Abstract
We tested the hypothesis that adenosine contributes to augmented skeletal muscle vasodilation during hypoxic exercise. In separate protocols, subjects performed incremental rhythmic forearm exercise (10% and 20% of maximum) during normoxia and normocapnic hypoxia (80% arterial O2 saturation). In protocol 1 (n = 8), subjects received an intra-arterial administration of saline (control) and aminophylline (adenosine receptor antagonist). In protocol 2 (n = 10), subjects received intra-arterial phentolamine (α-adrenoceptor antagonist) and combined phentolamine and aminophylline administration. Forearm vascular conductance (FVC; in ml·min−1·100 mmHg−1) was calculated from forearm blood flow (in ml/min) and blood pressure (in mmHg). In protocol 1, the change in FVC (ΔFVC; change from normoxic baseline) during hypoxic exercise with saline was 172 ± 29 and 314 ± 34 ml·min−1·100 mmHg−1 (10% and 20%, respectively). Aminophylline administration did not affect ΔFVC during hypoxic exercise at 10% (190 ± 29 ml·min−1·100 mmHg−1, P = 0.4) or 20% (287 ± 48 ml·min−1·100 mmHg−1, P = 0.3). In protocol 2, ΔFVC due to hypoxic exercise with phentolamine infusion was 313 ± 30 and 453 ± 41 ml·min−1·100 mmHg−1 (10% and 20% respectively). ΔFVC was similar at 10% (352 ± 39 ml·min−1·100 mmHg−1, P = 0.8) and 20% (528 ± 45 ml·min−1·100 mmHg−1, P = 0.2) hypoxic exercise with combined phentolamine and aminophylline. In contrast, ΔFVC to exogenous adenosine was reduced by aminophylline administration in both protocols (P < 0.05 for both). These observations suggest that adenosine receptor activation is not obligatory for the augmented hyperemia during hypoxic exercise in humans.
Keywords: aminophylline, systemic hypoxia, muscle blood flow
systemic hypoxia augments blood flow to contracting muscles. This increased perfusion is a product of local vasodilation and is proportional to the hypoxia-induced fall in arterial O2 content, thus preserving muscle O2 delivery and ensuring it is matched to metabolic demand. This means that the combination of exercise and hypoxia produces an augmented vasodilatation and blood flow relative to exercise under normoxic conditions (6, 31, 33, 40, 41). This augmented dilation occurs despite enhanced muscle sympathetic vasoconstrictor activity (21, 34, 35) but is not due to reduced α-adrenoceptor responsiveness associated with hypoxia (8, 41).
At rest, β-adrenergic receptor activation appears to be responsible for nearly half of the augmented hypoxic vasodilation in human skeletal muscle vascular beds (3, 38, 40). This β-adrenergic component includes a nitric oxide (NO)-dependent element (38), as hypoxic vasodilation in resting muscle is substantially reduced by NO synthase inhibition (4, 38). In addition to the β-adrenergic component, systemic hypoxia elevates resting skeletal muscle interstitial levels of adenosine (23), and there is a report (22) showing that adenosine receptor antagonists attenuate resting hypoxic forearm blood flow (FBF).
We recently reported that in the absence of overlying sympathetic vasoconstriction, β-adrenergic mechanisms contribute to the augmented hypoxic vasodilation during mild forearm exercise. However, the β-adrenergic component decreases with increased exercise intensity (40). This suggests that other local vasodilating factors are responsible for the augmented vasodilation during hypoxic exercise, especially at higher exercise intensities. In this context, adenosine released from the active muscles is thought to be a primary compensatory vasodilator signal during periods of reduced O2 availability (14, 18–20).
Based on this evidence, we tested the hypothesis that adenosine receptor activation contributes to the augmented vasodilation observed during hypoxic forearm exercise. Since the enhanced sympathetic outflow caused by systemic hypoxia can mask skeletal muscle vasodilation during exercise, we examined the role of adenosine-mediated vasodilation during forearm exercise with and without α-adrenoceptor blockade.
METHODS
Subjects
A total of eight healthy female subjects (mean age: 28 ± 2 yr) and 13 healthy male subjects (mean age: 27 ± 2 yr) volunteered to participate in two separate protocols. Subjects gave written informed consent and were nonobese, nonsmokers, and not taking any medications (except for oral contraceptives in some women). Studies were performed after subjects had fasted overnight and refrained from exercise and caffeine for at least 24 h. Female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives. All study protocols were approved by the Institutional Review Board.
Rhythmic Forearm Exercise
Forearm exercise was performed with a handgrip device by the nondominant arm at 10% and 20% (5 min each) of each subject's maximal voluntary contraction (MVC; mean: 40 ± 3 kg, range: 21–61 kg) determined at the beginning of each experiment. The weight was lifted 4–5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation (20 contractions/min) using a metronome to ensure correct timing. The average weight used for forearm exercise in protocol 1 was 3.8 ± 0.4 and 7.5 ± 0.9 kg for 10% and 20% MVC, respectively. The average weight used for forearm exercise in protocol 2 was 4.1 ± 0.3 and 8.2 ± 0.6 kg for 10% and 20% MVC, respectively.
Arterial and Venous Catheterization
A 20-gauge, 5-cm catheter was placed in the brachial artery of the exercising arm under aseptic conditions after local anesthesia (2% lidocaine) for the administration of study drugs and to obtain arterial blood samples. The catheter was connected to a three-port connector in series, as previously described in detail (7). One port was linked to a pressure transducer to allow measurement of arterial pressure and was continuously flushed (3 ml/h) with heparinized saline with a stopcock system to enable arterial blood sampling. The remaining two ports allowed arterial drug administration. Deep venous blood was sampled via an 18-gauge, 3-cm catheter inserted retrograde in an antecubital vein (16).
FBF
Brachial artery mean blood velocity and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole between contractions during steady-state conditions. FBF was calculated as the product of mean blood velocity (in cm/s) and brachial artery cross-sectional area (in cm2) and expressed as milliliters per minute.
Systemic Hypoxia
Hypoxia was generated using a self-regulating partial rebreathe system that clamps end-tidal CO2 at baseline levels despite large changes in minute ventilation during hypoxia (1, 38, 40, 41). During the hypoxic condition, the level of inspired O2 was titrated to achieve an arterial O2 saturation (assessed via pulse oximetry) of ∼80%. CO2 concentrations were monitored (Cardiocap/5, Datex-Ohmeda, Louisville, CO), and ventilation was assessed via a turbine (model VMM-2a, Interface Associates, Laguna Nigel, CA).
Pharmacological Infusions
Aminophylline (a nonselective adenosine receptor antagonist) was administered to the forearm via brachial artery catheter at a dose of 200 μg·dl forearm volume−1·min−1 (22, 24). To confirm adenosine receptor inhibition, exogenous adenosine was administered at three doses: 3.125, 6.25, and 12.5 μg·dl forearm volume−1·min−1 intra-arterially for 4 min at each dose before and after aminophylline infusion. Phentolamine (a nonselective α-adrenoceptor antagonist) was administered at the start of the study (protocol 2) to the forearm via the brachial artery catheter as a loading dose (10 μg·dl forearm volume−1·min−1 for 5 min) followed by a continuous maintenance dose (25 μg/min). This dose of phentolamine has been shown to effectively inhibit α-receptor vasoconstriction (9).
Blood Gas and Catecholamine Analysis
Brachial artery and deep venous blood samples were analyzed with a clinical blood gas analyzer (Bayer 855 Automatic Blood Gas System, Boston, MA) for Po2, Pco2, O2 content, pH, and O2 saturation. Arterial and venous plasma catecholamine (epinephrine and norepinephrine) levels were determined by HPLC with electrochemical detection.
Experimental Protocols
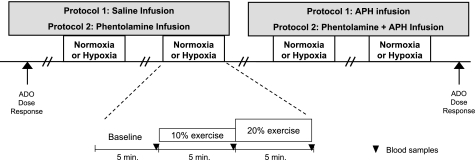
A schematic diagram of the general experimental design is shown in Fig. 1. Each subject completed a resting baseline condition (5 min) followed by rhythmic forearm exercise at 10%, which was immediately increased to 20% MVC (5 min each) during normoxia and normocapnic hypoxia. Exposure to normoxia or hypoxia was alternated and randomized.
Fig. 1.
Schematic diagram of the experimental protocols. Measurements were obtained at baseline and incremental exercise (10% and 20% of maximum voluntary contraction) under normoxic and hypoxic conditions. Protocol 1 was performed during control (saline) and aminophylline (Aph) infusions. Protocol 2 was performed during phentolamine infusion alone and combined phentolamine and aminophylline infusion. ADO, adenosine.
Protocol 1.
Resting baseline and forearm exercise (normoxia and hypoxia) were performed during a control (saline) infusion followed by an aminophylline infusion. Due to the long half-life of aminophylline, the aminophylline trial occurred last. A rest period of at least 20 min was allowed between conditions under each drug infusion.
Protocol 2.
Resting baseline and forearm exercise (normoxia and hypoxia) were performed during phentolamine infusion followed by an infusion of combined phentolamine and aminophylline. As in protocol 1, a rest period of at least 20 min was allowed between conditions under each drug infusion. During each infusion (saline or aminophylline in protocol 1 and phentolamine or phentolamine + aminophylline in protocol 2) and each condition (normoxia and hypoxia) arterial and venous blood was sampled at rest and at steady-state exercise for blood gas analysis and plasma catecholamine determination.
Data Analysis and Statistics
Data were collected at 200 Hz, stored on a computer, and analyzed offline with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial pressure was determined from the brachial artery pressure waveform, and heart rate was determined from the electrocardiogram. Values for minute ventilation, end-tidal CO2, and O2 saturation (pulse oximetry) were determined by averaging minutes 4 and 5 at rest and each exercise intensity. FBF and arterial pressure were determined by averaging values from the 4th minute at rest and each exercise bout. Forearm vascular conductance (FVC) was calculated as follows: FVC = (FBF/arterial pressure) × 100 and was expressed as ml·min−1·100 mmHg−1. For both rest and exercise, the changes in FBF (ΔFBF) and FVC (ΔFVC) due to hypoxia were calculated by subtracting resting FBF and FVC during normoxia from FBF and FVC values obtained during hypoxia within each drug infusion. Blood gas and catecholamine values were determined from blood samples obtained during normoxia and hypoxia with each drug infusion. The arteriovenous oxygen difference during forearm exercise was calculated by the difference between arterial and venous O2 content. Forearm O2 consumption (V̇o2) was calculated using the Fick equation as follows: V̇o2 = FBF × arteriovenous O2 difference. Norepinephrine spillover was estimated as the difference between venous and arterial norepinephrine levels during normoxic and hypoxic exercise.
All values are expressed as means ± SE. To determine the effect of hypoxia with each pharmacological treatment, differences in absolute FBF and FVC at rest (normoxia and hypoxia) and differences in ΔFBF and ΔFVC at rest and during each exercise intensity (normoxia and hypoxia) were determined via repeated-measures ANOVA. Hemodynamic, respiratory, blood gases, and catecholamine variables were compared via repeated-measures ANOVA to detect differences between responses during hypoxia at rest and during exercise across pharmacological infusions. Appropriate post hoc analysis determined where statistical differences occurred. Statistical difference was set a priori at P < 0.05.
RESULTS
Eight subjects (4 men and 4 women) completed protocol 1. One subject did not complete the protocol due to symptoms of vasovagal syncope (precipitous fall in blood pressure and heart rate) during hypoxia and was excluded from the analysis. Those subjects completing protocol 1 were 26 ± 1 yr of age, 171 ± 3 cm in height, and weighed 71 ± 4 kg (body mass index: 24 ± 1 kg/m2). Ten subjects (7 men and 3 women) completed protocol 2. Two subjects failed to complete the entire protocol due to outwardly evident physically labored breathing at some point during a hypoxic condition and voluntarily came off the mouth piece. Those subjects completing protocol 2 were 25 ± 2 yr of age, 175 ± 2 cm in height, and weighed 74 ± 3 kg (body mass index: 24 ± 1 kg/m2). Data collected from subjects not completing the protocols were excluded from group analysis.
Systemic Hemodynamic and Respiratory Responses
The group data (means ± SE) for hemodynamic and respiratory responses due to combined forearm exercise and hypoxia with each drug infusion (protocols 1 and 2) are shown in Table 1. As expected, during both protocols, heart rate and minute ventilation increased as a consequence of systemic hypoxia and incremental forearm exercise. There were no differences in mean arterial pressure between normoxia and hypoxia within drug infusions for both protocols (Table 1). By design, there were no differences in end-tidal CO2 between conditions for both protocols (Table 1).
Table 1.
Systemic hemodynamic and respiratory responses at rest and with incremental exercise during normoxia and hypoxia with each drug infusion
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| Protocol 1 | ||||||
| Control (saline) | ||||||
| MAP, mmHg | 85±2 | 88±2 | 90±3 | 88±2 | 88±2 | 90±3 |
| HR, beats/min | 62±3 | 67±3† | 70±3 | 75±3* | 78±4* | 79±4* |
| Minute ventilation, l/min (BTPS) | 7.1±0.9 | 8.1±1.0 | 10.0±1.2†‡ | 10.8±1.8* | 13.1±2.5* | 15.6±2.9*† |
| End-tidal CO2, % | 4.9±0.1 | 5.0±0.2 | 5.0±0.9 | 4.9±0.1 | 5.0±0.1 | 5.0±0.1 |
| Aminophylline | ||||||
| MAP, mmHg | 87±2 | 88±3 | 89±3 | 84±3 | 86±2 | 87±3 |
| HR, beats/min | 63±3 | 68±3† | 72±3† | 77±5* | 82±6*† | 88±6*† |
| Minute ventilation, l/min (BTPS) | 9.3±0.9 | 11.0±1.2 | 12.4±1.3† | 15.2±1.5* | 19.6±1.8*†‡ | 22.5±1.7*†‡ |
| End-tidal CO2, % | 5.0±0.1 | 5.0±0.1 | 5.1±0.1 | 5.0±0.1 | 5.0±0.1 | 5.1±0.1 |
| Protocol 2 | ||||||
| Phentolamine | ||||||
| MAP, mmHg | 87±2 | 90±2 | 93±2 | 90±2 | 90±3 | 90±3 |
| HR, beats/min | 57±3 | 61±3† | 65±3† | 73±4* | 80±5*† | 83±4* |
| Minute ventilation, l/min (BTPS) | 9.0±1.4 | 9.4±1.1 | 12.2±1.2 | 14.9±3.4 | 17.8±2.6*† | 21.7±2.9*† |
| End-tidal CO2, % | 5.4±0.1 | 5.4±0.1 | 5.4±0.1 | 5.3±0.1 | 5.4±0.1 | 5.4±0.1 |
| Combined phentolamine and aminophylline | ||||||
| MAP, mmHg | 90±2 | 91±2 | 94±3 | 89±2 | 88±3 | 90±3 |
| HR, beats/min | 60±4 | 66±4† | 70±4† | 78±5* | 83±4*† | 88±6*† |
| Minute ventilation, l/min (BTPS) | 15.0±1.7‡ | 17.4±1.6‡ | 21.8±2.1†‡ | 22.2±2.9*‡ | 27.9±2.7*‡ | 31.5±3.4*†‡ |
| End-tidal CO2, % | 5.4±0.1 | 5.4±0.1 | 5.5±0.1 | 5.4±0.1 | 5.5±0.1 | 5.5±0.1 |
Values are means ± SE; n = 8 subjects in protocol 1 and 10 subjects in protocol 2. MAP, mean arterial pressure; HR, heart rate; BTPS, body temperature pressure saturated.
Main effect of hypoxia, P < 0.05 vs. normoxia;
P < 0.05 vs. the previous intensity;
P < 0.05 vs. control (protocol 1) or phentolamine (protocol 2) administration.
Forearm Exercise
Table 2 shows group data (means ± SE) for forearm hemodynamics at rest and with increasing exercise intensity during control saline and aminophylline infusion (protocol 1) and phentolamine and combined phentolamine and aminophylline (protocol 2).
Table 2.
Forearm hemodynamics at rest and with incremental exercise during normoxia and hypoxia with each drug infusion
| Rest | 10% | 20% | Change From Normoxia at Rest |
||||
|---|---|---|---|---|---|---|---|
| 10% | 20% | ||||||
| Protocol 1 | |||||||
| FBF, ml/min | |||||||
| Control (saline) | |||||||
| Normoxia | 49±14 | 191±33† | 303±48† | 142±23 | 254±36† | ||
| Hypoxia | 58±16* | 205±37*† | 336±36*† | 157±29* | 287±35*† | ||
| Aminophylline | |||||||
| Normoxia | 133±35‡ | 231±28†‡ | 341±33† | 98±18 | 207±25† | ||
| Hypoxia | 183±52*‡ | 301±46*†‡ | 393±62*† | 167±25* | 259±44*† | ||
| FVC, ml·min−1·100 mmHg−1 | |||||||
| Control (saline) | |||||||
| Normoxia | 56±16 | 214±33† | 334±39† | 157±22 | 278±36† | ||
| Hypoxia | 65±17* | 229±38*† | 370±47*† | 172±39* | 314±34*† | ||
| Aminophylline | |||||||
| Normoxia | 154±38‡ | 263±29†‡ | 358±24† | 109±22 | 242±21† | ||
| Hypoxia | 218±60*‡ | 344±48*†‡ | 403±53*† | 190±29* | 287±48*† | ||
| Protocol 2 | |||||||
| FBF, ml/min | |||||||
| Phentolamine | |||||||
| Normoxia | 197±28 | 366±41† | 522±53† | 170±29 | 325±37† | ||
| Hypoxia | 320±53* | 488±49*† | 622±22*† | 291±34* | 425±46*† | ||
| Combined phentolamine and aminophylline | |||||||
| Normoxia | 270±28‡ | 415±39†‡ | 582±57†‡ | 144±21 | 312±40† | ||
| Hypoxia | 418±38*‡ | 586±57*†‡ | 749±62*†‡ | 316±39* | 478±43*† | ||
| FVC, ml·min−1·100 mmHg−1 | |||||||
| Phentolamine | |||||||
| Normoxia | 223±28 | 403±40† | 559±51† | 180±31 | 335±39† | ||
| Hypoxia | 347±50* | 536±42*† | 676±52*† | 313±30* | 453±31*† | ||
| Combined phentolamine and aminophylline | |||||||
| Normoxia | 298±25‡ | 451±36†‡ | 618±56†‡ | 153±23 | 320±43† | ||
| Hypoxia | 464±32*‡ | 650±49*†‡ | 826±56*†‡ | 352±39* | 528±45*† | ||
Values are means ± SE; n = 8 subjects in protocol 1 and 10 subjects in protocol 2. FBF, forearm blood flow; FVC, forearm vascular conductance.
Main effect of hypoxia, P < 0.05;
P < 0.05 vs. the previous exercise intensity);
P < 0.05 vs. control (protocol 1) or phentolamine (protocol 2) administration.
Protocol 1.
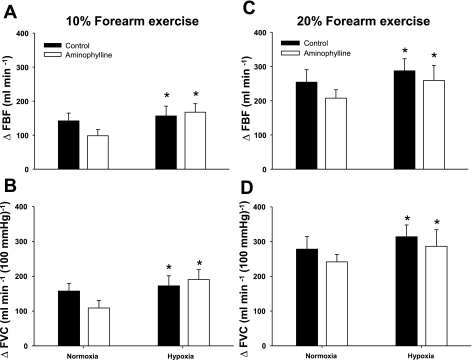
Systemic hypoxia increased resting FBF and FVC (relative to normoxic baseline values) during the control saline infusion (P < 0.05). Likewise, ΔFBF and ΔFVC (relative to normoxic baseline values) to incremental hypoxic exercise were higher compared with normoxic exercise of the same intensity during the control saline infusion (main effect of hypoxia, P < 0.05; Fig. 2, A–D). The intra-arterial administration of aminophylline increased normoxic baseline (resting) and 10% MVC exercise blood flows above the values observed during control saline infusion (P < 0.01; Table 2). However, ΔFBF or ΔFVC during normoxic forearm exercise with aminophylline administration was not different than the changes observed during control saline infusion (P > 0.05; Table 2). Notwithstanding the aminophylline-induced elevation in resting blood flow, ΔFBF due to acute systemic hypoxia at rest was 10 ± 4 and 49 ± 23 ml/min during the saline and aminophylline infusions, respectively (P = 0.11). ΔFVC due to systemic hypoxia was 9 ± 4 and 64 ± 29 ml·min−1·100 mmHg−1 for the saline and aminophylline infusions, respectively (P = 0.09). Aminophylline administration did not reduce either ΔFBF (P = 0.4) or ΔFVC (P = 0.5) during hypoxic forearm exercise at 10% MVC (Fig. 2, A and B) compared with control saline. Similarly, during hypoxic exercise at 20% MVC, aminophylline infusion did not statistically reduce ΔFBF (P = 0.2) or ΔFVC (P = 0.3; Fig. 2, C and D). This finding was not due to ineffective drug dosage, as adenosine receptor inhibition was confirmed via exogenous adenosine infusions after the aminophylline administration (P < 0.05; see Fig. 4, A and B).
Fig. 2.
Changes in forearm blood flow (ΔFBF) and forearm vascular conductance (ΔFVC) due to hypoxic exercise during saline (control) and aminophylline administration (n = 8). At 10% or 20% forearm exercise, the adenosine receptor antagonist (aminophylline) did not reduce FBF (A and C) or FVC (B and D) compared with control (saline) during normoxic or hypoxic exercise. *Main effect of hypoxia, P < 0.05 vs. normoxia.
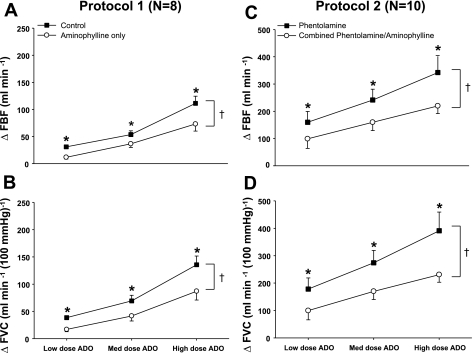
Fig. 4.
ΔFBF and ΔFVC due to incremental adenosine administration during saline and aminophylline administration (protocol 1, n = 8) and during phentolamine administration and combined phentolamine and aminophylline administration (protocol 2, n = 10). In protocol 1, the adenosine receptor antagonist (aminophylline) reduced FBF (A) and FVC (B) at all doses of exogenous adenosine compared with control (saline). In protocol 2, combined α-adrenoceptor inhibition (phentolamine) and the adenosine receptor antagonist (aminophylline) reduced FBF (C) and FVC (D) at all doses of exogenous adenosine compared with phentolamine alone. *P < 0.05 vs. aminophylline (protocol 1) or combined phentolamine and aminophylline (protocol 2); †main effect of adenosine dose, P < 0.05.
Forearm V̇o2 increased (P < 0.05) to 24 ± 3 and 37 ± 6 ml/min with normoxic exercise at 10% and 20% MVC, respectively, during the control saline infusion. This was similar to V̇o2 values obtained during hypoxic exercise (17 ± 6 and 40 ± 6 ml/min for 10% and 20%, respectively, P < 0.05 for exercise intensity). During the aminophylline administration, forearm V̇o2 increased (P < 0.05) to 27 ± 4 and 36 ± 6 ml/min with normoxic exercise at 10% and 20% MVC, respectively. Values obtained during normoxic exercise with aminophylline infusion were similar to those obtained during hypoxic exercise (24 ± 7 and 38 ± 7 ml/min for 10% and 20%, respectively, P < 0.05 for exercise intensity). There were no difference in V̇o2 between control saline and aminophylline infusions.
Protocol 2.
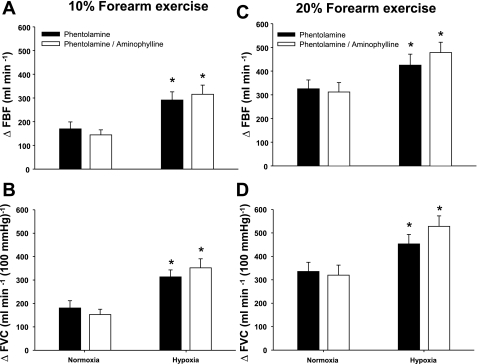
At rest, phentolamine infusion increased baseline blood flow to 197 ± 28 ml/min. This was higher than resting baseline FBF values obtained during the control saline infusion from protocol 1 (P < 0.01) and similar to baseline FBF values observed during aminophylline infusion (P = 0.24, independent measures ANOVA). Systemic hypoxia increased resting FBF and FVC during the phentolamine infusion (P < 0.05). That is, in the absence of overlying vasoconstriction, ΔFBF and ΔFVC due to hypoxia at rest were 124 ± 31 and 124 ± 29 ml·min−1·100 mmHg−1, respectively. Likewise, ΔFBF and ΔFVC during incremental hypoxic exercise were higher compared with normoxic exercise of the same intensity with phentolamine infusion (main effect of hypoxia, P < 0.05; Fig. 3, A–D).
Fig. 3.
ΔFBF and ΔFVC due to hypoxic exercise during phentolamine administration and combined phentolamine and aminophylline administration (n = 10). At 10% or 20% forearm exercise, combined α-adrenoceptor inhibition (phentolamine) and the adenosine receptor antagonist (aminophylline) did not reduce FBF (A and C) or FVC (B and D) compared with phentolamine alone during normoxic or hypoxic exercise. *Main effect of hypoxia, P < 0.01 vs. normoxia.
The combined phentolamine and aminophylline infusion increased baseline resting and exercise (10% and 20% MVC) blood flows compared with values observed during phentolamine alone (P < 0.05; Table 2). However, ΔFBF or ΔFVC during normoxic forearm exercise with combined phentolamine and aminophylline administration was similar to the changes observed during phentolamine alone (P > 0.05; Table 2). Systemic hypoxia increased resting FBF and FVC during phentolamine and aminophylline infusion (P < 0.05; Table 2). ΔFBF due to hypoxia at rest (148 ± 20 ml/min) during the combined phentolamine and aminophylline infusion was similar to ΔFBF during phentolamine alone (P = 0.31). Furthermore, ΔFVC due to hypoxia at rest (166 ± 19 ml/min) during combined phentolamine and aminophylline administration was not different than ΔFBF during phentolamine alone (P = 0.11).
During hypoxic exercise, inhibition of overlying vasoconstriction via phentolamine infusion led to a significant vasodilation (ΔFBF and ΔFVC) at 10% and 20% MVC (Fig. 3, A–D). However, the combination of phentolamine and aminophylline did not blunt ΔFBF (Fig. 3, A and C) or ΔFVC (Fig. 3, B and D) relative to incremental hypoxic exercise with phentolamine alone. This finding was not due to ineffective blockade of adenosine receptors, because the vasodilation elicited by exogenous adenosine infusions after combined phentolamine and aminophylline administration was blunted (P < 0.05; Fig. 4, C and D).
Forearm V̇o2 increased (P < 0.05) to 28 ± 5 and 48 ± 7 ml/min with normoxic exercise at 10% and 20% MVC, respectively, during the phentolamine administration. This was similar to V̇o2 values obtained during hypoxic exercise (29 ± 5 and 47 ± 5 ml/min for 10% and 20%, respectively, P < 0.05 for exercise intensity). During the combined phentolamine and aminophylline administration, forearm V̇o2 increased (P < 0.05) to 19 ± 3 and 39 ± 5 ml/min with normoxic exercise at 10% and 20% MVC, respectively. In protocol 2, the values obtained during normoxic exercise with combined phentolamine and aminophylline infusion were similar to those obtained during hypoxic exercise (17 ± 4 and 37 ± 8 ml/min for 10% and 20%, respectively, P < 0.05 for exercise intensity). There were no statistical differences in V̇o2 between phentolamine and combined phentolamine and aminophylline infusions.
Blood gases and Catecholamines
Protocol 1.
Systemic hypoxia reduced arterial Po2 (P < 0.01) and arterial O2 content (P < 0.01) at rest and with incremental forearm exercise. An increased O2 extraction during normoxic exercise led to a similar arteriovenous O2 difference between the control and aminophylline infusions, despite mildly lower arterial O2 content with aminophylline (Table 3). During hypoxic exercise, values for venous O2 content (extraction of O2) were similar between aminophylline and control infusions, which led to a lower arteriovenous O2 difference associated with hypoxic exercise during aminophylline administration (P < 0.05; Table 3).
Table 3.
Arterial and venous blood gas responses at rest and with incremental exercise during normoxia and hypoxia with each drug infusion
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| Protocol 1 | ||||||
| Control (saline) | ||||||
| O2 saturation, % | 97±0 | 97±0 | 97±0 | 82±1* | 82±1* | 83±0* |
| Arterial Po2, Torr | 103±3 | 107±2 | 107±2 | 47±1* | 47±0.3* | 48±1* |
| Venous Po2, Torr | 34±1 | 27±1† | 27±1 | 29±1* | 21±0*† | 23±0* |
| Arterial O2 content, ml/l | 188±6 | 190±7 | 191±7 | 160±5* | 162±6* | 170±8* |
| Venous O2 content, ml/l | 107±5 | 79±6† | 79±6 | 94±5* | 60±3*† | 66±6* |
| Arteriovenous O2, ml/l | 81±5 | 111±6† | 112±5 | 65±4* | 101±4*† | 103±4* |
| Aminophylline | ||||||
| O2 saturation, % | 97±0 | 97±0 | 97±0 | 84±0* | 84±1* | 84±1* |
| Arterial Po2, Torr | 110±1 | 110±3 | 111±2 | 50±1* | 49±1* | 50±1* |
| Venous Po2, Torr | 46±0‡ | 24±0†‡ | 25±0 | 37±1*‡ | 26±0*† | 23±0*† |
| Arterial O2 content, ml/l | 175±6‡ | 173±6‡ | 175±7‡ | 152±6*‡ | 147±6*‡ | 154±5*‡ |
| Venous O2 content, ml/l | 152±5 | 65±5† | 60±7 | 129±5‡ | 69±5† | 61±4 |
| Arteriovenous O2, ml/l | 23±1‡ | 108±4† | 115±6 | 23±2*‡ | 78±3*†‡ | 95±4*† |
| Protocol 2 | ||||||
| Phentolamine | ||||||
| O2 saturation, % | 97±0 | 97±0 | 97±0 | 83±1* | 82±1* | 83±1* |
| Arterial Po2, Torr | 105±3 | 109±3 | 107±2 | 48±1* | 47±1* | 52±1* |
| Venous Po2, Torr | 54±3 | 33±3† | 30±2† | 39±1 | 30±1† | 29±2 |
| Arterial O2 content, ml/l | 185±4 | 187±4 | 188±4 | 157±3* | 159±3* | 169±4*† |
| Venous O2 content, ml/l | 164±5 | 110±8† | 94±6† | 140±3* | 99±5*† | 94±7* |
| Arteriovenous O2, ml/l | 22±4 | 77±8† | 94±7† | 18±3* | 59±6*† | 76±6*† |
| Combined phentolamine and aminophylline | ||||||
| O2 saturation, % | 97±0 | 97±0 | 97±0 | 83±1* | 83±1* | 83±1* |
| Arterial Po2, Torr | 110±2 | 110±2 | 111±3 | 49±2* | 48±1* | 51±1* |
| Venous Po2, Torr | 57±3 | 35±2† | 32±2 | 43±2‡ | 32±1† | 32±0.3 |
| Arterial O2 content, ml/l | 166±4‡ | 169±4‡ | 170±3‡ | 144±4*‡ | 143±3*‡ | 153±3*†‡ |
| Venous O2 content, ml/l | 160±5 | 116±6† | 104±6 | 138±6 | 108±5† | 103±8 |
| Arteriovenous O2, ml/l | 7±3‡ | 53±8†‡ | 65±6‡ | 6±6*‡ | 35±6*†‡ | 50±8*†‡ |
Values are means ± SE; n = 8 subjects in protocol 1 and 10 subjects in protocol 2.
Main effect of hypoxia, P < 0.05 vs. normoxia;
P < 0.05 vs. the previous intensity;
P < 0.05 vs. control (protocol 1) or phentolamine (protocol 2) administration.
Systemic hypoxia increased arterial epinephrine during control saline and aminophylline infusions (P < 0.05; table 4). Further changes in arterial epinephrine did not occur with incremental exercise with control saline administration for both normoxic and hypoxic conditions (P = 0.18). However, arterial epinephrine increased progressively with hypoxic exercise intensity during aminophylline administration (P < 0.05). Systemic hypoxia did not increase norepinephrine spillover (P = 0.52); however, norepinephrine spillover was higher with incremental hypoxic exercise during aminophylline infusion (P < 0.01; Table 4).
Table 4.
Venous and arterial epinephrine and norepinephrine at rest and incremental exercise during normoxia and hypoxia with each drug infusion
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| Protocol 1 | ||||||
| Control (saline) | ||||||
| Arterial norepinephrine, pg/ml | 143±16 | 153±18 | 159±18† | 152±14 | 168±18† | 170±16† |
| Venous norepinephrine, pg/ml | 206±18 | 199±17 | 201±15 | 227±20 | 200±20† | 188±16† |
| Venous-arterial norepinephrine difference, pg/ml | 64±14 | 48±13† | 42±14† | 75±8 | 25±5† | 19±4† |
| Arterial epinephrine, pg/ml | 44±8 | 41±4 | 40±4 | 65±10* | 78±13* | 79±11* |
| Venous epinephrine, pg/ml | 11±1 | 26±3† | 30±3† | 16±1 | 43±2*† | 58±2*†‡ |
| Aminophylline | ||||||
| Arterial norepinephrine, pg/ml | 138±17 | 137±17 | 141±18 | 159±17 | 158±15 | 176±18 |
| Venous norepinephrine, pg/ml | 220±17 | 184±14† | 183±16† | 230±16 | 223±15* | 220±18* |
| Venous-arterial norepinephrine difference, pg/ml | 83±5 | 49±4† | 42±5† | 72±4 | 65±5§ | 44±7†‡§ |
| Arterial epinephrine, pg/ml | 51±9 | 54±8 | 67±14 | 76±7* | 97±6*† | 135±11*†§ |
| Venous epinephrine, pg/ml | 20±2§ | 39±1†§ | 41±1†§ | 40±1*§ | 67±3*†§ | 112±3*†‡§ |
| Protocol 2 | ||||||
| Phentolamine | ||||||
| Arterial norepinephrine, pg/ml | 162±20 | 162±24 | 166±24 | 180±27 | 176±21 | 184±24 |
| Venous norepinephrine, pg/ml | 222±27 | 203±32 | 209±31 | 261±32* | 247±26* | 234±28† |
| Venous-arterial norepinephrine difference, pg/ml | 59±11 | 41±9† | 43±10† | 81±8* | 70±8* | 50±7†‡ |
| Arterial epinephrine, pg/ml | 37±5 | 40±6 | 47±7 | 65±13* | 78±14*† | 93±17*† |
| Venous epinephrine, pg/ml | 27±5 | 32±5 | 39±5 | 44±6 | 63±11*† | 77±13*†‡ |
| Combined phentolamine and aminophylline | ||||||
| Arterial norepinephrine, pg/ml | 180±19 | 185±21§ | 192±21§ | 175±25 | 173±20 | 195±23†‡ |
| Venous norepinephrine, pg/ml | 290±30§ | 289±34§ | 285±31§ | 276±37 | 268±30 | 284±31 |
| Venous-arterial norepinephrine difference, pg/ml | 110±13§ | 105±16§ | 93±13†§ | 101±13 | 95±13§ | 89±11†§ |
| Arterial epinephrine, pg/ml | 50±3 | 62±5§ | 69±6†§ | 91±11* | 103±13* | 115±16*† |
| Venous epinephrine, pg/ml | 37±5 | 53±4§ | 67±5†§ | 70±7* | 92±12*†§ | 113±15*†‡§ |
Values are means ± SE; n = 8 subjects in protocol 1 and 10 subjects in protocol 2.
Main effect of hypoxia, P < 0.05 vs. normoxia;
P < 0.05 vs. rest;
P < 0.05 vs. 10 % exercise;
main effect of drug, P < 0.05 vs. control (protocol 1) or phentolamine (protocol 2) administration.
Protocol 2.
Systemic hypoxia reduced arterial Po2 (P < 0.01) and arterial O2 content (P < 0.01) at rest and with increasing exercise intensity. There was a statistical main effect of drug infusion on arterial O2 content. That is, the combined phentolamine and aminophylline administration reduced arterial O2 content at rest and incremental exercise under normoxic and hypoxic conditions compared with phentolamine alone (P < 0.05; Table 3). In contrast to protocol 1, extraction of O2 was similar during normoxic and hypoxic exercise with phentolamine and combined phentolamine and aminophylline infusions. This led to a significantly reduced arteriovenous O2 difference (mainly attributable to arterial O2 content) with the phentolamine and aminophylline condition compared with phentolamine alone (P < 0.05). The observed difference between drug infusions was likely due to baseline flow discrepancies, as V̇o2 values were statistically similar.
Systemic hypoxia increased arterial epinephrine at rest and incremental exercise during both phentolamine and combined phentolamine and aminophylline infusions (P < 0.05; Table 4). There was a statistical main effect of drug infusion on arterial epinephrine concentration during normoxic exercise. That is, the combined phentolamine and aminophylline infusion resulted in higher levels of arterial epinephrine at each level of exercise during normoxia (P < 0.01). There was a main effect of drug on norepinephrine spillover. Norepinephrine spillover was higher during incremental normoxic and hypoxic exercise with the combined phentolamine and aminophylline infusion (P < 0.01).
DISCUSSION
The primary novel finding from this study is that adenosine receptor inhibition with aminophylline (confirmed with the exogenous adenosine administration; Fig. 4) failed to reduce the augmented hypoxic vasodilation in human skeletal muscle during forearm exercise. Thus, adenosine is not obligatory for the augmented vasodilation seen during hypoxic exercise in humans. This was observed both with (protocol 1) and without (protocol 2) overlying α-adrenergic vasoconstriction, suggesting that the enhanced sympathetic outflow associated with systemic hypoxia (21, 34, 35) did not mask any underlying adenosine-mediated vasodilation.
In addition to the findings obtained during hypoxic exercise, our data suggest hypoxia-induced skeletal muscle vasodilation in resting humans does not require an adenosine component. This finding contrasts with the conclusions of Leuenberger et al. (22), who previously reported that adenosine contributes to hypoxia-induced forearm vasodilation in resting humans. These inconsistent results may be explained by the differing approaches to analysis used in the two studies. In the report from Leuenberger et al. (22) and in the present study, aminophylline alone increased baseline resting blood flow during normoxia. Leuenberger et al. (22) reported changes in muscle blood flow relative (%change) to normoxic baseline values during aminophylline administration. In the present study, we reported absolute changes in muscle blood flow from normoxic baseline during aminophylline administration. If we were to calculate our FBF data as a percentage of baseline blood flow during control saline administration, hypoxia increased FBF at rest by 21 ± 8% relative to the normoxia baseline. Hypoxia increased FBF by 40 ± 20% relative to the normoxia baseline during aminophylline administration. Thus, comparing our data during hypoxia at rest relative to normoxia baseline values during drug infusions does not change our conclusions.
In this investigation, intra-arterial phentolamine (protocol 2) increased baseline FBF similar to aminophylline alone (protocol 1; Fig. 2), which allowed us to mathematically compare percent changes in FVC during hypoxia to a “flow control” obtained during α-adrenergic inhibition. As an example, during the control saline administration (protocol 1), hypoxic exercise at 10% MVC increased FVC by 357 ± 56% from normoxia baseline values. With phentolamine administration alone, hypoxic exercise at 10% MVC increased FVC by 157 ± 19% relative to the normoxia baseline (protocol 2). However, the absolute changes in forearm flow or conductance were similar in the two conditions. Therefore, we chose to report the absolute change in muscle blood flow and conductance from the normoxic baseline and believe that these values mostly represent the vasodilator responses during exercise and systemic hypoxia. Along these lines, it was shown in the 1950s that when baseline FBF was intentionally increased before handgrip exercise, the elevated baseline flow had no effect on the change in flow caused by exercise (28).
Potential Mechanisms for Augmented Exercise Hyperemia During Hypoxia
Our previous report (40) and that of Weisbrod et al. (38) demonstrated the contribution of systemic epinephrine release as a signal for augmented hypoxic vasodilation at rest. We extended these findings to describe the intensity-dependent contribution of β-adrenergic vasodilation during hypoxic exercise (40), where the direct contribution of epinephrine to the augmented hypoxic exercise hyperemia decreases with increasing exercise intensity. We suggested a local vasodilator signal or signals would be responsible for the precisely controlled matching of O2 delivery to demand and to compensate for the reduction in the β-adrenergic-mediated component.
The results from the present study suggest that adenosine release is not a major local vasodilator signal ensuring appropriate matching of O2 delivery to demand. Along these lines, β-adrenergic receptor-mediated NO release is known to contribute to augmented hypoxic vasodilation at rest (38), and, therefore, an enhanced release of NO via β-adrenergic mechanisms during adenosine receptor inhibition could maintain optimal O2 delivery. One potential confound related to β-adrenergic-mediated vasodilation is that both aminophylline and β-adrenergic receptors affect intracellular cAMP levels via different mechanisms. Thus, aminophylline might potentially augment β-adrenergic-mediated vasodilation even though we have previously shown that β-adrenergic-mediated vasodilation does not contribute to hypoxic vasodilation during higher-intensity forearm exercise.
Deoxygenation of red blood cells can lead to ATP release (2, 12, 15). Circulating ATP from red blood cells may play a role in the augmented hypoxic vasodilation. In fact, venous plasma levels of ATP tend to be elevated with hypoxic exercise compared with normoxic exercise (12). The release of ATP can elicit vasodilation through binding of purinergic P2Y receptors located on vascular endothelial cells. This may lead to the release of NO, prostaglandins, and/or EDHFs (11, 17, 26, 32, 39). The circulating ATP hypothesis remains attractive because this would allow precise matching of O2 delivery to demand.
Experimental Considerations
There are five potential limitations to our study that should be mentioned. First, in a condition where O2 is limited, such as systemic hypoxia, an adenosine vasodilator signal normally present during exercise (14, 18–20) may be augmented. In the present study, when ΔFBF during hypoxic exercise was calculated from baseline values obtained during hypoxia at rest (response to exercise per se during hypoxia), there was no effect of aminophylline administration on exercise-mediated ΔFBF or ΔFVC.
Second, in the present study, the nonspecific adenosine receptor antagonist aminophylline (antagonizes all four adenosine receptor isoforms: A1, A2A, A2B, and A3) was chosen on the basis of availability for human use. Previous data in the rat hindlimb have suggested that the adenosine-mediated component of muscle vasodilation during hypoxia is mediated by A1 receptors (5), whereas adenosine is thought to play a role in skeletal muscle vasodilation during exercise via A2A receptors (30). Whether the actions of adenosine become additive when the two stimuli are combined (hypoxia and muscle contractions) is unclear. There are also potential issues related to drug and adenosine distribution. In animal models, the extravascular release of adenosine from active muscle is thought to be a signal for exercise hyperemia per se (30). In this context, the modest effect of intraarterial aminophylline on exercise hyperemia during normoxia (24, 27, 29) and the lack of effect during hypoxic exercise might reflect an inability to block extravascular adenosine receptors using the intra-arterial administration of aminophylline. However, during systemic hypoxia in rats, intraluminal adenosine release from the endothelium appears to contribute to hypoxic vasodilation (5). Thus, there are complex issues related to the site of where any adenosine released during hypoxic exercise is originating from, and there are also issues about the intravascular versus extravascular distribution of aminophylline.
Third, as discussed above, aminophylline administration resulted in significant increases in resting baseline FBF and FVC (Table 2). Aminophylline is a known phosphodiesterase inhibitor thus increasing cAMP levels in the vascular smooth muscle, leading to increases in FBF (37). This elevated baseline FBF with aminophylline administration could alter the responsiveness of vascular smooth muscle during hypoxic exercise. However, we observed a similar elevation in baseline blood flow with phentolamine administration alone (protocol 2; Table 2) and aminophylline administration alone (protocol 1; Table 2). The responses to hypoxia and hypoxic exercise were also similar during either phentolamine or aminophylline; therefore, it is unlikely that the elevated baseline FBF alone during aminophylline infusion masked any changes in FBF due to adenosine receptor inhibition. Furthermore, despite the aminophylline-induced vasodilation observed in the resting forearm vasculature, we demonstrated effective adenosine receptor inhibition to exogenous adenosine infusions (Fig. 4). If adenosine was necessary for the full expression of the augmented exercise hyperemia during hypoxia, at least some reduction in ΔFBF or ΔFVC should have been observed.
Fourth, the present study could not differentiate between changes in muscle and skin circulation. However, previous research has suggested that changes in FBF during hypoxia at rest are primarily due to increases in skeletal muscle, and not skin, blood flow (21). However, Heinonen et al. (13) demonstrated that adenosine plays a role in muscle blood flow heterogeneity even in the absence of changes in bulk blood flow during one-leg intermittent isometric exercise.
Finally, the goal of the intra-arterial administration of aminophylline was to isolate the effects of adenosine receptor blockade in the experimental forearm without producing systemic effects. However, we observed an exaggerated ventilatory response at rest and with exercise during aminophylline infusions in both protocols. While systemic infusion of aminophylline has been shown to increase minute ventilation (25, 36), it also has profound effects on heart rate and blood pressure (10, 25). In the present study, the local administration of aminophylline did not increase heart rate, blood pressure, or arterial levels of norepinephrine. Furthermore, the total amount of locally infused aminophylline used in the present study (223 ± 15 mg infused over 120 min) was substantially less than the systemic bolus doses (450–550 mg) used in previous studies (10, 25, 36). Additionally, the timing of the hemodynamic and respiratory measurements was such that they were made after only 60–140 mg (depending on the order of the trials) of aminophylline had been infused. Since the aminophylline trials were always performed last, the elevated minute ventilation might be a result of the second hypoxic exposure. We have observed this exaggerated ventilatory response to the second hypoxic exposure with other drugs such as phentolamine (40) and the l-arginine analog N-monomethyl-l-arginine (unpublished observations).
Conclusions
We found that adenosine receptor activation was not obligatory for the augmented vasodilation seen during hypoxic forearm exercise. This occurred in the presence and absence of overlying sympathetic vasoconstriction.
GRANTS
This work was supported by National Institutes of Health Grants AR-55819 (to D. P. Casey), HL-46493 (to M. J. Joyner), and HL-78019 (to B. W. Wilkins) and by Clinical and Translational Science Award RR-024150. The Caywood Professorship via the Mayo Foundation also supported this research.
ACKNOWLEDGMENTS
The authors are grateful to the study volunteers for participation in the study. The authors also thank Branton Walker, Rachel Elvebak, Brianna Vaa, Christopher Johnson, Lakshmi (Madhuri) Somaraju, Pam Engrav, Karen Krucker, and Shelly Roberts for technical assistance.
REFERENCES
- 1. Banzett RB, Garcia RT, Moosavi SH. Simple contrivance “clamps” end-tidal Pco2 and Po2 despite rapid changes in ventilation. J Appl Physiol 88: 1597–1600, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Blauw GJ, Westendorp RG, Simons M, Chang PC, Frolich M, Meinders AE. β-Adrenergic receptors contribute to hypoxaemia induced vasodilation in man. Br J Clin Pharmacol 40: 453–458, 1995 [PMC free article] [PubMed] [Google Scholar]
- 4. Blitzer ML, Lee SD, Creager MA. Endothelium-derived nitric oxide mediates hypoxic vasodilation of resistance vessels in humans. Am J Physiol Heart Circ Physiol 271: H1182–H1185, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol 514: 151–162, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol 284: R291–R303, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt α-adrenergic vasoconstriction in the human forearm. J Physiol 549: 985–994, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eklund B, Kaijser L. Effect of regional α- and β-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol 262: 39–50, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elliott CG, Nietrzeba RM, Adams TD, Crapo RO, Jensen RL, Yanowitz FG. Effect of intravenous aminophylline upon the incremental exercise performance of healthy men. Respiration 47: 260–266, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Heinonen I, Nesterov SV, Kemppainen J, Nuutila P, Knuuti J, Laitio R, Kjaer M, Boushel R, Kalliokoski KK. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J Appl Physiol 103: 2042–2048, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98: 6–8, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol 263: H1078–H1083, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klabunde RE. Conditions for dipyridamole potentiation of skeletal muscle active hyperemia. Am J Physiol Heart Circ Physiol 250: H62–H67, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Koch LG, Strick DM, Britton SL, Metting PJ. Reflex versus autoregulatory control of hindlimb blood flow during treadmill exercise in dogs. Am J Physiol Heart Circ Physiol 260: H436–H444, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol 257: H1507–H1515, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol Heart Circ Physiol 261: H1659–H1664, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol 87: 2218–2224, 1999 [DOI] [PubMed] [Google Scholar]
- 23. MacLean DA, Sinoway LI, Leuenberger U. Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation 98: 1990–1992, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol 101: 1678–1684, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Morice AH, Schofield P, Keal EE, Sever PS. A comparison of the ventilatory, cardiovascular and metabolic effects of salbutamol, aminophylline and vasoactive intestinal peptide in normal subjects. Br J Clin Pharmacol 22: 149–153, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296: R1140–R1148, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Patterson GC, Shepherd JT. The effects of continuous infusions into the brachial artery of adenosine triphosphate, histamine and acetylcholine on the amount and rate of blood debt repayment following rhythmic exercise of the forearm muscles. Clin Sci (Lond) 13: 85–91, 1954 [PubMed] [Google Scholar]
- 29. Radegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 171: 177–185, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Ray CJ, Marshall JM. Elucidation in the rat of the role of adenosine and A2A-receptors in the hyperaemia of twitch and tetanic contractions. J Physiol 587: 1565–1578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558: 351–365, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986 [DOI] [PubMed] [Google Scholar]
- 34. Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 65: 1548–1552, 1988 [DOI] [PubMed] [Google Scholar]
- 35. Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol 67: 2095–2100, 1989 [DOI] [PubMed] [Google Scholar]
- 36. Stroud MW, 3rd, Lambertsen CJ, Ewing JH, Kough RH, Gould RA, Schmidt CF. The effects of aminophylline and meperidine alone and in combination on the respiratory response to carbon dioxide inhalation. J Pharmacol Exp Ther 114: 461–469, 1955 [PubMed] [Google Scholar]
- 37. Taddei S, Pedrinelli R, Salvetti A. Theophylline is an antagonist of adenosine in human forearm arterioles. Am J Hypertens 4: 256–259, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol 138: 1451–1458, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of β-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101: 1343–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]