Abstract
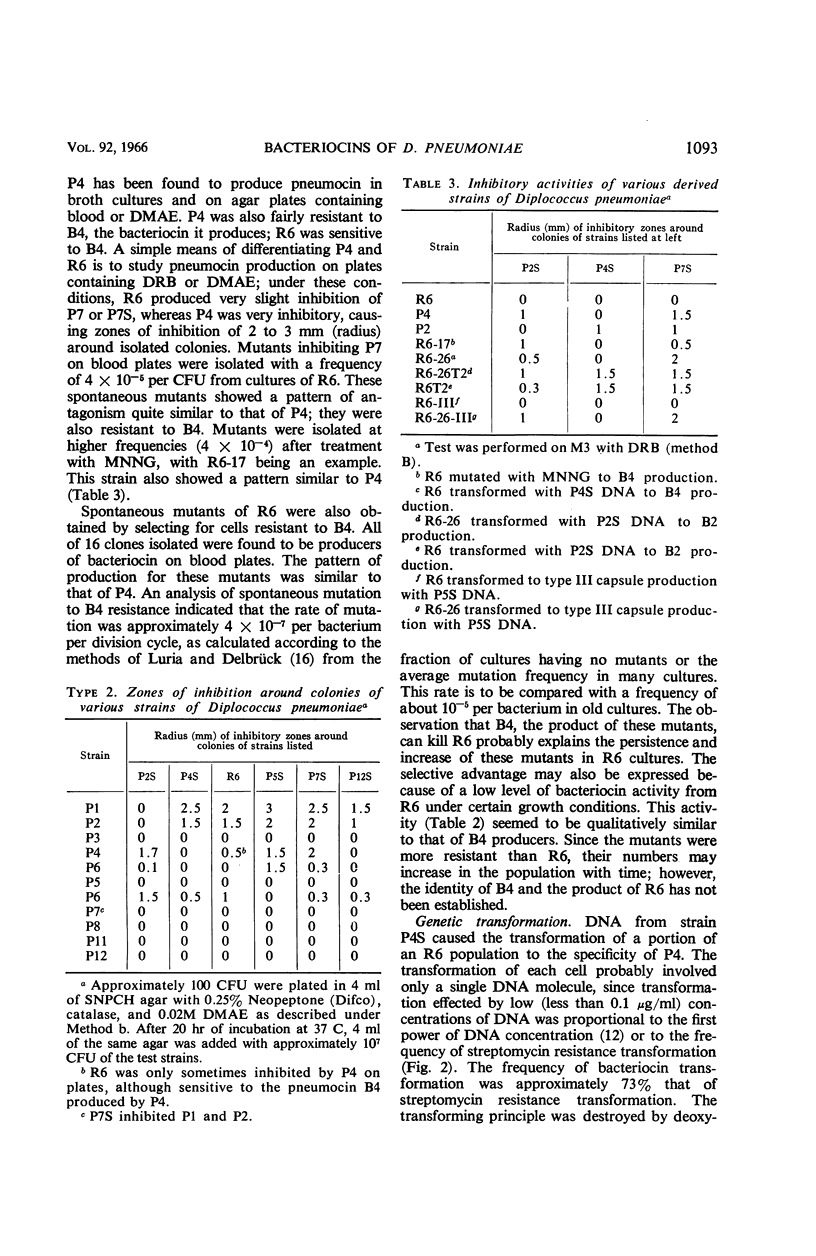
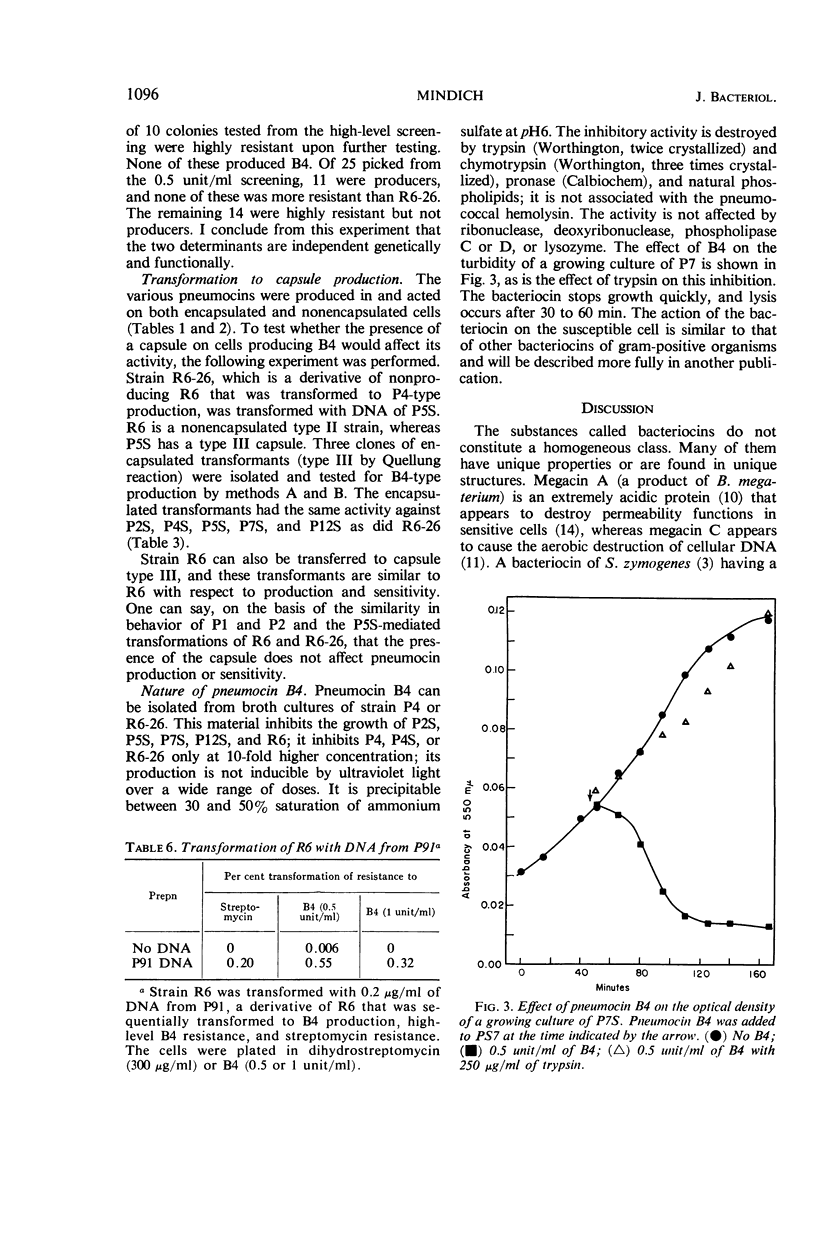
Mindich, Leonard (The Public Health Research Institute of the City of New York, Inc., New York, N.Y.). Bacteriocins of Diplococcus pneumoniae. I. Antagonistic relationships and genetic transformations. J. Bacteriol. 92:1090–1098. 1966.—Several strains of Diplococcus pneumoniae produced bacteriocins inhibiting the growth of other strains of pneumococcus but inactive upon themselves. Strain R6, which did not produce pneumocin under most conditions, was changed to a producing state by mutation or by transformation with deoxyribonucleic acid from other pneumococci. The genetic determinants of several different bacteriocins were introduced into the same cell, where they acted independently. Determinants active in bacteriocin production were also constructed by crosses involving two nonproducing parents. Resistance to pneumocin B4 was a property of cells of the P4 type which produced it. On selection of pneumocin-resistant cells resulting from mutation or transformation in strain R6, resistant cells, all of which were pneumocin producers, were obtained. The genetic determinants of B4 production and resistance were transferred together in transformation. A gene for high level B4 resistance was found in strain P7S. This gene was not associated with B4 production and did not induce production when introduced into R6, although it did increase resistance to B4. Pneumocin B4 was bactericidal and caused a rapid cessation of growth. Its action was inhibited by pronase, trypsin, chymotrypsin, and phospholipids.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D., DAVIE J. M. PROBABLE IDENTITY OF A GROUP D HEMOLYSIN WITH A BACTERIOCINE. J Bacteriol. 1963 Oct;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S. Phenotypic expression of a genetic property introduced by deoxyribonucleate. J Gen Physiol. 1959 Mar 20;42(4):737–748. doi: 10.1085/jgp.42.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- HOLLAND I. B. Further observations on the properties of megacin, a bacteriocine formed by Bacillus megaterium. J Gen Microbiol. 1962 Dec;29:603–614. doi: 10.1099/00221287-29-4-603. [DOI] [PubMed] [Google Scholar]
- IVANOVICS G., ALFOLDI L., NAGY E. Mode of action of megacin. J Gen Microbiol. 1959 Aug;21:51–60. doi: 10.1099/00221287-21-1-51. [DOI] [PubMed] [Google Scholar]
- JACOB F., LWOFF A., SIMINOVITCH A., WOLLMAN E. Définition de quelques termes relatifs a la lysogénie. Ann Inst Pasteur (Paris) 1953 Jan;84(1):222–224. [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]




