central nuclei, the presence of inflammation and/or inflammatory cells, and other abnormalities. In this context, myopathic myofibers were defined as those with features of single-myofiber injury by displaying necrotic or regenerating basophilic myofibers (1). The number of myopathic fibers, number of fibers with central nuclei, total fiber number, and average fiber size on a single section of quadriceps muscle were quantified by investigators blinded to genotype and cohort for each mouse. The whole cross-sectional area of the muscle was examined to account for regional variability. Photomicrographs were taken using a Zeiss Axioskop microscope (Carl Zeiss, Oberkochen, Germany) equipped with a QImaging Retiga EXi digital camera (Surrey, Canada) using the AxioVision software package.
Immunostaining for embryonic myosin heavy chain was conducted using the F1.652 monoclonal antibody obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). Quadriceps were frozen in liquid nitrogen-cooled isopentane and sectioned at a thickness of 10 μm. The mouse on mouse (MOM) immunodetection kit was used with the avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) to reduce background reactivity. Sections were fixed in ice-cold methanol for 2 min and stained with 1:5 dilution of F1.652 primary antibody. Secondary antibody staining using FITC-Avidin (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted at 1:60 was then conducted, and fluorescent images were viewed and collected using the Axioskop, AxioCam, and AxioVision microscope, camera, and software systems (Carl Zeiss, Oberkochen, Germany). Embryonic myosin heavy-chain positive fibers were quantified on a single section of quadriceps from mice of the two sedentary cohorts by investigators blinded to genotype and cohort for each mouse.
Measurement of serum creatine kinase.
Blood was collected in anesthetized mice by eye bleed on cohorts of age-matched sedentary female SUR2 mutant and control mice. Serum was isolated using serum separator tubes and sent to an outside laboratory (RADIL, Columbia, MO) for analysis.
Statistical analysis.
Data are reported as means ± SE, and statistical analysis was conducted using GraphPad Prism version 4.0 for Macintosh (GraphPad Software, San Diego CA, www.graphpad.com). Student's t-tests, one-way ANOVA with post hoc Tukey's multiple-comparison tests, or two-way ANOVA with repeated measures and post hoc Bonferroni comparison of means were used where appropriate. Significance was set at a level of P = 0.05.
RESULTS
SUR2 mutant mice display reduced exercise capacity.
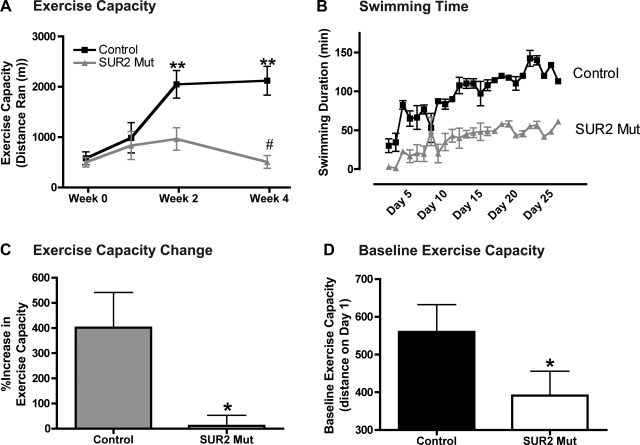
Female SUR2 mutant (12 ± 2 wk of age) and age-matched, female control mice underwent 4 wk of daily exercise that include both treadmill running and swimming. We studied female mice since male SUR2 mutant mice do not survive as well (3). Mice were subjected daily to swimming until exhaustion, and exercise capacity was measured by treadmill running over time (weeks 0, 1, 2, and 4), as described previously (15, 24). Control mice (n = 5) significantly increased exercise capacity from 580 ± 130 m at week 0 to 2,121 ± 287 m at week 4 (P < 0.001, Fig. 1A). SUR2 mutant mice (n = 5) did not increase exercise capacity after 4 wk (501 ± 96 m and 508 ± 125 m at week 0 and week 4, respectively; Fig. 1A). SUR2 mutant mice displayed a slight, insignificant increase in exercise capacity after week 2 (963 ± 224 m) but were unable to maintain this trend. After 4 wk, control mice had a significantly greater exercise capacity at week 4 (2,121 ± 287 m) than SUR2 mutant mice (501 ± 125 m, P < 0.001). Additionally, control mice consistently were able to swim longer than SUR2 mutant mice throughout the exercise period (Fig. 1B). Overall, control mice increased exercise capacity by 401 ± 140%, in which SUR2 mutants had an 11 ± 42% increase (Fig. 1C). It is interesting to note that baseline exercise ability (exercise capacity prior to the 4-wk regimen) was also less in SUR2 mutants compared with controls (n = 10, 390 ± 65 m vs. 560 ± 72 m, respectively, P < 0.05, Fig. 1D). For this study, we included the sedentary cohort, as well as that cohort destined to complete the 4-wk program. All control mice were able to complete the 4-wk exercise protocol, while one SUR2 mutant mice died, while swimming on day 9 of exercise (n = 1/5), presumably from sudden cardiac death previously described in SUR2 mutant mice (3).
Fig. 1.
SUR2 mutant mice cannot increase exercise capacity. Female SUR2 mutant (n = 5) and control (n = 5) underwent 4 wk of daily exercise by swimming or treadmill running. A: exercise capacity was determined by treadmill running at week 0 (baseline), week 1, week 2, and week 4 (conclusion of study). Mice ran at 20 m/min at a 20° incline until they were unable to maintain pace despite physical prodding. Control mice significantly increased exercise capacity, whereas SUR2 mutant mice were unable to do so. **P < 0.001 vs. week 0, #P < 0.001 vs. week 4 control, both by ANOVA. B: control mice were able to consistently swim longer than SUR2 mutant mice. C: control mice demonstrated a four-fold increase exercise capacity, while SUR2 mutant mice were unable to increase exercise capacity. *P < 0.05 vs. control by Student's t-test. D: baseline exercise capacity for mice in both sedentary and exercised cohorts was measured. SUR2 mutant mice (n = 10) exhibit reduced exercise capacity at baseline compared with match control mice (n = 10). *P < 0.05 vs. control by Student's t-test.
Sedentary SUR2 mutant mice have skeletal muscle myopathy.
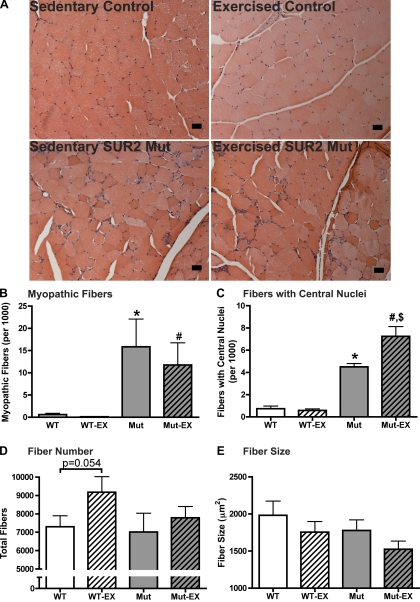
Quadriceps muscles were isolated from control and SUR2 mutant, exercised, and sedentary cohorts. Quadriceps muscle from both sedentary and exercised SUR2 mutant mice contained myopathic fibers at significantly higher numbers than either control cohort (P < 0.05, Fig. 2). Myopathic myofibers were defined as those with features of single-myofiber injury by displaying necrotic or regenerating basophilic myofibers (1). Sedentary control (n = 5) and sedentary SUR2 mutant mice (n = 5) had 0.6 ± 0.3 and 16 ± 6.2.1 myopathic fibers per thousand total fibers, respectively (P < 0.05, Fig. 2B). Exercised control (n = 5) and exercised SUR2 mutant mice (n = 4) had 0.1 ± 0.03 and 12 ± 5.0 myopathic fibers, respectively (P < 0.05, Fig. 2B). This degree of exercise did not increase pathological evidence of muscle damage in either control or SUR2 mutant cohorts. In exercised and sedentary SUR2 mutants, myopathic fibers were typically present in foci around the periphery of the quadriceps muscle. Sedentary SUR2 mutant mice (n = 5) had increased numbers of fibers with central nuclei compared with sedentary controls (n = 5, 4.5 ± 0.3 and 0.7 ± 0.2 fibers with central nuclei per thousand, respectively, P < 0.001, Fig. 2C). Similarly, exercised SUR2 mutants had increased centrally nucleated fibers (n = 4, 7.3 ± 0.9 fibers with central nuclei per thousand, P < 0.01, Fig. 2C). This level of exercise had no effect on control mice (n = 5, 0.5 ± 0.1 fibers with central nuclei per thousand), consistent with a more subtle damage that is sufficient to promote regeneration in SUR2 mutant muscles. While overall fiber number was not significantly different between any of the cohorts (7,301 ± 602 and 7,020 ± 1,018 fibers for sedentary control (n = 5) and SUR2 mutant (n = 5) cohorts, respectively, Fig. 2D), exercise did promote a trend toward increased fiber number in control animals (9,178 ± 848 vs. 7,301 ± 602 total fibers for exercised (n = 4) and control (n = 5) sedentary cohorts, respectively, P = 0.0544). Average fiber size was similar between sedentary control (n = 5, 1,982 ± 191 μm2, Fig. 2E) and SUR2 mutant (n = 5, 1,778 ± 143 μm2) mice and was not significantly affected by exercise (1,755 ± 143 μm2 and 1,524 ± 110 μm2 for control and SUR2 mutant mice, respectively). In addition, there was no evidence of dystrophic muscle pathology, such as gross fibrosis or fatty replacement.
Fig. 2.
Skeletal muscle myopathy in unexercised SUR2 mutant mice. Quadriceps muscle was isolated from sedentary and exercised cohorts of control (n = 5 and 5, respectively) and SUR2 mutant mice (n = 4 and 4, respectively). A: representative photomicrographs of paraffin-embedded quadriceps muscle from sedentary and exercised cohorts of control and SUR2 mutant mice. Sedentary SUR2 mutant mice display skeletal muscle myopathy that does not worsen with exercise. Myopathic fibers were typically present in foci around the muscle periphery. (Scale bar = 200 μm or 10 μm). B: myopathic fibers are increased in SUR2 mutant mice. *P < 0.05 vs. sedentary control, #P < 0.05 vs. exercised control by Student's t-test. C: centrally nucleated fibers are increased in SUR2 mutant muscle. *P < 0.001 vs. sedentary control, #P < 0.001 vs. exercised control, $P < 0.01 vs. sedentary SUR2 mutant, all by ANOVA and Tukey's multiple-comparison tests. D: quantification of total fiber number. E: quantification of fiber size.
The tibialis anterior muscles were also examined and showed evidence of myopathic pathology. Foci of necrotic and regenerating fibers were present across the muscle body where sedentary control (n = 5) and SUR2 mutant mice (n = 5) had 2.4 ± 1.8 and 10.9 ± 7.8 myopathic fibers per 1,000, respectively (see Supplemental Fig. 1 in the online version of this article). Exercise did not worsen the myopathy between the exercised control (n = 5, 0.3 ± 0.1 myopathic fibers per 1,000) and SUR2 mutant (n = 4, 12.1 ± 8.8 myopathic fibers per 1,000). The tibialis anterior muscles from sedentary SUR2 mutant mice had significantly increased numbers of centrally nucleated fibers compared with sedentary control mice (n = 5, 24 ± 10 and 2 ± 0.3 fibers with central nuclei per thousand, respectively, P < 0.05). As in quadriceps muscles, total fiber number was comparable between all cohorts (sedentary control = 2,404 ± 105, n = 5; exercised control = 2,739 ± 253, n = 5; sedentary SUR2 mutant = 2,468 ± 88, n = 5; exercised SUR2 mutant 3,494 ± 1,105, n = 4). However, for tibialis anterior, sedentary SUR2 mutant mice (n = 5) did have significantly decreased fiber size compared with controls (n = 5) (1,280 ± 69 vs. 1,499 ± 36 μm2, respectively, P < 0.05). Exercise did not affect fiber size in either SUR2 mutant (n = 3) or control mice (n = 5) (1,161 ± 56 and 1,310 ± 113 μm2, respectively). Like quadriceps muscle, there were no dystrophic changes with or without exercise in SUR2 mutant tibialis anterior muscles.
The extensor digitorum longus (EDL) muscle showed similar changes in SUR2 mutants. EDL from sedentary control (n = 5) and SUR2 mutant mice (n = 4) had an increase in myopathic fibers between genotypes, 0.3 ± 0.3 and 2.2 ± 1.6 myopathic fibers per 1,000, respectively, (see Supplemental Fig. 2 in the online version of this article). EDL from SUR2 mutant mice had comparatively fewer myopathic fibers than either quadriceps or tibialis anterior muscles. Exercise did not worsen the myopathy in control (n = 5, 1.4 ± 1.4 myopathic fibers per 1,000) and SUR2 mutant (n = 4, 0.5 ± 0.5 myopathic fibers per 1,000) cohorts. Sedentary SUR2 mutant mice (n = 4) had significantly increased centrally nucleated fibers compared with sedentary control mice (n = 5, 14.6 ± 4.2 and 6.6 ± 1.9 fibers with central nuclei per thousand, respectively, P < 0.05). Like the other muscle groups, exercise did not exacerbate the number of myopathic fibers in the SUR2 mutant muscle [22.5 ± 8.0 and 4.7 ± 1.8 fibers with central nuclei per thousand for SUR2 mutant (n = 4) and control (n = 5), respectively]. Diaphragm muscles from control mice (n = 5) were relatively free of damage, as evidenced by lack of myopathic fibers, fibrosis, and inflammatory infiltrate. In contrast, diaphragm skeletal muscle from SUR2 mutant mice contained myopathic fibers qualitatively similar to that observed in quadriceps muscle (see Supplemental Fig. 3 in the online version of this article). Exercise did not improve or worsen diaphragm muscle damage in either control or SUR2 mutant mice, and diaphragm muscle from exercised SUR2 mutant control mice did not show the extensive fibrosis and inflammatory infiltrate observed in exercised Kir6.2-null mice (24).
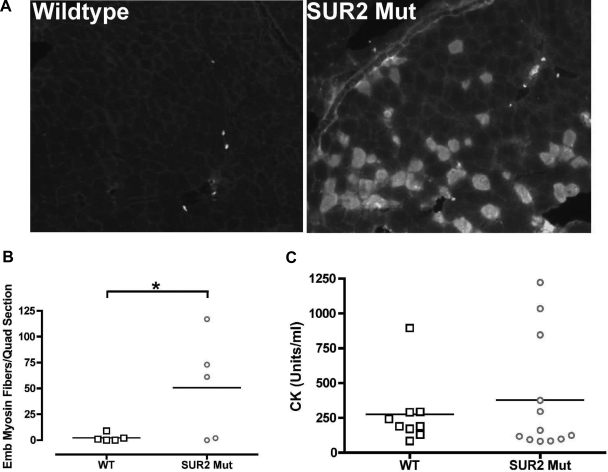
As a secondary measure of muscle regeneration, we studied expression of embryonic myosin heavy chain. Quadriceps muscle from sedentary control mice contained few embryonic myosin heavy-chain-positive fibers (2 ± 1.7 fibers per section of quadriceps, n = 5), while SUR2 mutant mice had considerably more (51 ± 22 fibers per section of quadriceps, n = 5, Fig. 3, A and B). Mirroring what was seen for the degenerative fibers distribution, in SUR2 mutant muscle, regenerative embryonic myosin heavy-chain-positive fibers were often present in groups along the periphery of the muscle. Serum creatine kinase (CK) is an intracellular enzyme released following plasma membrane disruption in skeletal muscle damage. Serum CK was similar between sedentary control (278 ± 81 U/ml; n = 9) and SUR2 mutant mice (378 ± 119 U/ml; n = 12), although more SUR2 mutant mice had CK levels greater than 500 U (Fig. 3C). These data suggest that a profound disruption of skeletal muscle membrane integrity, such as that seen in forms of muscular dystrophy, is not present in SUR2 mutant muscle.
Fig. 3.
Enhanced regeneration in SUR2 mutant muscle. Embryonic myosin heavy chain staining was performed on quadriceps muscles from sedentary control and SUR2 mutant mice. A: embryonic myosin heavy-chain positive fibers are present in SUR2 mutant mice, typically in clusters along the muscle periphery. B: embryonic myosin heavy-chain positive fibers are increased in SUR2 mutant muscle. Control (n = 5) and SUR2 mutant (n = 5) mice. *P < 0.05 by Student's t-test. C: creatine kinase measurements from serum of female control (n = 9) and SUR2 mutant (n = 12) mice were similar (P = 0.2602).
With exercise, SUR2 mutant mice lose less weight than control mice.
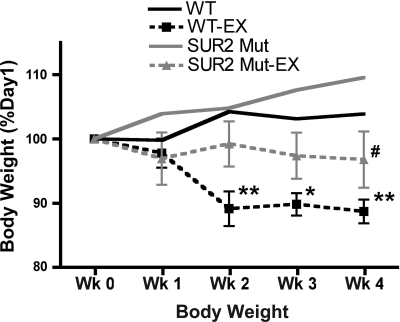
Body mass was measured weekly in mice from all cohorts. Sedentary cohorts of control and SUR2 mutant mice both continued to gain weight throughout the 4-wk exercise period (Fig. 4). Exercised control mice had significant weight loss by week 2 that was maintained for the remainder of the exercise period (P < 0.01, Fig. 4). Gross examination of exercised control mice revealed much less abdominal fat than any other cohort (data not shown). In contrast, exercised SUR2 mutant mice did not lose weight over the 4-wk exercise period, although they did weigh less than their sedentary cohorts due to lack of normal weight gain (P < 0.05, Fig. 4).
Fig. 4.
Chronic exercise does not affect SUR2 body mass. Control mice loss body mass with increased exercise capacity, and this loss is maintained for the duration of the exercise period. SUR2 mutant mice maintain body mass with chronic exercise but do not gain weight, as do sedentary SUR2 mutant mice. **P < 0.01 vs. sedentary control, *P < 0.05 vs. sedentary control, and #P < 0.05 vs. sedentary SUR2 mutant all by two-way ANOVA with repeated measures and Bonferroni post hoc tests.
SUR2 mutant mice show normal glucose homeostasis after exercise training.
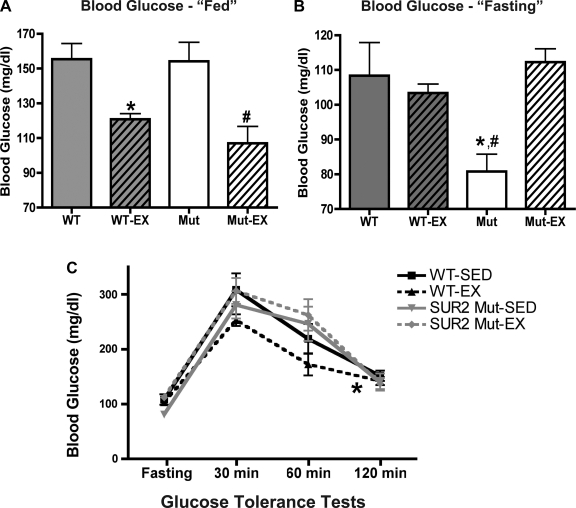
Serum glucose was measured at baseline (“fed”) and after a 16-h overnight fast. Baseline serum glucose was similar between sedentary control (155 ± 9 mg/dl, n = 5) and SUR2 mutant (154 ± 11 mg/dl, n = 5) mice, and exercise significantly reduced baseline blood glucose concentration in both control (n = 5, 121 ± 3 mg/dl, P < 0.05) and SUR2 mutant (n = 4, 107 ± 10 mg/dl, P < 0.01) mice (Fig. 5A). After an overnight 16-h fast, sedentary SUR2 mutant mice had lower fasting glucose than sedentary control mice (81 ± 5 vs. 108 ± 10 mg/dl, respectively, P < 0.05, Fig. 5B). However, fasting blood glucose was similar in exercised control (103 ± 3 mg/dl) and SUR2 mutant (112 ± 4 mg/dl, P < 0.05 vs. sedentary SUR2 mutant) cohorts.
Fig. 5.
Serum glucose levels are not altered in exercised SUR2 mutant mice. A: baseline “fed” serum glucose levels were similar for control and SUR2 mutant mice. Exercise significantly reduced baseline serum glucose in both control and SUR2 mutant mice. *P < 0.05 vs. sedentary control, #P < 0.01 sedentary SUR2 mutant both by ANOVA. B: serum glucose was measured after a 16-h overnight fast. Fasting blood glucose was significantly reduced in sedentary SUR2 mutant mice. *P < 0.05 vs. sedentary control, #P < 0.05 vs. exercised SUR2 mutant both by ANOVA. C: following an overnight fast, glucose challenge tests were conducted (2 mg/g ip glucose). Response to glucose challenge was similar between sedentary control and SUR2 mutant mice. Exercise significantly improved response to glucose challenge in control mice, but not SUR2 mutant mice. *P < 0.05 by two-way ANOVA comparison of control cohorts.
A glucose bolus was given, and serum glucose was measured in a glucose tolerance tests (2 mg glucose/g body wt, intraperitoneal injection) conducted on both sedentary cohorts yielded similar results (Fig. 5C). Although exercise mildly improved response in control mice (P < 0.05 vs. sedentary control, two-way ANOVA comparison of cohorts), no differences were present between sedentary and exercised SUR2 mutant mice.
Reduced cardiac function in SUR2 mutant mice.
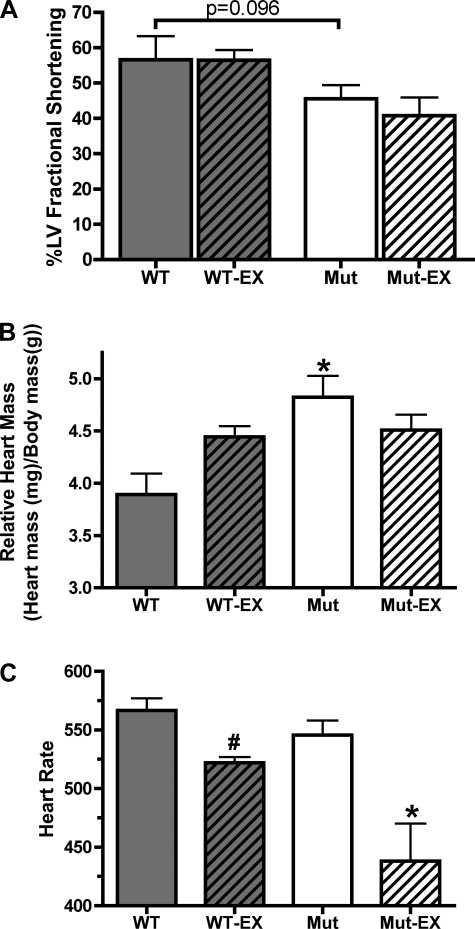
The effect of exercise training on in vivo cardiac function was analyzed by echocardiography on mice under light isoflurane anesthesia. Exercise did not affect cardiac function as measured by percent left ventricular fractional shortening (FS). Sedentary control mice showed a FS of 57 ± 7% (n = 5), and this was not altered by chronic exercise (FS = 56 ± 3%, n = 5, Fig. 6A). Likewise, SUR2 mutant mice did not show a significant decline in FS with exercise (46 ± 4% and 41 ± 5% in sedentary and exercise cohorts, respectively) (n = 5 and n = 4 for sedentary and exercise cohorts, respectively, Fig. 6A). SUR2 mutant mice do exhibit a trend of reduced cardiac function compared with controls (46 ± 4% and 57 ± 7%, respectively); however, this finding did not reach significance (P = 0.096).
Fig. 6.
The effect of exercise training on cardiac function in SUR2 mutant mice. A: echocardiography was used to measure in vivo cardiac function in lightly anesthetized mice. Exercise did not produce a significant effect on cardiac function as measured by left ventricular fractional shortening, although SUR2 mutant mice exhibit a trend of reduced cardiac function compared with control (P = 0.096). B: exercise did not have a significant effect on heart size. SUR2 mutant mice have significant cardiac hypertrophy at baseline. *P < 0.01 by ANOVA. C: exercise reduced heart rate in both control (n = 5) and SUR2 mutant (n = 4) cohorts (n = 5 for sedentary control and SUR2 mutant). #P < 0.01 vs. sedentary control, *P = 0.01 vs. sedentary SUR2 mutant both by Student's t-tests.
Upon completion of the exercise protocol, hearts were harvested, weighed, and sectioned for analysis. SUR2 mutant mice [n = 5, 4.8 ± 0.2 heart mass (mg)/body mass (g)] had significant cardiac hypertrophy at baseline compared with controls [n = 5, 3.9 ± 0.2 heart mass (mg)/body mass (g), P < 0.01, Fig. 3B]. However, exercise did not affect heart mass in either cohort in contrast to exercise-induced cardiac hypertrophy in Kir6.2-null mice (15). No cardiomyocyte damage, fibrosis, or other abnormality was noted (data not shown).
Heart rate under anesthesia was similar between sedentary control [n = 5, 567 ± 10 beats per minute (bpm)] and SUR2 mutant mice (n = 5, 546 ± 12 bpm, Fig. 6C). Exercise resulted in significantly reduced heart rate in both control (n = 5, 522 ± 5 bpm, P = 0.0023) and SUR2 mutant mice (n = 4, 438 ± 32 bpm, P = 0.0057, Fig. 3C). ECG telemetry was conducted on a subset of SUR2 mutant mice following the exercise period (n = 2 sedentary and n = 2 exercised SUR2 mutant mice). All SUR2 mutant mice displayed coronary vasospasm, as evidenced by ST segment elevation, an indicator of acute myocardial injury, although no obvious difference was present between sedentary and exercised SUR2 mutant mice (data not shown).
DISCUSSION
We previously found mice engineered to lack full-length SUR2 displayed improved tolerance to acute sympathetic stress. However, we now find that SUR2 mutant mice tolerate exercise training as a form of chronic stress less well than control mice. The mice used for these studies harbor a gene deletion that removes exons 14 to 18 of the ABCC9 gene that encodes SUR2. This gene deletion removes the first nucleotide binding fold of SUR2, and muscles from these mice have been previously shown to lack sulfonylurea-sensitive KATP channels (4). SUR2 mutant mice were unable to increase their exercise capacity above nonexercised baseline levels, and this finding mirrors what has been described for Kir6.2-null mice (15, 24). However, unlike Kir6.2-null mice, we find that SUR2 mice have a baseline skeletal myopathy. Kir6.2-null mice develop a myopathic process only with chronic exercise. However, in both cases, it is likely that this myopathic process limits exercise capacity. Signs of skeletal muscle damage and regeneration were present in both mouse Kir6.2 and SUR2 mutant models after exercise (15, 24). It is notable that both Kir6.2-null and SUR2 mutant mice had these skeletal muscle changes across multiple muscle groups. Tibialis anterior, extensor digitorum longus, and diaphragm muscle were characterized in both mouse models, and while some differences exist between Kir6.2-null and SUR2 mutants, together, the data support that KATP channels are required for normal skeletal muscle function (24). Given the broad expression of SUR2 and Kir6.2, including expression in the heart, a cardiovascular contribution to exercise intolerance is also likely present.
In the careful study by Thabet et al. (24), skeletal muscle damage in exercised Kir6.2 mutant mice targeted glycolytic type IIB fibers. With exercise, there was an increase in centrally nucleated fibers, and interestingly, these fibers were nearly all (98–99%) type IIB. Although SUR2 mutant muscle showed increased numbers of central nucleated fibers compared with control cohorts in some muscle groups, the percentage of centrally nucleated fibers was considerably lower in SUR2 mutant muscles. In exercised Kir6.2-null mice, it was reported that between 5 and 20% of total fibers contained central nuclei in tibialis and extensor digitorum longus muscle, with the extensor digitorum longus muscle most affected (24). In contrast, SUR2 mutant mice had much lower percentages of central nucleated fibers, typically in the 1–2% range. In addition, no changes in fiber-type composition were observed between SUR2 mutant and control mice (data not shown). These data suggest that these two partner proteins, Kir6.2 and SUR2, have differential effects on skeletal muscle function. These differences may derive from alternative partnering, for example, with Kir6.1 or SUR1, or may also relate to the shorter protein produced from the SUR2 gene (20). Also, the genetic backgrounds of the mice used in these studies differ, and this could account for some differences.
Glucose homeostasis in SUR2 mutant mice.
KATP channels regulate insulin production and glucose utilization. We found that serum glucose levels were not different between exercised SUR2 mutant and control mice, and as such, likely do not have a significant contributory role in the response to exercise. Using in vivo insulin clamp experiments, Chutkow et al. (4) previously demonstrated that glucose uptake was increased 1.5-fold in sedentary SUR2 mutant mice compared with control mice. It is notable that fasting serum glucose levels were no longer reduced in SUR2 mutant mice after being subjected to exercise. Even the modest amount of exercise accomplished by SUR2 mutant mice in this study was sufficient to overcome the reduction in fasting blood glucose. In the current study, glucose tolerance was not significantly enhanced in SUR2 mutant mice. This difference between studies may relate to different mouse strains (FVB vs. 129/C57BL6) and a smaller cohort size.
Skeletal muscle KATP channels have known roles in glucose uptake and protection during fatigue by preserving energy stores (19, 21). However, exercise training had only mild effects on serum glucose levels in both SUR2 mutant and as reported for Kir6.2-null mice (15). In addition to these defects, impaired calcium handling may be present in skeletal muscle lacking KATP channels. Skeletal muscle fibers from Kir6.2-null mice demonstrate increased intracellular calcium levels (6). In addition, the effects of a lack of KATP channels worsen with age; Kir6.2-null more than 1 year of age display significantly less force recovery after fatigue compared with younger Kir6.2-null mice aged 2–3 mo (9). Cifelli et al. (6) demonstrated that while Kir6.2-null fibers had contractile dysfunction, membrane integrity was preserved as measured by Evans blue dye uptake. Our study provides additional support for preserved membrane integrity as serum creatine kinase levels were similar between SUR2 mutant and control cohorts. An intact skeletal muscle membrane supports that metabolic defects may account for the impaired exercise tolerance that derives from lack of KATP channels in skeletal muscle.
Potential mechanisms of skeletal myopathy in SUR2 mutant muscle.
The lack of KATP channels may impair functional sympatholysis. In response to exercise, sympathetic tone is increased to maintain elevated blood pressure and heart rate, thereby allowing an adequate supply of essential nutrients to active organs (10). Increased sympathetic tone leads to vasoconstriction in resting muscle, whereas arteries supplying active, contracting muscle undergo relaxation. Inhibition of sympathetic vasoconstriction, known as functional sympatholysis, has been suggested to involve KATP channels (10, 25). Pharmacologic activation of KATP channels by diazoxide inhibits sympathetic vasoconstriction in resting muscle, while muscle with glibenclamide blockade of KATP channels produces continued sympathetic vasoconstriction (25). Contracting skeletal muscle in SUR2 mutant mice may face continued vasoconstriction and impaired oxygen and nutrient supply, leading to focal ischemia and cell death. Lack of KATP channels may alter sympathetic innervation of skeletal muscle leading to the development of myopathy. SUR2 mutant mice also lack KATP channels in vascular smooth muscle and have hypertension and coronary vasospasm at baseline. It is plausible that vasospasm of arteries and arterioles supplying skeletal muscle occurs, resulting in focal ischemia and muscle damage. Such defects could easily account for an impaired ability to chronically exercise.
Cardiac function deficits in SUR2 mutant mice are not exacerbated by exercise.
Forced, involuntary physical exertion to near exhaustion by swimming or treadmill running is stressful and can have adverse consequences (2, 7, 11). Exercise did not worsen cardiac function in SUR2 mutant mice and is in contrast to what was reported for Kir6.2-null mice (15). We found a relatively small deficit in SUR2 mutant hearts, as measured by left ventricular fractional shortening. Unaffected by exercise, this decrease is the first observation of impaired cardiovascular function in SUR2 mutant mice. Male SUR2 mutant mice were previously reported to have cardiac hypertrophy, presumably because of baseline hypertension, and this finding was confirmed here in sedentary female SUR2 mutant mice (22). Kir6.2-null mice also display cardiac hypertrophy, reduced cardiac function, and reduced cardiac output, but only after exercise and not in sedentary mice (15). On the basis of these findings, it is hypothesized that compromised cardiac function may directly impair adaptation to chronic exercise in Kir6.2-null mice. The role of cardiac dysfunction in the impaired exercise response of SUR2 mutant mice is less clear. Reduced cardiac function did develop after exercise training but was present at baseline, and neither cardiac function nor hypertrophy was worsened by exercise. Both of these features are distinct from what has been reported in Kir6.2-null mice. SUR2 mutant mice also demonstrate cardioprotection when faced with acute adrenergic stress and ischemia. Taken together, these data suggest that the mild reduction in cardiac function may have limited global effects (22). Of note, Pu et al. (20) recently described the existence of additional SUR2 splice variants whose expression is retained in SUR2 mutant mice. It is possible that these SUR2 splice forms contribute to cardiac KATP channels and that lack of this subset of KATP channels in Kir6.2-null mice underlies the observed impaired cardiovascular response to exercise.
Sex differences in SUR2 mutant mice.
KATP channels have been implicated in the sex-based responses to ischemic injury (12). Known sex differences are present in SUR2 mutant mice, as male SUR2 mutant mice have a shorter lifespan than female SUR2 mutant mice (3). Female SUR2 mutant mice were used for the current investigation, and we selected these mice as to be able to complete the study. However, although the underlying cause of this sex difference is currently unknown, key characteristics of the SUR2 mutant phenotype are present in both sexes. Coronary vasospasm, measured by ST segment elevation over time, is present in similar levels in both male and female mice, as is baseline cardioprotection against ischemic and adrenergic stress (Kakkar R, Stoller DA, McNally EM, unpublished data). Thus, we would expect male SUR2 mutant mice also to have skeletal muscle myopathy and an impaired response to exercise.
Perspectives and Significance
This study reinforces the emerging and important role of KATP channels in adaptation to chronic stress and exercise. Impaired exercise tolerance was previously reported for Kir6.2-null mice, and we now report a similar finding for SUR2 mutant mice. However, there are interesting and important differences between these models. Notably, SUR2 mutant mice have baseline skeletal muscle myopathy that is not reported for Kir6.2 mice, and in SUR2 mutant mice, chronic exercise does not exacerbate the myopathic process. Exercise-induced damage in Kir6.2-null mice led to a marked increase in centrally nucleated fibers, consistent with enhanced damage and regeneration from exercise. In contrast, SUR2 mice have a much lower fraction of centrally nucleated fibers, and a very modest effect of exercise was evident only in some muscle groups. The difference in baseline myopathy present in SUR2 mutant, but not described in Kir6.2-null animals, may derive from alternative subunit partnering, genetic background differences, sex differences, or alternative transcripts produced from the gene encoding SUR2. Further, Kir6.2 mutant mice have impaired cardiovascular responses to acute and chronic stress, while we did not detect similar findings in SUR2 mutant mice (22, 27). SUR2 mutant mice, unlike Kir6.2-null mice, have cardioprotection to acute stress. Together, the concordance of experimental data from these mouse models highlights a major role for KATP channels in adaptation to stress and exercise. The interplay and contribution of KATP channels of different compositions from multiple cell and tissue types argues for tissue-specific investigations of KATP channels function. The availability of pharmacological agents that target these channels suggests that these agents could alter exercise response in addition to their known regulation of cardiovascular function.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health R01 HL078926.
REFERENCES
- 1.Banker B, Engel A. Basic reactions of muscle. In: Myology: Basic and Clinical ( 3rd ed.), edited by Engel A, Franzini-Armstrong C. New York: McGraw-Hill, Medical Pub. Division, 2004, p. 691 [Google Scholar]
- 2.Carmichael MD, Davis JM, Murphy EA, Brown AS, Carson JA, Mayer EP, Ghaffar A. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol 291: R1344–R1348, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest 110: 203–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci USA 98: 11760–11764, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cifelli C, Boudreault L, Gong B, Bercier JP, Renaud JM. Contractile dysfunctions in ATP-dependent K+ channel-deficient mouse muscle during fatigue involve excessive depolarization and Ca2+ influx through L-type Ca2+ channels. Exp Physiol 93: 1126–1138, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cifelli C, Bourassa F, Gariepy L, Banas K, Benkhalti M, Renaud JM. KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and force development during fatigue in vitro. J Physiol 582: 843–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis JM, Murphy EA, Brown AS, Carmichael MD, Ghaffar A, Mayer EP. Effects of oat beta-glucan on innate immunity and infection after exercise stress. Med Sci Sports Exerc 36: 1321–1327, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Gong B, Legault D, Miki T, Seino S, Renaud JM. KATP channels depress force by reducing action potential amplitude in mouse EDL and soleus muscle. Am J Physiol Cell Physiol 285: C1464–C1474, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Gong B, Miki T, Seino S, Renaud JM. A KATP channel deficiency affects resting tension, not contractile force, during fatigue in skeletal muscle. Am J Physiol Cell Physiol 279: C1351–C1358, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Hansen J, Sander M, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiol Scand 168: 489–503, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Ikeuchi M, Koyama T, Takahashi J, Yazawa K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol Pharm Bull 29: 2106–2110, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Johnson MS, Moore RL, Brown DA. Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol 290: H2644–H2647, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res 98: 682–689, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet 15: 2285–2297, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kane GC, Behfar A, Yamada S, Perez-Terzic C, O'Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes 3: S169–S175, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol 38: 937–943, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, Minokoshi Y, Renaud JM, Seino S. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 283: E1178–E1184, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA 95: 10402–10406, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol 38: 917–925, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J Mol Cell Cardiol 44: 188–200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81: 133–176, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Stoller D, Kakkar R, Smelley M, Chalupsky K, Earley JU, Shi NQ, Makielski JC, McNally EM. Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. J Mol Cell Cardiol 43: 445–454, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest 109: 509–516, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thabet M, Miki T, Seino S, Renaud JM. Treadmill running causes significant fiber damage in skeletal muscle of KATP channel-deficient mice. Physiol Genomics 22: 204–212, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest 99: 2602–2609, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol 577: 1053–1065, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA 99: 13278–13283, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.