Abstract
Skeletal muscles, especially weight-bearing muscles, are very sensitive to changes in loading state. The aim of this paper was to characterize the dynamic changes in the unloaded soleus muscle in vivo following a short bout of hindlimb suspension (HS), testing the hypothesis that transcriptional events respond early to the atrophic stimulus. In fact, we observed that after only 1 day of HS, primary transcript levels of skeletal α-actin and type I myosin heavy chain (MHC) genes were significantly reduced by more than 50% compared with ground control levels. The degree of the decline for the mRNA expression of actin and type I MHC lagged behind that of the pre-mRNA levels after 1 day of HS, but by 2 and 7 days of HS, large decreases were observed. Although the faster MHC isoforms, IIx and IIb, began to be expressed in soleus after 1 day of HS, a relatively significant shift in mRNA expression from the slow MHC isoform type I toward these fast MHC isoforms did not emerge until 7 days of HS. One day of HS was sufficient to show significant decreases in mRNA levels of putative signaling factors serum response factor (SRF), suppressor of cytokine signaling-3 (SOCS3), and striated muscle activator of Rho signaling (STARS), although transcription factors yin-yang-1 (YY1) and transcriptional enhancing factor-1 (TEF-1) were not significantly affected by HS. The protein levels of actin and type I MHC were significantly decreased after 2 days of HS, and SRF protein was significantly decreased after 7 days HS. Our results show that after only 1 day of unloading, pre-mRNA and mRNA expression of muscle proteins and muscle-specific signaling factors are significantly reduced, suggesting that the downregulation of the synthesis side of the protein balance equation that occurs in atrophying muscle is initiated rapidly.
Keywords: myosin heavy chain, skeletal α-actin, hindlimb suspension, soleus muscle, SOCS3, SRF, STARS
skeletal muscle is a plastic tissue adapting readily to altered activity states through changes in muscle mass, muscle protein expression, and metabolic and contractile fiber type shifts. Recently, evidence has accumulated to show that a well-regulated series of events occurs in skeletal muscle concerning the control of net protein balance, which is mediated by the relative rates controlling protein synthesis vs. protein degradation (16). Findings suggest that the anabolic signaling pathway(s) linking the IGF-1, protein kinase B (PKB)/Akt-mammalian target of rapamycin (mTOR) cascade plays a key (but not necessarily the only) role in mediating hypertrophic adaptations (15, 30), whereas atrophic stimuli, such as unloading or disuse, are associated with decreased protein synthesis and increased protein degradation. Booth and colleagues were, to our knowledge, the first investigators to systematically examine the role of protein synthesis and pretranslational markers [e.g., β-myosin heavy chain (MHC) and actin] on the muscle atrophy processes using limb immobilization models (3, 36) focusing on the fast-twitch muscle, gastrocnemius, and a hindlimb suspension model, examining soleus and gastrocnemius muscles (35). Their findings suggested that protein synthesis deficits were early contributors to the muscle atrophy during the first several hours through at least 7 days in gastrocnemius muscle. More recently, factors regulating catabolic processes such as the FOXO-ubiquitin (atrogin related)-proteasome cascade (19, 23, 31) have been shown to play a pivotal role in protein degradation (28). In the context of the above, which for the most part has focused primarily on translational/posttranslation events (2, 15, 23), it appears that transcriptional-pretranslational events have received less attention; in fact, early experiments performed by Booth et al. indicated that transcriptional events, e.g., actin (3, 36) or type I MHC mRNA (35) expression, contributed little to the atrophy response until 7 or more days of either immobilization or suspension, respectively. However, we have reported findings that suggest that pretranslational events can be important players in the regulation of protein turnover (20).
Previous studies concerning the plasticity of skeletal muscle in response to altered activity states have focused heavily on the MHC gene family, which plays a pivotal role in the remodeling of the muscle phenotype (e.g., fast to slow and vice versa) (4, 5). In contrast, relatively less attention has been focused on the role of skeletal α-actin (acta-1) in the context of adaptive processes impacting muscle homeostasis even though this protein is one of the most abundant proteins expressed in striated muscle (10). Acta-1 comprises ∼40% of the myofibril (contractile element) pool and ∼25% of the total muscle protein pool. Unlike MHC, acta-1 is expressed mainly as a single isoform and thus it is not fiber-type specific, yet when muscle mass is altered in response to different loading states that cause either hypertrophy or atrophy, acta-1 does undergo significant quantitative changes that contribute to the total amount of myofibril protein that is expressed in any given fiber (7, 8, 20, 26, 36). Thus acta-1, like the MHC isoforms, is in a dynamic state of regulation as the muscle goes from one physiological state to another. The primary goal of this report was to examine the time course of the dynamic changes in actin, as well as slow, type I MHC gene expression, at both the transcriptional/pretranslational and posttranslational level of expression, focusing on the earlier time points of HS-induced atrophy of the rat slow soleus muscle.
Previous studies have implicated several transcription factors such as serum response factor (SRF), yin-yang-1 (YY1), and transcriptional enhancing factor-1 (TEF-1) in the regulation of the acta-1 promoter (7, 24, 27, 33). In addition, signaling mediators, suppressor of cytokine signaling-3 (SOCS3) and striated muscle activator of Rho signaling (STARS), appear to be indirectly linked to regulation of acta-1 transcriptional activity via modulation of SRF (1, 33). SRF has been shown to regulate expression of acta-1 by directly binding to regulatory elements in the proximal region of its promoter (7), and there is evidence suggesting that SOCS3 regulates SRF-1 transcriptional expression (33). STARS is a protein that binds to the actin filament, but in the unbound state stimulates SRF-dependent transcription regulation, in part via Rho activation (24, 34, 37), the latter of which serves as a SRF coactivator for transcription targets such as acta-1 (6, 24). YY1 has been implicated as both an activating and repressing factor concerning acta-1 regulation (27), and TEF-1 is a known transcription factor for slow, type I MHC (13), although there is a prominent TEF-1 binding site on the acta-1 promoter (7). Within the current report, we tested the hypothesis that regulation of transcriptional events affecting muscle protein genes and their putative regulators occur very early in response to HS, thereby contributing to net protein balance.
METHODS
Experimental design and treatment protocols.
This study was conducted in conformity with the “Guiding Principles in the Care and Use of Animals” of the American Physiological Society, and the protocol was approved by the University of California, Irvine Institutional Animal Care and Use Committee. Young adult female Sprague-Dawley rats weighing 160 ± 5 g were randomly assigned to two experimental groups, designated as ground control (GC) and hindlimb suspended (HS; n = 24). Rats were allowed access to food and water ad libitum. HS animals were allocated into three groups (n = 8) that were euthanized after 1, 2, or 7 days of suspension. Another set of rats was euthanized after 4 and 16 days of HS only for comparison of muscle weight-to-body weight (MW/BW) ratio. For all time points, GC animals were processed along with the HS animals. Our aim was to examine the cellular effects of unloading at three points in the early, yet acute, phase of muscle atrophy.
The HS model is adapted from the original HS model (38) and involves a tail traction method using a noninvasive casting procedure. The base portion of rats' tails are prepared with a cast composed of SkinTrac skin traction strips and Tensoplast elastic adhesive bandages. The distal portion of the casting materials utilized a swivel harness system which is attached to a hook on the top of the cage. The hook is adjusted to allow only the front legs of the animal to reach the floor.
Tissue collection.
At the specific time points, the rats were killed via an injection of Pentosol euthanasia solution (Med-Pharmex) at a dose of 0.4 ml/kg (∼160 mg/kg pentobarbital sodium) ip. At the cessation of heart beat, a skin incision was made and the soleus muscles of both legs were dissected free of connective tissue, weighed, snap-frozen between blocks of dry ice, and stored at −80°C for later analysis.
Muscle and myofibril protein analyses.
A preweighed portion of each soleus muscle sample was homogenized in 20 vol of a homogenization buffer, which contained 250 mM sucrose, 100 mM KCl, 5 mM EDTA, and 10 mM Tris·HCl, pH 7.0. Myofibrillar proteins were quantitatively extracted from a known volume of the total homogenate by a modification of the original procedure described by Solaro et al. (32) and were suspended into a known volume of 100 mM KCl, 10 mM Tris, and 1 mM EDTA, pH 7.4. Protein concentration in the homogenate and myofibril suspension was determined using the Bio-Rad protein assay with gamma globulin as the standard. Muscle protein and myofibril content was calculated based on the homogenized muscle piece weight and total muscle weight.
MHC and actin protein analysis.
Soleus muscle MHC and actin proteins were separated on acrylamide gels (10% T, 2.5% C) using a standard SDS-PAGE technique according to the Laemmli method (25) and as described previously (20). The gels were stained with Brilliant blue G 250 (Sigma Chemical), destained, and then scanned using a Molecular Dynamics Laser Scanning Personal Densitometer (Sunnyvale, CA). The MHC and actin bands were identified on the digitized image, and their intensity was calculated via volume integration of density within a rectangle containing the entire band with local background correction. Using this method, MHC and actin proteins were expressed as arbitrary units per milligram of total protein.
Total RNA isolation.
Total RNA was extracted from preweighed frozen soleus muscle samples using the TRI Reagent (Molecular Research Center, Cincinnati, OH) according to the company's protocol. This procedure is based on the method described by Chomczynski (9). Extracted RNA was precipitated from the aqueous phase with isopropanol, and after washing with ethanol, the extract was dried and suspended in a known volume of nuclease-free water. The RNA concentration was determined in duplicates by optical density at 260 nm (using an OD 260 unit equivalent to 40 μg/ml). The muscle total RNA concentration was calculated based on total RNA yield and the weight of the analyzed sample.
The extracted RNA was DNase-treated using RQ1 RNase-free DNase (Promega) for 30 min at 37°C followed by a second RNA extraction using TriReagent LS (MRC). The RNA concentration was determined in duplicates by OD 260, and its integrity was assessed by gel electrophoresis and ethidium bromide staining of 1 μg total RNA on 1% agarose gel. Only RNA samples that were not degraded were utilized for the RNA analyses. The RNA solutions were stored frozen at −80°C and were used subsequently in RT-PCR procedures.
RNA analyses.
The expression of skeletal α-actin (acta-1) and type I MHC pre-mRNA, and acta-1, type I, IIa, IIx, and IIb MHC mRNA were analyzed using One Step RT-PCR system (Qiagen kit). In this assay system, the reactions were carried out using 100 ng total RNA in a 25-μl reaction. In the reverse transcription (RT) step, only the reverse primer was added (15 pmol/reaction). The RT reactions were carried out at 50°C for 30 min, followed by denaturing the RT enzyme for 15 min at 95°C. After this step, the missing PCR primer was added to the reaction tube, and this step was followed by PCR for an optimized number of cycles (17–27 cycles) so that the product yield is in the linear range of detection.
In contrast, a two-step RT-PCR system was used to study the expression of a collection of mRNA markers, including SRF, SOCS3, STARS, TEF-1, and YY1. Methods for the two step RT-PCR are as described previously (19). Briefly, one microgram of total RNA was reverse transcribed for each muscle sample using the SuperScript II RT from Invitrogen (Carlsbad, CA) in a 20-μl total reaction volume at 45°C for 50 min, according to the provided protocol using an oligo(dT) and random primer mix. One microliter of each RT reaction (0 to 20-fold dilution, depending on target mRNA abundance) was used for the PCR amplification. The PCR reactions were carried out in the presence of optimized concentration of MgCl2 using standard PCR buffer (Bioline), 0.2 mM dNTP, 1 μM specific primer set, and 0.75 unit of Biolase DNA polymerase (Bioline, Genesee, San Diego, CA) in 25 μl total volume.
RT-PCR products were separated by gel electrophoresis on a 2.5% agarose gel using 1× TAE buffer. The gels were stained with ethidium bromide and then the gel was exposed to UV light source and a digital image was captured. Band intensity was analyzed using Image Quant Software.
RT-PCR data for a specific RNA expression are reported as concentration (arbitrary units/mg muscle). The initial data collection represents arbitrary scan units per nanogram RNA, and these were normalized to muscle weight using the muscle RNA concentration as the correction factor. This same approach was used previously by Heinemeier et al. (21), and we are adapting this method here because it is the most logical way to report specific mRNA expression in muscles that are undergoing dynamic change in RNA concentration as the muscles rapidly atrophy. PCR primers (Table 1) were designed using Primer Select Software (Lasergene, DNAStar) and based on available GenBank sequences, and they were purchased from Operon Biotechnologies (Huntsville, AL).
Table 1.
PCR primers
| Target mRNA | PCR Primer Sequence, 5′→3′ | Product Size, bp |
|---|---|---|
| Acta-1 mRNA | Fwd: GCACCCGACCCTGCTCACTGA | 225 |
| Rev: ATGGCGTGTGGCAGGGCATAAC | ||
| Acta-1 pre-mRNA | Fwd: CAGCCCCTGGGAGACTGACTTGAAGA | 194 |
| Rev: CCAACTCTGGAAAGAATTGCCCGTTTGAAG | ||
| Type I MHC mRNA | Fwd: GGAGCTCACCTACCAGACAGA | 308 |
| Rev: CTCAGGGCTTCACAGGCATCC | ||
| Type I MHC pre-mRNA | Fwd: CCTGGTCCTATGTGCCGATCTCTAACGA | 215 |
| Rev: CGGTCCCCAATGGCAGCAATAAC | ||
| Type IIa MHC mRNA | Fwd: CCTCTTACTTCCCAGCTGCACCTTCT | 239 |
| Rev: ACTTTCCCTGCGTCTTTGCTCTGAAT | ||
| Type IIx MHC mRNA | Fwd: ACGGTCGAAGTTGCATCCCTAAAG | 263 |
| Rev: CACCTTCGGTCTTGGCTGTCAC | ||
| Type IIb MHC mRNA | Fwd: AGCCTGCCTCCTTCTTCATCTGG | 229 |
| Rev: CACGGTTGCTTTCACATAGGACTC | ||
| SRF | Fwd: TGCGGCGTTACACGACCTTC | 224 |
| Rev: GTCTGAGCGGGGTGGAGAGTCT | ||
| SOCS3 | Fwd: TCACGGCTGCCAACATCTGG | 228 |
| Rev: CGGCGGCGGGAAACTTG | ||
| STARS | Fwd: GAGGAGCCCAAGTGGAAGAGTGACA | 215 |
| Rev: TGCTGCCACCTGCCTTTCAAGTT | ||
| TEF-1 | Fwd: CTGGAGCGGCAGCGAGAGC | 234 |
| Rev: TTTCCCGTCCTCAGTTTGATGTATCTG | ||
| YY1 | Fwd: GCCCTCATAAAGGCTGCACAAAGAT | 223 |
| Rev: GTGCGCAAATTGAAGTCCAGTGAA |
Primers were designed with PrimerSelect software (DNAStar, Lasergene). Note that for suppressor of cytokine signaling-3 (SOCS3), reported rat mRNA has only 1 exon, whereas mouse and human report 2 exons. We used the mouse sequence blast to rat genome for deducing rat SOCS3-exon-1 (GenBank accession no. NM_053565). Primers were designed from exon 1/exon 2 (E1/E2). For transcriptional enhancing factor-1 (TEF-1) mRNA, there are many alternative splice variants. Primers are from exons 2 and 3, which are common for all the reported isoforms in GenBank and separated by approximately 82-kb intron (GenBank accession no. XM_001078008). Acta-1, skeletal α-actin; MHC, myosin heavy chain; SRF, serum response factor; STARS, striated muscle activator of Rho signaling; YY1, yin-yang-1; Fwd, forward; Rev, reverse.
Western blot analyses.
Western blot methods were applied for the analyses of protein expression of SRF using a commercially available antibody (SRF: Santa Cruz SRF, sc-13029; H-300 rabbit polyclonal). Muscle samples were extracted by homogenization in 10 vol of ice-cold buffer A (50 mM Tris·HCl pH 7.8, 2 mM potassium phosphate, 2 mM EDTA, 2 mM EGTA, 50 mM β-glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mM DTT, 3 mM benzamidine, 1 mM sodium orthovanadate, 10 μM leupeptin, 5 μg/ml aprotinin, 200 μg/ml soybean trypsin inhibitor, and 1 mM AEBSF) using a motor-driven glass pestle. The homogenate was immediately centrifuged at 12,000 g for 30 min at 4°C. The supernatant was immediately saved in aliquots at −80°C for subsequent use in immunoblotting. The supernatant protein concentration was determined using the Bio-Rad protein assay with gamma globulin as the standard. Approximately 50 μg of supernatant proteins was subjected to SDS-PAGE (12.5% T), according to standard protocol (25), then electrophoretically transferred to a PVDF membrane (Immobilon-P) using 10% methanol, 1 mM orthovanadate, 25 mM Tris, 193 mM glycine, pH 8.3. The enhanced chemiluminescence (ECL) method was used for signal detection (Amersham, Piscataway, NJ) after incubations with the primary antibody and horseradish peroxidase-conjugated secondary antibody. Signal intensity was determined by laser scanning densitometry (Molecular Dynamics/Image Quant). All the samples were run under identical (previously optimized) conditions, including the transfer on the membrane, the reaction with the first and secondary antibodies, washing conditions, ECL detection, and film exposure. To ensure the consistency of this analysis at least one representative sample from each group was included in the gel run and Western analysis. In addition, a positive control, provided by the antibody supplier, was run on the gel to allow for normalization. For Western blotting and detection conditions, the detected signal was directly proportional to the amount of protein loaded on the gel over a range 20–150 μg (data not shown).
Plasmid DNA injection into the soleus muscle.
This procedure was performed under general anesthesia as described previously (12, 14). A skin incision was made in posterior hindlimb to expose the soleus muscle. Twenty microliters of PBS containing a mixture of two supercoiled DNA plasmids—two picomoles of type I MHC promoter-pGL3 construct [type I MHC promoter sequence (−3500/+34 relative to the transcription start site) subcloned into a firefly luciferase (Fluc) reporter vector (pGL3 basic, Promega)] and 1 picomole of acta-1 promoter-pRL construct [2-kb promoter sequence of the human skeletal α-actin (a gift from S. Swoap, Williams College, MA), subcloned into a Renilla luciferase reporter (pRLnull, Promega)]—was injected into the soleus muscles using a 29-gauge needle attached to a 0.5-ml insulin syringe. Rats undergoing HS were prepared for tail casting immediately following plasmid injection procedure. Experimental period was 7 days following the DNA injection, which is the necessary time for maximal reporter expression.
Reporter expression assay.
Luciferase assay was performed as described previously (12). Briefly, frozen soleus muscle tissues were homogenized in 1 ml ice-cold passive lysis buffer from Promega, using a glass homogenizer. The homogenate was centrifuged and the supernatant was reserved for the luciferase activity assay using Promega's Dual Luciferase Assay kit, which is designed for sensitive detection of both firefly and Renilla luciferase activities in a single-extract aliquot. Luciferase activities were measured as light output (as measured by a Monolight 2010-C luminometer) integrated over 10-s intervals generated by 10 μl supernatant and are expressed as relative light units. Background levels, based on luciferase activities of noninjected tissue, are subtracted from the activities of test samples. These experiments are predicated on the assumption that the level of luciferase activity is proportional to the degree of promoter activity.
It should be noted that because we injected the type I MHC promoter-pGL3 and the actin promoter-pRL constructs together, we did not transfect an additional housekeeping gene as a reference promoter to correct for uptake. Instead, we measured the amount of plasmid DNA within the transfected tissue at the time of the analysis. Plasmid amount in the extract was determined by PCR targeting the luciferase sequence in the plasmid as previously described in Huey et al. (22). We found that, on average, the plasmid content in the muscle at the time of analyses is similar between control and HS samples (data not shown), which verifies that the difference in luciferase activities between GC and HS was not because of less plasmid uptake by the muscles of the HS group but because the HS caused a reduction in the transcriptional regulation of these muscle protein gene promoters.
Statistical analysis.
All values are reported as means and standard error of the mean (SE). Treatment effects were determined by one-way ANOVA with post hoc testing (Neuman-Keuls multiple comparison tests) using the Prism software package (Graphpad). For all statistical tests the 0.05 level of confidence was accepted for statistical significance. For each group, n = 8.
RESULTS
Muscle weight.
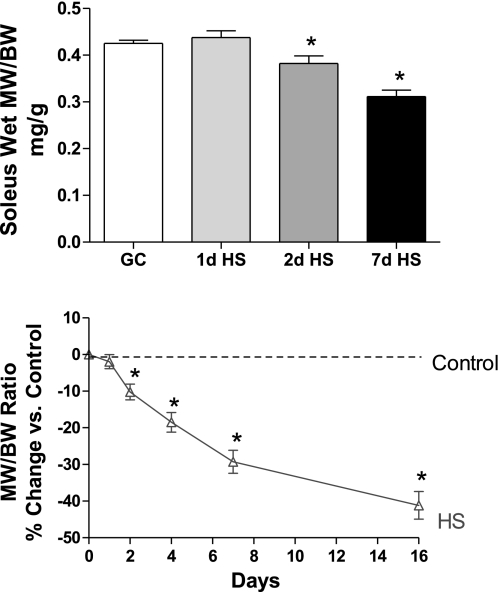
GC groups were analyzed in parallel with the HS experimental groups. Statistical analyses revealed that there were no differences between the GC groups (P > 0.05); thus they were combined for performing the battery of analyses presented below. Since body weight was decreased in response to HS (8% after 7 days) relative to the GC values, we focused on the relative muscle mass, which is the ratio of muscle weight to body weight, to correct for this change (Fig. 1). Although relative muscle mass was unchanged after 1 day of HS, it was significantly reduced by ∼10% and 25% after 2 and 7 days of HS, respectively. To put these data into greater perspective, we analyzed separately two additional groups of rats for relative muscle weight at 4 and 16 days of HS, which are also presented in Fig. 1. It is important to note that relative muscle mass appears to decrease very early following unloading and although the relative muscle mass continues to decrease through this time course, the degree of atrophy is more severe in the first week of unloading.
Fig. 1.
Soleus muscle weight, comparing ground control (GC) rats and hindlimb-suspended (HS) rats suspended for 1, 2, or 7 days. Relative muscle weight is ratio of soleus muscle wet weight (MW, mg) and rat body weight (BW, g). Line graph represents a time course depicting the effect of suspension on relative muscle weight. The y-axis is percent change compared with control. Control muscle weight at all time points corresponds to 0 % (dashed line). Solid line drawn from 5 time points: relative muscle weights at 1, 2, 4, 7, and 16 days of hindlimb suspension. *P <0.05 vs. GC; n = 8.
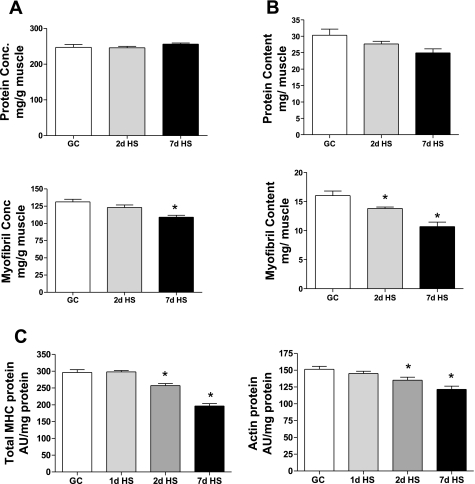
Total and myofibril protein concentration and content.
Total protein concentration in soleus muscle was not different among GC vs. 2-day and 7-day HS groups (Fig. 2A). However, the yield of myofibril protein concentration was significantly decreased in the 7-day HS group relative to the GC group, suggesting that the myofibrillar fraction was the main target of the atrophy process (Fig. 2A).When the protein data were expressed as content [e.g., concentration (mg/g) × muscle weight (g) = mg/muscle], one can observe a different pattern in both the total protein and myofibril protein pools, respectively (Fig. 2B). Although both pools of protein tend to decrease in response to HS, a significant loss of the myofibrillar pool is evident after 2 days and 7 days of HS and the loss of this specific pool is consistent with the net loss in relative muscle mass as seen in Fig. 1. There was no change in total or myofibrillar protein concentrations or contents after 1 day of HS (data not shown).
Fig. 2.
Soleus muscle protein content and concentration. A: protein concentration (conc). Amount of protein (mg) from total soleus muscle or myofibril fraction (mg) [ratio of protein (mg) to muscle weight (g)]. B: protein content. Ratio of amount of protein (mg) from total soleus muscle or myofibril fraction (mg) extracted from each muscle. C: myosin and actin protein. Amount of protein (mg) from myofibril fraction. GC vs. HS for 1, 2, or 7 days. *P <0.05 vs. GC.
Actin and total MHC protein concentration.
Given the above observation that myofibril protein content was decreased at both the 2- and 7-day time points of unloading, we ascertained whether there also was a specific alteration in either total MHC or skeletal actin protein concentration per unit of total protein as determined by their relative band densities using SDS gel analyses. As presented in Fig. 2C, there was a significant reduction in both total MHC and actin band densities after 2 and 7 days of HS relative to the GC group, with the MHC protein concentration changes responding to a slightly greater extent especially at 7 days than that of the actin protein. There was no significant change in MHC or actin protein concentration after 1 day of HS.
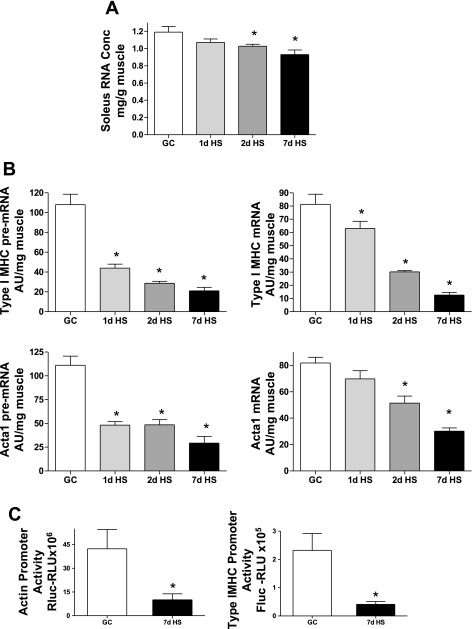
Total RNA concentration.
Previously we have reported that the total RNA pool is sensitive to altered loading stimuli (20). As presented in Fig. 3A, HS caused a decrease in total RNA concentration relative to GC with significant reductions at the 2- and 7-day time points. Thus the total RNA concentration deficit is a putative marker of events negatively impacting the protein balance state in response to HS.
Fig. 3.
Actin and type I myosin heavy chain (MHC) expression affected by HS. A: total RNA concentration. Amount of total RNA (mg) in soleus muscle (g) decreases following HS. B: skeletal α-actin (acta 1) and type I MHC, pre-mRNA (left) and mRNA (right) (AU/mg muscle). C: promoter activity. Actin Renilla luciferase (Rluc; left) and type I MHC firefly luciferase (Fluc). RLU, relative light units. GC vs. HS for 1, 2 or 7 days. *P <0.05 vs. GC.
Acta-1 and slow MHC pre- and mature mRNA.
In this study, we used two pretranslational markers of gene regulation. The first (pre-mRNA) represents the primary product of transcriptional activity, whereas the second (mRNA) represents the product following processing of the primary transcript. As shown in Fig. 3B, we observed a marked reduction in acta-1 and type I MHC pre-mRNA after only 1 day of HS, and an even larger reduction at the 7-day time point. This large reduction in the amount of pre-mRNA of these two sarcomeric proteins after only 1 day of unloading indicates that the suppression of the transcriptional activity of these major sarcomeric proteins is a very early event in the atrophy process. The reduction in the slow type I MHC mRNA was not as robust as that seen for the pre-mRNA at the 1-day time point, yet both acta-1 and type I MHC mRNA levels showed significant decreases after 2 days of HS. We attribute this response to the longer turnover time needed to degrade this RNA pool, which becomes evident by the marked reduction in acta-1 and type I MHC mRNA observed at the 7-day time point (Fig. 3B). Given the pre-mRNA responses of both the acta-1 and type I MHC genes in response to HS, we hypothesized that the promoters of these two genes would also respond to HS, promoter activity being the second marker of pretranslational gene regulation. As shown in Fig. 3C, there was a very large reduction in activity of acta-1 and type I MHC promoters following 7 days HS. These results showing that pre-mRNA level and promoter activity of acta-1 and type I MHC genes responded to HS suggest that these sarcomeric genes are predominantly regulated at the level of transcription.
Although this report focuses on the unloading-induced changes in the expression of acta-1 and type I MHC, the contractile proteins predominantly expressed in soleus muscle, it should be mentioned that HS affected fast MHC isoforms as well. Table 2 shows a comparison of the fast MHC isoform mRNA levels in GC soleus and after 1, 2, and 7 days after HS. This percent distribution of the four adult MHC isoforms illustrates the shift from slow toward fast isoforms, which is characteristic of unloaded slow muscle.
Table 2.
Mean percent distribution of mRNA in soleus muscle
| MHC Isoform Type | GC | HS |
||
|---|---|---|---|---|
| 1 day | 2 day | 7 day | ||
| Slow I | 83 | 76* | 71* | 42* |
| Fast IIa | 13 | 19 | 17* | 3* |
| Fast IIx | 0 | 2* | 9* | 37* |
| Fast IIb | 0 | 0 | 1* | 18* |
P < 0.05 vs. ground control (GC) group.
Expression of putative mediators likely regulating acta-1 and slow, type I MHC genes.
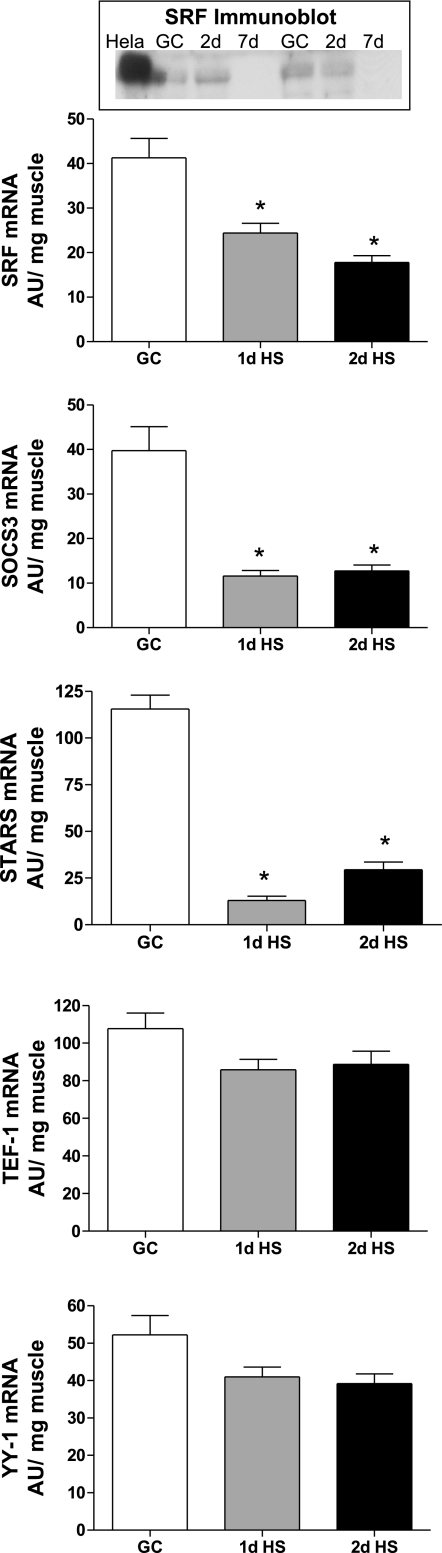
Several transcription factors such as SRF, YY1, and TEF-1 have been linked to the regulatory processes of the acta-1 promoter (7, 24, 27, 33). In addition, signaling proteins, SOCS3 and STARS, are associated with acta-1 transcriptional activity via the activation of SRF (1, 33). These factors for the most part have been examined mainly in cultured cells, so we aimed to determine if the expression of these putative regulatory proteins are early responders to HS by measuring their expression in GC and after 1 and 2 days of HS in soleus muscles in vivo. As shown in Fig. 4, the mRNA levels of SRF, SOCS3, and STARS were greatly depressed after the onset of HS, decreasing by 41, 71, and 89%, respectively, after only 1 day of HS compared with GC levels. A Western blot (Fig. 4, inset) compared the protein content of SRF in the GC and HS groups and showed a significant decrease (55%; n = 8) after 7 days of HS. Whether these other factors show changes in response to HS at the protein level was not able to be determined due to either the unavailability or lack of fidelity of specific antibodies. The mRNA levels of YY1 and TEF-1 did not change significantly in response to 1 or 2 days of HS, and TEF-1 mRNA expression levels are decreased significantly only after 7 days of HS (data not shown). Unlike YY1 and TEF-1, the expression of mediators SRF, SOCS3, and STARS responded markedly to HS and early in the time course, which may indicate that their regulation is similar to, or possibly vital to, the response of type I MHC or acta-1 mRNA expression.
Fig. 4.
HS affects transcription factor expression. Serum response factor (SRF), suppressor of cytokine signaling-3 (SOCS3), striated muscle activator of Rho signaling (STARS), transcriptional enhancing factor-1 (TEF-1), and yin-yang 1 (YY1) mRNA (AU/mg muscle). GC vs. HS for 1 and 2 days. *P <0.05 vs. GC. Inset: Western Blot of SRF protein, GC vs. 2 and 7 days of HS.
DISCUSSION
Contributing factors impacting protein balance.
We maintain that transcriptional/pretranslational regulatory processes are important in the response to an atrophic stimulus and impact muscle phenotype, and atrophy stems not solely from posttranslation regulation, e.g., degradation, as traditionally thought. We observed a marked decrease in the total RNA concentration at the 2-day time point (Fig. 3), in addition to the reduced transcriptional activity and associated decline in mRNA levels of the two key sarcomeric genes. It is thought that ∼85% of the total RNA pool is composed of ribosomal RNA, which suggests that there was a significant reduction in the translational capacity of the muscle to maintain the total muscle protein content and myofibril protein concentration and content (Fig. 2) after unloading soleus muscles. Taken together, one could speculate that the reduced pretranslational substrate (mRNA) and the potentially reduced translational capacity (ribosomal RNA) both combine to downregulate the synthesis side of the protein balance equation in atrophying muscle, with the net result that the myofibril fraction of the protein pool is reduced significantly, accounting for the loss in muscle weight. While a previous study by Thomason et al. (35) suggested that there was a trend for a reduction in protein synthesis during the early stages of HS (5 h), it is likely that if such analysis were performed after 1 day of HS, it would have resulted in a significant decrease in protein translation based on the findings reported herein.
Transcriptional inhibition of acta-1 and type I MHC.
Available evidence suggests that the pre-mRNA expression of a gene correlates well with its transcriptional activity based on comparisons with nuclear run on assays (11). Thus the pre-mRNA can be used as an indirect measure or a marker of the corresponding gene transcriptional activity (29). In the present study, the pre-mRNA analyses revealed a marked downregulation of both the acta-1 and slow, type I MHC genes in response to unloading, indicating a marked decrease in these genes' transcriptional activities. This is also corroborated by the marked decrease in both type I and skeletal α-actin promoter activity in transfected muscles subjected to 7 days of HS. The large decrease in both slow type I MHC (60%) and acta-1 (56%) pre-mRNA that was measured at the 1-day time point of HS suggests that the reduced transcription of these two genes, whose protein content comprise the major portion of the myofibril fraction, is a major target of the initial atrophy response. One day of HS is sufficient to cause a significant decrease in the level of the mature mRNA species of type I MHC, whereas actin mRNA levels decrease but not significantly until the 2-day time point. The mRNA levels of both type I MHC and actin continue to decrease further with a longer period of unloading.
To our knowledge, we are not aware of previous findings that conclude that these two pivotal genes are the initial indicators marking the early stages of atrophy response. Booth's group studying atrophic models, limb immobilization, and hindlimb suspension, examined protein synthesis rate in slow (soleus) and fast muscles at early (6 h) and later time points (7 days). Although they found that protein synthesis rates were affected very early, they did not detect changes in the mRNA expression of actin until after 7 days of immobilization in gastrocnemius (3) or 7 days of HS in soleus muscle (35). Moreover, they did not detect significant changes in type I MHC mRNA even after 7 days of HS, whereas we report that type I MHC mRNA was particularly affected by HS, even at the 1-day time point. Their findings on HS soleus muscle, as illustrated in Fig. 3 of Thomason et al. (35), demonstrated that protein synthesis rates rapidly decreased during the “first few days” of suspension, and that later on there was an increase in degradation that accounted predominately for the loss in myofibril protein as a new steady state was reached at about day 24. They proposed that much of the rapid decrease in myofibril protein synthesis could not be due to transcriptional regulation, because of their inability to detect a decrease in type I MHC mRNA. Accounting for the differences between our findings and theirs in regard to mRNA expression of type I MHC and actin in HS soleus is difficult, yet with the new PCR methods for measuring mRNA and pre-mRNA transcripts, that are more sensitive, duplicatable, and quantifiable, we are confident in our results relative to their observations (35).
Although we contend that mRNA reductions contribute greatly to the overall atrophic response, it is obvious that protein degradation is at work to some degree during the early phase of atrophic stimuli, especially with regard to the myosin protein pool. Type I MHC and actin showed a significant decrease in their protein levels after only 2 days of HS, which is likely attributed to protein degradation. The unloading stimulus causes an atrophy response and phenotype remodeling of the MHC gene family, i.e., the downregulation of the slower isoforms (type I and IIa) and the upregulation of the faster (type IIx and IIb) isoforms (4, 5). In other words, the unloaded muscle becomes markedly smaller while at the same time shifting to a relatively faster phenotype (4). In this report, we showed that after only 2 days of HS, the mRNA levels of all MHC isoforms in soleus have been altered significantly, and even after only 1 day, the slow type I and IIx isoforms showed changes in their expression. These data illustrate how particularly sensitive the slow isoform type I is to unloading. The increase in fast isoforms not only portrays the plasticity of muscle following the induction of the unloading stimulus but also demonstrates that despite the strong atrophic stimuli, not all mRNAs are downregulated in response to unloading. This indicates that it is not just a generalized global shutdown of transcription/translation processes that are responsible for the atrophic response but that a specific regulatory cascade mediating the atrophic signaling pattern is clearly exhibited in this model. Despite the fact that the mRNA expression of fast isoforms increased after 2 days of HS, we still observed a decrease in total MHC protein at 2 and 7 days, larger than that seen for actin. One possible explanation for the decreased total MHC protein is that the myosin isoform pool is likely in considerable flux. We speculate that there is an immediate selective degradation of the predominant type I isoform such that, in combination with the probable lag in the accumulation of the “replacement” fast isoform pool, the amount of total MHC protein will be significantly lower than that of actin in the early phase of unloading.
Expression of transcription factors relevant to muscle atrophy.
Potential key signaling factors relevant to the regulation of these two genes were examined in order to address the hypothesis that for such a rapid reduction of acta-1 and slow MHC transcriptional activity to occur, a very early alteration in the transcriptional regulatory factors would be necessary. As anticipated, the factors SRF, SOCS3, and STARS showed a very large decrease of 41%, 71%, and 89%, respectively, in their expression levels at the 1-day time point. It should be noted that both SOCS and STARS ultimately stimulate SRF activation; thus both affect acta-1 transcription indirectly. Available evidence suggests that SRF is likely a key player in modulating acta-1 transcriptional activity in mammalian skeletal muscle, given the existence of several SRF binding sites in both the distal and proximal regions of the acta-1 promoter (7). Additionally, Spangenburg has reported that overexpression of SOCS3 in differentiating C2C12 myoblasts results in stimulation of SRF responsive promoters including the acta-1 promoter (33). These observations, coupled to our in vivo findings that the mRNA expression of SOCS3 responds positively to loading in rat skeletal muscle (17), suggest that these two signaling factors could be involved in directly or indirectly regulating the acta-1 gene. STARS is an actin binding protein that has also been implicated in the transcriptional regulation of acta-1 via RhoA/SRF activation (1, 37). Therefore, the dramatic reduction in the expression of these three factors that occurs within the first days of HS shows that these mediators in the signaling cascade also respond initially to unloading. On the other hand, when we examined the expression of both TEF-1 and YY1 (Fig. 4), the response to HS of these two genes did not parallel the early decline that we observed for the other mediators or acta-1 and type I MHC. The fact that the regulatory mediators SRF, SOCS3, STARS are considered to be activating factors for acta-1, it is not surprising that their level of expression decreased dramatically in response to HS. However, the YY1 has been implicated as a “repressor” of acta-1 transcription by competing with SRF in the binding of SRE site on acta-1 promoter (27), and one may speculate that as a repressor, it may have increased its expression, yet here YY1 showed no significant response to HS. The expression patterns of TEF-1 and YY1 may indicate that they are not responsive to atrophy cascade at the mRNA level, yet of course, it is possible that their expression levels, and those of SRF, SOCS3, and STARS as well, do not determine their activity as transcription regulators. In fact, changes in SRF protein levels were not consistently reduced at the 2-day time point but were clearly repressed at the 7-day time point of HS (Fig. 4, inset). It is still to be determined if an atrophic stimulus causes these “activators” to become unbound or detached from their regulatory sites/cofactors or modulated by posttranscriptional modifications such as phosphorylation. Nonetheless, it makes sense that the stimuli of unloading would induce an atrophy cascade that would include the downregulation of acta-1 as well as its activating transcription factors.
In summary, although the hindlimb suspension procedure involving rodents has been studied extensively as a model of unloading-induced skeletal muscle atrophy (5, 18), there were several novel findings delineated in this particular study. These include the following: 1) a significant decrease in relative muscle mass occurred within days following unloading and may be attributed to a specific loss in both myofibril concentration and content, and acta-1 and MHC protein (Fig. 2); 2) there was a very rapid decrease in transcriptional activity of both acta-1 and slow type I MHC (the predominant isoform expressed in soleus muscle), as determined by pre-mRNA levels after 1 day applying the unloading stimulus, suggesting that downregulation of these two genes is an early event in the atrophy process (Fig. 3); 3) paralleling the rapid reduction in expression of acta-1 and slow MHC was the significant reduction in total RNA concentration (Fig. 3A), indicating a contribution by pretranslational processing to the atrophy cascade; and 4) the expression of the transcription factor, SRF, and possible coregulators (SOCS3, STARS) of acta-1 transcription responds very early to the HS manipulation. Delineating the exact signaling pathway that translates the unloading stimuli directly to the regulation of muscle genes remains the topic of further investigations.
GRANTS
We appreciate the support of National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-30346 (K. M. Baldwin).
ACKNOWLEDGMENTS
We thank Weihua Jiang, Anqi Qin, Gelareh Nikpour, Li Ying Zhang, and Alvin Yu for excellent technical assistance.
REFERENCES
- 1. Arai A, Spencer JA, Olson EN. STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. J Biol Chem 277: 24453–24459, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem 42: 61–74, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Babij P, Booth FW. Alpha-actin and cytochrome c mRNAs in atrophied adult rat skeletal muscle. Am J Physiol Cell Physiol 254: C651–C656, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Baldwin KM. Effects of altered loading states on muscle plasticity: what have we learned from rodents? Med Sci Sports Exerc 28: S101–S106, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Baldwin KM, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil 81: S40–S51, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Barrientos T, Frank D, Kuwahara K, Bezprozvannaya S, Pipes GC, Bassel-Duby R, Richardson JA, Katus HA, Olson EN, Frey N. Two novel members of the ABLIM protein family, ABLIM-2 and -3, associate with STARS and directly bind F-actin. J Biol Chem 282: 8393–8403, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Carson JA, Schwartz RJ, Booth FW. SRF and TEF-1 control of chicken skeletal α-actin gene during slow-muscle hypertrophy. Am J Physiol Cell Physiol 270: C1624–C1633, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Carson JA, Yan Z, Booth FW, Coleman ME, Schwartz RJ, Stump CS. Regulation of skeletal α-actin promoter in young chickens during hypertrophy caused by stretch overload. Am J Physiol Cell Physiol 268: C918–C924, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Chomoczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol 18: 637–706, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Elferink CJ, Reiners JJ. Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Giger J, Haddad F, Qin A, Baldwin KM. In vivo regulation of the β-myosin heavy chain in soleus muscle of suspended and weight-bearing rats. Am J Physiol Cell Physiol 278: C1153–C1161, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Giger J, Haddad F, Qin A, Baldwin KM. Functional overload increases beta-MHC promoter activity in rodent fast muscle via the proximal MCAT (betae3) site. Am J Physiol Cell Physiol 282: C518–C527, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Giger J, Haddad F, Qin AX, Zeng M, Baldwin KM. The effect of unloading on type I MHC gene regulation in rat soleus muscle. J Appl Physiol 98: 1185–1194, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37: 1974–1984, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86: E19–E35, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Haddad F, Adams GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 100: 1188–1203, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Haddad F, Qin A, Zeng M, McCue SA, Baldwin KM. Interaction of hyperthyroidism and hindlimb suspension on skeletal myosin heavy chain expression. J Appl Physiol 85: 2227–2266, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity II. Molecular markers of protein deficits. J Appl Physiol 95: 791–802, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. I. Cellular markers of protein deficits. J Appl Physiol 95: 781–790, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol 102: 573–581, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Huey KA, Haddad F, Qin A, Baldwin KM. Transcriptional regulation of the type I myosin heavy chain gene in denervated rat soleus. Am J Physiol Cell Physiol 284: C738–C748, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33: 155–165, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Kuwahara K, Barrientos T, Pipes GC, Li S, Olson EN. Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol Cell Biol 25: 3173–3181, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 26. Lamon S, Wallace MA, Leger B, Russell AP. Regulation of STARS and its downstream targets suggest a novel pathway involved in human skeletal muscle hypertrophy and atrophy. J Physiol 587: 1795–1803 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee TC, Shi Y, Schwartz RJ. Displacement of BrdUrd-induced YY1 by serum response factor activates skeletal alpha-actin transcription in embryonic myoblasts. Proc Natl Acad Sci USA 89: 9814–9818, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol 585: 203–215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peters DG, Mitchell-Felton H, Kandarian SC. Unloading induces transcriptional activation of the sarco(endo)plasmic reticulum Ca2+-ATPase 1 gene in muscle. Am J Physiol Cell Physiol 276: C1218–C1225, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nature Cell Biol 3: 1009–1013, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solaro RJ, Pang DC, Briggs FN. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta 245: 259–262, 1971 [DOI] [PubMed] [Google Scholar]
- 33. Spangenburg EE. SOCS-3 induces myoblast differentiation. J Biol Chem 280: 10749–10758, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Takano H, Komuro I, Oka T, Shiojima I, Hiroi Y, Mizuno T, Yazaki Y. The Rho family G proteins play a critical role in muscle differentiation. Mol Cell Biol 18: 1580–1589, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomason DB, Biggs RB, Booth FW. Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol Regul Integr Comp Physiol 257: R300–R305, 1989 [DOI] [PubMed] [Google Scholar]
- 36. Watson PA, Stein JP, Booth FW. Changes in actin synthesis and alpha-actin-mRNA content in rat muscle during immobilization. Am J Physiol Cell Physiol 247: C39–C44, 1984 [DOI] [PubMed] [Google Scholar]
- 37. Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, Schwartz RJ. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J Biol Chem 273: 30287–30294, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Wronski TJ, Morey ER. Skeletal abnormalities in rats induced by simulated weightlessness. Metab Bone Dis Relat Res 4: 69–75, 1982 [DOI] [PubMed] [Google Scholar]