Abstract
The contribution of sympathetic nerves arising from the superior cervical ganglia (SCG) toward the growth and function of cerebral blood vessels is pertinent throughout maturation as well as in response to cardiovascular stress imposed by high-altitude long-term hypoxia (LTH). The function of SCG sympathetic neurons is dependent on intracellular Ca2+ concentration ([Ca2+]i) signaling, which is strongly influenced by a process known as Ca2+-induced Ca2+ release (CICR) from the smooth endoplasmic reticulum (SER). In this study, we used the sheep SCG neuronal model to test the hypotheses that maturation decreases CICR and high-altitude LTH depresses CICR in fetal SCG neurons but not in those of the adult. We found that the contribution of CICR to electric field stimulation (EFS)-evoked [Ca2+]i transients was greatest in SCG cells from normoxic fetuses and was abolished by LTH. The decline in CICR was associated with a reduction in sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) function in fetal SCG cells during LTH, reducing SER Ca2+ levels below the threshold needed for the coupling of Ca2+ influx and CICR. With respect to the maturation from the fetus to adult, the decrease in CICR may reflect both a reduction in the levels of ryanodine receptor isoforms 2 and 3 and SERCA function. In response to LTH and in contrast to the fetus, CICR function in adult SCG cells is maintained and may reflect alterations in other mechanisms that modulate the CICR process. As CICR is instrumental in the function of sympathetic neurons within the cerebrovasculature, the loss of this signaling mechanism in the fetus may have consequences for the adaptation to LTH in terms of fetal susceptibility to vascular insults.
Keywords: function of superior cervical ganglia, sympathetic development, electric field stimulation-evoked calcium transients, intracellular calcium stores, intracellular calcium release
calcium signaling controls numerous processes, including contraction, secretion, and gene expression (30, 36, 38). In superior cervical ganglia (SCG) neurons, Ca2+ signals govern the release of norepinephrine (NE) (7, 42, 48). In turn, NE release plays a critical role in the regulation of blood pressure and cerebral blood flow distribution (9, 17, 24, 54). Thus, the study of intracellular Ca2+ concentration ([Ca2+]i) dynamics in peripheral and central neurons has become a useful tool in the investigation of many physiological functions including the neurovascular contribution to the function of cerebral blood vessels (7, 47).
In SCG neurons, [Ca2+]i signaling and neurotransmitter release begin with the opening of voltage-gated Ca2+ channels, whereby much of the influx intensity of the [Ca2+]i signal is dampened by Ca2+-buffering proteins (35, 52). However, signaling, as defined by increases in [Ca2+]i, is sustained and amplified by Ca2+-induced Ca2+ release (CICR) through the activation of ryanodine receptors (RyRs) (38, 49, 50, 51). RyRs are Ca2+-permeable ion channels that can exist as three isoforms (RyR1, RyR2, and RyR3) on the smooth endoplasmic reticulum (SER) (14). To sustain repetitive CICR with ongoing neuronal function, SER Ca2+ stores must be continually refilled. This function is fulfilled by sarco(endo)plasmic reticulum Ca2+-ATPases (SERCAs), which simultaneously buffer [Ca2+]i transients and refill SER Ca2+ stores so that CICR can continue (48, 52). Another component in the refilling of SER Ca2+ stores are plasma membrane storage-operated Ca2+ channels (SOCCs). These channels open in response to the depletion of SER Ca2+ stores and provide for extracellular Ca2+ influx (4, 52). As CICR is a prominent mode of [Ca2+]i signaling for processes such as the release of neurotransmitters and hormones and smooth muscle tone, it is under tight regulation by intracellular modulators acting on the RyRs (18, 45, 51). These modulators include calmodulin, phosphorylation, SER Ca2+ levels, neuronal nitric oxide (NO) synthase (nNOS), cADP-ribose (cADPr), and the protein modulators FK506-binding protein (FKBP)12 and FKBP12.6 (14, 26, 30, 50, 57).
Given that sympathetic nerves play an important role in cerebrovascular responses when exposed to stressors such as high-altitude long-term hypoxia (LTH), our research group studied the function of these neurons in fetal and adult sheep cerebral arteries in response to LTH (28). We have shown the function of cerebrovascular sympathetic nerves to be greater in near-term fetuses compared with adults (5, 39). During the adaptation to high-altitude LTH, the capacity of the nerves to release NE declines in fetal but not adult arteries (6). Overall, the mechanism(s) for this alteration in stimulation-evoked NE release is unknown. However, as CICR is critical to neurotransmitter release, LTH may well alter [Ca2+]i signaling (18, 36, 38, 45).
Using the sheep SCG neuronal model, we tested the hypotheses that 1) maturation decreases CICR and 2) high-altitude LTH depresses CICR in fetal SCG neurons but not in those of the adult. Furthermore, the observed alterations in CICR may possibly be accounted for by RyR abundance, cellular levels of CICR modulators nNOS and cADPr, and/or changes in SER Ca2+ filling levels. We carried out this study in isolated SCG cells and SCG tissue homogenates within four treatment groups: fetal normoxic, adult normoxic, fetal LTH, and adult LTH. To determine the contribution of CICR to electrical field stimulation (EFS)-evoked [Ca2+]i transients, we used fura-2 microfluorometry, the RyR agonist caffeine, and the RyR antagonist ryanodine. As refilling of SER Ca2+ stores is necessary to maintain CICR and is mediated in part by the function of SERCA, we used the SERCA blocker cyclopiazonic acid (CPA) to estimate the function of SERCA in SCG cells. CICR is, in part, determined by the levels of RyRs and their endogenous modulators nNOS and cADPr; thus, we also measured their cellular levels in whole SCG homogenates. Understanding the [Ca2+]i dynamics within these SCG neurons with respect to CICR provides insight as to their function and role in the regulation of the maturing cerebral vasculature and the impact of stressors such as LTH on the function of these neurons.
MATERIALS AND METHODS
All procedures in this study were approved by the Institutional Animal Care and Use Committee of Loma Linda University. Forty pregnant ewes and forty nonpregnant ewes of mixed breed were obtained from a single supplier (Nebeker Ranch, Lancaster, CA). These animals were randomly assigned to two groups: control normoxic (20 pregnant and 20 nonpregnant ewes) and LTH (20 pregnant and 20 nonpregnant ewes). Animals in the LTH group were transported to the White Mountain Research Station (Bishop, CA; altitude 3,280 m), where they were maintained for ∼100 days. Ewes in the normoxic group remained at Nebeker ranch (altitude: 718 m). Pregnant animals were studied at 138–142 days of gestation. At the appropriate time, LTH animals were transported to the Center for Perinatal Biology at Loma Linda University, where they underwent immediate study. To maintain arterial Po2 at ∼60 Torr in LTH animals, tracheal tubes were surgically implanted to allow for the administration of N2 gas (19). Arterial Po2 values were measured in 0.5-ml blood samples from near-term fetuses and adults (ABL300, Radiometer, Copenhagen, Denmark). The mean Po2 for normoxic and LTH adults was 101 ± 2 and 61 ± 3 Torr, respectively. The mean Po2 for normoxic and LTH fetuses was 22.4 ± 0.8 and 19.1 ± 0.6 Torr, respectively. These measurements indicate a significant decline in Po2 for both the fetus and adult with LTH treatment (P < 0.01). On the day of tissue harvest, ewes were euthanized by an intravenous injection of pentobarbital sodium (100 mg/kg), and the fetuses were delivered by cesarean section. Fetal weights were not significantly altered (P > 0.05) by LTH. The weights were 4,187 ± 183 and 4,148 ± 255 g for normoxic and LTH fetal sheep, respectively (n = 30 for fetal normoxic sheep and n = 25 for fetal LTH sheep).
SCG Preparation
After death, SCGs were dissected by making incisions in the neck along the trachea, tracing superior and posterior to expose the carotid artery, vagal nerve, and medial angle of the mandible. The vagal nerve was traced superior and posterior toward the medial angle of the mandible to the cervical sympathetic trunk to expose the ganglion body. After dissection, SCGs were placed in ice-cold Krebs solution (bubbled with a 95% O2-5% CO2 gas mixture, pH 7.4) containing (in mM) 118 NaCl, 4.8 KCl, 1.6 CaCl2, 1.2 KH2PO4, 25 NaHCO3, 1.2 MgSO4, 0.3 ascorbic acid, and 11.5 glucose. Tissues were then transported to the laboratory within 15 min of dissection to be immediately processed as described below or snap frozen in liquid nitrogen until molecular analysis.
For Ca2+ imaging, the ganglia were acutely dissociated in 5 ml of Earle's balanced salt solution (EBSS) containing trypsin (6,000 U/ml), collagenase D (1 mg/ml), DNAse-1 type IV (0.1 mg/ml), HEPES (20 mM), glucose (10 mM), and NaHCO3 (10 mM) and adjusted to pH 7.4 with NaOH (1 M). After an incubation at 4°C overnight, the digestion was continued at 34°C for 40 min and was subsequently stopped by the addition of 5 ml of modified HBSS with 10% FCS, 1.3 mM CaCl2, and 5 mM HEPES and adjusted to pH 7.4 with NaOH (1 M).
Acutely dissociated cells were centrifuged at 60 g for 5 min and resuspended in 5 ml of fresh HBSS. Cells were centrifuged again at 60 g for 5 min and redispersed in 5 ml of HBSS [containing 10% FCS and 5 mM HEPES and adjusted to pH 7.4 with NaOH (1 M)]. Cells were centrifuged again, and HBSS was decanted to 1 ml. Cells were dispersed in sterile Pasteur pipettes, and a 0.5-ml volume of the dispersed cells was placed onto Cell-Tak (3.5 mg/cm2, BD Biosciences, Bedford, MA)-coated glass coverslips. The coverslips were modified by attaching an oval 2-cm ring to the surface with Sylgard adhesive (Dow Corning). To facilitate cell attachment, the coverslips were placed in 35-mm culture dishes and centrifuged at 60 g for 5 min (Beckman S4180). Measurements of intracellular Ca2+ were completed within 6 h.
Measurement of Intracellular Ca2+
Our methods for measuring intracellular Ca2+ have been previously described. As we studied the cumulative effects of LTH, SCG cells were derived from acute dissociation, and the data reflect the acquisition of global cytosolic [Ca2+]i transients from the soma of the SCG neuron (1, 41, 52). Cells were loaded with 10 μM fura-2 AM for 20 min at room temperature and then washed with low-K+ Tyrode buffer containing (in mM) 138 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 10 HEPES, and 10 glucose and adjusted to pH 7.4 with NaOH (1 M). Incubation was continued for an additional 20 min to allow intracellular esterases to convert the fura-2 AM dye into the free acid form. We addressed the potential problem of an age-related difference in the amount of fura-2 uptake or a difference in the activity of the nonspecific esterases that convert fura-2 AM to the free salt, which was assessed by monitoring the 510-nm emission fluorescence signal when fura-2 was excited at 380 nm (F380) in resting SCG cells in each age and treatment group (37). We consistently observed equivalent dye loading in SCG cells from all animal treatment groups in the present study (near-term fetus: 75.50 ± 2.18, adult: 76.30 ± 4.46, fetal LTH: 76.36 ± 4.57, adult LTH: 76.71 ± 4.37) as well as in adult rats from previous studies (1, 41, 52). These data suggest that there are no significant differences in dye loading in SCG cells isolated from fetal and adult normoxic and LTH animals. Coverslips were mounted into a superfusion chamber attached to the stage of a Nikon inverted microscope (Nikon Instruments, Melville, NY). The microscope was attached to a Universal Imaging System running MetaFluor version 6.1 (Molecular Devices, Sunnyvale, CA). The perfusion system allowed the chamber volume (∼250 μl) to be exchanged at the rate of 2 times/s (500 μl/s). Fura-2 was illuminated by a xenon lamp, and the fluorescence was excited alternately at wavelengths of 340 and 380 nm by a Lambda DG-4 (Sutter Instruments, Novato, CA). A Photometric Cool Snap 12-bit digital camera (Roper Scientific) was used to measure the emission fluorescence at 510 nm. Adjusting the microscope stage where no cells were in the field of view corrected for background light levels. Before fura-2 AM was loaded, cellular autofluorescence was examined in SCG cells as previously described (52). Autofluorescence was below the limits of detection by our imaging system and did not significantly alter our [Ca2+]i measurements. During the experiment, background fluorescence was subtracted, the 340- and 380-nm fluorometric signals were collected, and the Ca2+ concentration was calculated and logged to a Dynamic Data Exchange Excel file every ∼300 ms. Ambient light levels were minimized, and SCG cells were illuminated only during data acquisition to minimize dye photobleaching.
Intracellular Ca2+ was estimated by both in vitro and in vivo calibration methods as we have previously reported (1, 41, 52). We used the experimental fluorescent intensity ratios (R) to calculate [Ca2+]i over the physiological range by iterative fit to the following equation: [Ca2+]i = Kd(R − Rmin)/(Rmax − R)Sf, where Rmin is the 340-to-380-nm ratio at zero [Ca2+] and Rmax is the 340-to-380-nm ratio at 40 μM [Ca2+]i. Kd is the dissociation constant of fura-2, and Sf is a correction factor relating the ratio of Fmin to Fmax, which is the emission intensity at 380 nm when fura-2 is in the free form (Fmin; 0 nM [Ca2+]) or bound form (Fmax; 40 μM [Ca2+]) (22). We routinely carried out calibrations and kept a running average of the Rmin, Rmax, Sf, and Kd values for fura-2 for over 10 yr. For this report, we used multiple calibrations and [Ca2+]i was estimated using averaged in vitro values for Sf (15.60), Rmin (0.35), Rmax (3.13), and Kd (266 nM).
EFS Apparatus
EFS was delivered to isolated cells through platinum electrodes as previously described (1). EFS-evoked [Ca2+]i transients were elicited by square wave pulses via a Grass S48 Stimulator (Grass Medical Instruments, Quincy, MA). A 10-Ω resistor was placed in series with the parallel platinum electrodes, and the delivered current was monitored with a Tektronix TDS 2024B oscilloscope (Tektronix, Wilsonville, OR). Subsequently, graded stimulation trains from a minimum of 3 pulses to a maximum of 27 pulses at 3 Hz were delivered at 300 mA in the presence and absence of either the RyR agonist caffeine (5 mM, Sigma, St. Louis, MO) or the RyR antagonist 9,21-dehydro-ryanodine (100 μM, Calbiochem, La Jolla, CA) to analyze the enhancement and blockade of Ca2+ release from SER stores, respectively. Given the differential sensitivity between the near-term fetus and adult (i.e., the number of pulses to reach one-half the maximum of the Δ[Ca2+]i response), fetal cells were typically stimulated up to 24 pulses and the adult up to 27 pulses. A 3-Hz frequency and graded trains were observed to be physiologically sufficient with respect to the application of repetitive stimulation and the prevention of the induction of toxic [Ca2+]i levels and cellular stress (1, 50, 51). In the case of SERCA blockade, two to three trains of stimulation (50 pulses, 5 Hz, 300 mA) were applied in the absence and presence of 10 μM CPA (Sigma).
EFS Protocols
EFS protocol 1: progressive pulse stimulation at maximal current in the absence and presence of 100 μm ryanodine.
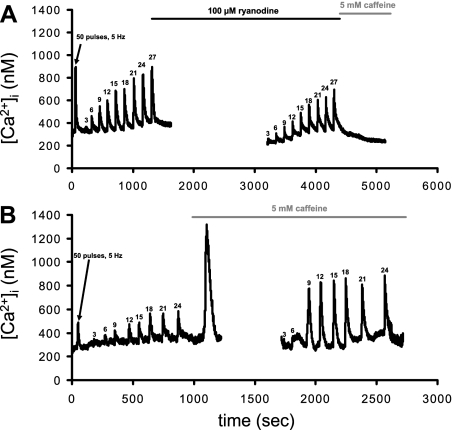
EFS protocol 1 is shown in Fig. 1A. At relatively low (nM) concentrations, ryanodine activates the channel, locking it into an open low conductance state, whereas high (μM to mM) concentrations block the channel (14). We initially performed dose-response experiments and found that a concentration of 100 μM resulted in maximal RyR blockade in both fetal and adult SCG cells. A priming supermaximal stimulus (50 pulses, 10 s, 5 Hz, 300 mA) was delivered to cells to load SER Ca2+ stores and to ensure uniform SER loading from cell to cell (33). Only cells that responded and recovered from this stimulus were considered for further stimulation. A 2-min equilibration period separated each stimulation train. During the last control supermaximal stimulation, Tyrode buffer containing 100 μM ryanodine was rapidly superfused onto the cells followed by a 30-min incubation period. This insured that RyRs were fully blocked as ryanodine has greater efficacy when the RyR is activated (36). EFS was repeated again after the drug was applied. Note in Fig. 1A that the addition of 5 mM caffeine after the last EFS [Ca2+]i transient in the presence of ryanodine did not evoke any further rises in [Ca2+]i. These data establish that this protocol blocks RyR activity.
Fig. 1.
Representative electrical field stimulation (EFS)-evoked intracellular Ca2+ concentration ([Ca2+]i) transients in fetal normoxic superior cervical ganglia (SCG) cells showing the application of EFS protocol 1 (A) and protocol 2 (B). A: protocol 1. A priming stimulus of square wave EFS (50 pulses at 5 Hz, 1-ms duration, 300 mA) was used to ensure filling of smooth endoplasmic reticulum Ca2+ stores. This was followed by a set of graded pulses (3–27 pulses at 3 Hz, 1-ms duration, 300 mA). Ryanodine was added during the last supermaximal transient with a subsequent equilibration period of 30 min. To ensure maximal blockade of ryanodine receptors (RyRs), caffeine was applied and failed to evoke a [Ca2+]i transient. B: protocol 2. Control stimulations were as described in A followed by the addition of caffeine, as indicated by the large transient that returned to baseline [Ca2+]i. EFS was then repeated 10 min after the formation of the caffeine-evoked [Ca2+]i transient. All electrical stimulations were followed by at least 2 min for recovery.
EFS protocol 2: progressive pulse stimulation at maximal current in the presence and absence of 5 mm caffeine.
EFS protocol 2 is shown in Fig. 1B. This protocol was developed on the basis that caffeine can enhance the contribution of CICR to EFS-evoked [Ca2+]i transients (50, 51). After the last train (∼24 pulses), Tyrode buffer containing 5 mM caffeine was rapidly superfused onto the cells. The application of caffeine caused a rapid increase of [Ca2+]i due to release from [Ca2+]i stores, which declined to baseline. These data are consistent with our previous study (1) and with data obtained from dorsal root ganglia cells (50). SCG cells were then allowed to incubate in the presence of caffeine for 10 min to sensitize RyRs and to allow SERCA to refill the SER Ca2+ stores. While still in the presence of 5 mM caffeine, cells were exposed to the series of pulses (3–24 pulses, 3 Hz) again.
EFS protocol 3: measurement of SERCA function using the SERCA antagonist CPA.
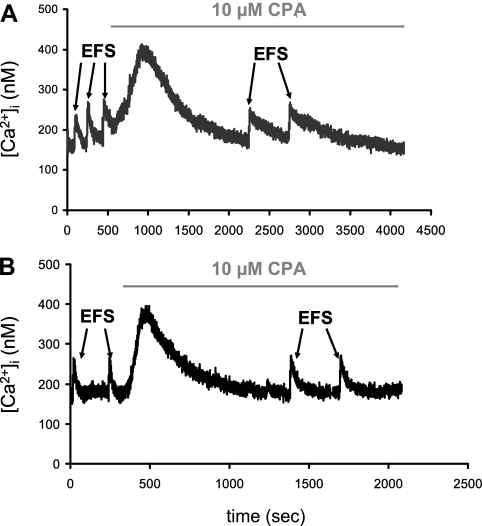
In EFS protocol 3 (see Fig. 6), two to three EFS trains (5 Hz, 50 pulses, 300 mA) were applied followed by the introduction of 10 μM CPA to the bathing solution. The initial increase in [Ca2+]i induced by the presence of CPA demonstrates that SERCA was blocked and that the SER was depleted of Ca2+. This is consistent with our previous data in rodent SCG cells (48). The [Ca2+]i transient that resulted from SERCA blockade was allowed to return back to baseline [Ca2+]i before EFS was repeated. As the recovery rate constant is in part determined by SERCA function, we analyzed the first-order recovery rate constant (τ) in the absence and presence of CPA using Origin 6.1 software. The difference in τ in the absence and presence of the SERCA antagonist provides a surrogate measure for SERCA function.
Fig. 6.
Representative EFS-evoked [Ca2+]i transients in an isolated normoxic fetal SCG cell (A) and LTH fetal SCG cell (B) in the absence and presence of the sarco(endo)plasmic reticulum Ca2+-ATPase antagonist cyclopiazonic acid (CPA). Two to three supramaximal EFS trains (5 Hz, 50 pulses, 300 mA; see materials and methods, protocol 3) were applied in the absence (control) and presence of 10 μM CPA. The initial [Ca2+]i rise induced by the presence of CPA was allowed to return back to baseline [Ca2+]i before EFS was repeated. The time constant for recovery (τ) was obtained for all [Ca2+]i transients as a first-order exponential decay fit from the peak [Ca2+]i to 95% of basal [Ca2+]i.
Validation of the EFS Protocol
In a previous study (1), we demonstrated that EFS-evoked increases in [Ca2+]i are lost if Ca2+ influx is blocked with 100 μM La3+. Similarly, in this study, we used the same control protocol whereby EFS-evoked increases in [Ca2+]i were lost when Ca2+ influx was blocked with 100 μM La3+ (data not shown). These data from the former and present studies suggest that Ca2+ influx is necessary to activate CICR and that the release of Ca2+ from the SER is not occurring by some other mechanism.
ELISA Assays for RyR1, RyR2, RyR3, and nNOS
We have developed a very selective and sensitive ELISA assay used in this study to quantify the relative levels of RyR1, RyR2, RyR3, and nNOS in the SCG (53). We validated the selectivity of our antibodies for the RyR subtypes and nNOS using Western blot analysis to ensure that under appropriate conditions the antibodies would yield single bands (32, 53). The ELISA has advantages over traditional Western blot analysis especially when attempting to quantify proteins with relatively high molecular weights. RyRs have a molecular weight of >500 kDa and, thus, do not transfer well to blotting membranes, and incomplete transfer will affect the quantification of RyR protein levels (21).
To isolate cell protein, SCGs were snap frozen in liquid nitrogen, pulverized to a fine powder using a metal mortar and pestle, and then placed in 200 μl of ice-cold lysis buffer containing (in mM) 107 NaCl, 50 Tris·HCl, 10 EDTA, and 0.5% Tween 20. One milliliter of a protease inhibitor cocktail (10 ml cocktail stock containing bestatin, E-64, leupeptin, and aprotinin, Sigma) was added to the lysis buffer to inhibit the degradation of proteins of interest. In all protocols, 10 μl of the sample were removed for protein analysis, and proteins were solubilized in 25 μl of 1 N NaOH. A bicinchoninic acid protein assay kit and BSA standards obtained from Pierce (Rockford, IL) were used to quantify total protein content in SCGs (46).
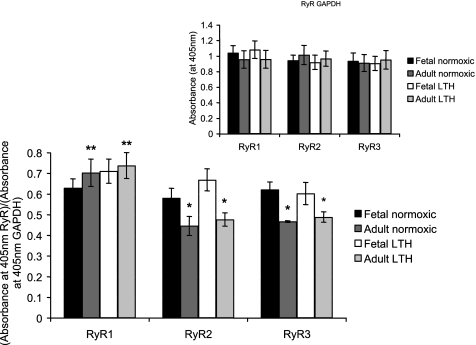
ELISA analysis of RyRs and nNOS was performed as previously developed and validated by our laboratory (53). Briefly, 500 ng (RyR assays) or 250 ng (nNOS assays) of protein were placed in high-affinity 96-well binding plates (Corning, Corning, NY) for 16–24 h at 4°C. Unbound proteins were washed away with PBS-Tween 20 [PBS-T; 138 NaCl, 2.7 KCl (pH 7.4), and 0.1% Tween 20, Sigma]. Proteins were blocked for 1 h at room temperature using 1% BSA in PBS (Sigma). Antigen-coated plates were then incubated for 16 h at 4°C with selective primary antibodies (1:500 dilution) for RyR1, RyR2, and RyR3 (Chemicon, Temecula, CA) or nNOS (BioMol, Plymouth Meeting, PA). Plates were washed three times with PBS-T and incubated for 1 h at 37°C with horseradish peroxidase-conjugated secondary antibodies (Zymed, San Francisco, CA, 1:2,000 dilution). The unbound antibodies were washed away three times with PBS-T, and plates were incubated in a mixture of hydrogen peroxide and 2,2′-azino-di-(ethylbenz-thiazoline)sulfonic acid (ABTS; Zymed) for 15–20 min. ABTS was oxidized in the reaction to yield a green chromophore, and the absorbance was measured at 405 nm via a microplate reader. To determine the relative levels of RyRs, the absorbance at 405 nm was normalized to GAPDH levels also determined by the ELISA assay (53) (see Fig. 5, inset).
Fig. 5.
Impact of maturation and LTH on cellular levels of RyR1, RyR2, and RyR3 isoforms in ovine SCG cells. RyR isoforms levels were quantified using ELISA and normalized to GAPDH (see materials and methods). Inset: GAPDH controls. Data are means ± SE; n = 8 ganglia from 8 normoxic adults and 8 LTH adults, 16 ganglia (2 ganglia pooled for each experiment) from 8 normoxic fetuses, and 16 ganglia (2 ganglia pooled for each experiment) from 8 LTH fetuses. Each experiment was performed in triplicate. *Significantly different from the normoxic or hypoxic fetus (P < 0.05 by ANOVA and Fisher's PLSD test); **significantly different from two other isoforms (P < 0.01 by ANOVA and Fisher's PLSD test).
The use of GAPDH as a normalizing protein has been a source of controversy in hypoxic studies. The gene promoter of GAPDH has been reported to have an inducible hypoxic responsive element using prostate adenocarcinoma cell line models with acute hypoxic exposure (48 h) (29). However, another report using human glioblastoma cells indicated no alteration in GAPDH expression with acute hypoxic exposure (48 h) and concluded that GAPDH was an ideal “housekeeping gene” for their study (43). Although our particular study used a chronic hypoxic approach, we found no significant alteration in the expression of GAPDH protein with development or LTH.
The abundance of nNOS was determined using a modification of our previously published method (32). A purified recombinant nNOS standard (2–15 ng, Zymed) was added to the high-affinity binding plates, and the ELISA assay was performed in the same manner as with the samples in all experiments. This method yields a linear relation (r = 0.99; data not shown) that allows absorbance to be converted to mass of nNOS in each sample.
Tissue Preparation for Total DNA Measurements
In this study, cADPr measurements were normalized to total DNA content as the quantity of soluble protein is variable with development and chronic hypoxia (27). The DNA assay has been previously described, and this technique has been packaged into DNeasy tissue kits from Qiagen (Valencia, CA) (56). Briefly, 10–15 mg of snap-frozen pulverized SCGs were placed in a 1.5-ml microcentrifuge tube, lysed, and then treated with RNAse. DNA was isolated using DNeasy minispin columns and premade buffers according to the manufacturer's instructions. The integrity of the DNA was determined by 260-to-280-nm ratio, and samples yielding a ratio of 1.8–2.0 indicate high-purity DNA.
Fluorimetric Assay for cADPr
Quantification of tissue levels of cADPr, a modulator of the CICR process, was done by a previously developed fluorometric assay (20). This cycling assay works on the principle that ribosyl cyclase will work in reverse in the presence of excess nicotinamide, which then converts all tissue cADPr to NAD. NAD is consumed in a cycling assay by alcohol dehydrogense, reducing NAD to NADH, which is subsequently used by diaphorase to reduce resazurin to resorufin (20). The final product, resorufin, fluoresces at 590 nm when activated at 544 nm. Briefly, snap-frozen SCGs (1 SCG/assay for the adult and 2 SCGs/assay for the fetus) were pulverized and placed in 500 μl of 0.6 M perchloric acid, homogenized, sonicated, and extracted with 500 μl of 3:1 chloroform and tri-n-octylamine. Samples were centrifuged for 10 min at 1,500 g, and the aqueous top layer containing cADPr was decanted. Samples were adjusted to pH 8.0 with 20 mM sodium phosphate buffer. Samples were then treated with an enzyme solution to remove contaminating nucleotides. This buffer solution contained 0.44 U/ml nucleotide pyrophosphatase, 12.5 U/ml alkaline phosphatase, 0.0625 U/ml NADase, 2.5 mM MgCl2, and 20 mM sodium phosphate (pH 8.0). Samples were incubated for 3 h at 37°C and then centrifuged at 3,000 g for 30 min in Centricon-3 tubes (Bedford, MA). Next, 100 μl of the samples were added to 96-well microliter plates, 50 μl of ribosyl cyclase solution (containing 0.3 μg/ml purified ribosyl cyclase and 30 mM nicotinamide) were then added, and samples were incubated for 15 min at room temperature to convert cADPr to NAD. After the conversion of cADPr to NAD, 100 μl of cycling reagent (containing 0.1 mg/ml BSA, 10 mM nicotinamide, 100 μg/ml alcohol dehydrogenase, 2% ethanol, 10 μg/ml diaphorase, 10 μM flavin mononucleotide, and 5 μg/ml resazurin) were added to each sample and incubated for 4 h at room temperature. Plates were placed on a Biotec FLX800 fluorometer and illuminated at 544 nm with emitted light recorded at 590 nm. A series of pure cADPr standards (0.2–100 nM) were prepared and treated with ribosyl cyclase and cycling reagent solutions in the same manner as the samples. The 590-nm intensity versus concentration of cADPr standards yields a linear relation, which was used to convert emission intensity at 590 nm in each sample to cADPr concentration (r = 0.99; data not shown). The lower limit of detection in this assay was ∼0.5 nM. All assays in this study were run in triplicate, and the SE was <3% of the mean for each triplicate.
Data Analysis
All Ca2+ transients were analyzed using customized algorithms in Origin 6.1. Peak [Ca2+]i (Δ[Ca2+]i) was determined by subtracting basal [Ca2+]i from maximum stimulation-evoked [Ca2+]i for all transients. Plots of pulse number versus percentage of maximal Δ[Ca2+]i were generated and fitted by a Boltzmann sigmoid function using Origin 6.1. τ was obtained for all [Ca2+]i transients as a first-order exponential decay fit from the peak [Ca2+]i to basal [Ca2+]i using Origin 6.1.
Statistics
The impact of development and hypoxia on all parameters was determined by ANOVA and Fisher's protected least-significant difference test for a comparative analysis between treatment groups. Within-group analysis for [Ca2+]i parameters before and after drug treatments in the single cell experiments were compared using a Student's paired t-test. Statistical analysis was done using StatView 5.0 software (Abacus Concepts, Berkeley, CA).
RESULTS
Impact of Maturation and LTH on the Contribution of CICR to EFS-Evoked [Ca2+]i Transients in Single SCG Neurons
To measure the contribution of CICR to EFS-evoked [Ca2+]i transients, we used the RyR antagonist ryanodine to block CICR and the RyR agonist caffeine to sensitize CICR to EFS-evoked increases in [Ca2+]i. Figure 1A shows a representative recording of EFS-evoked Ca2+ transients for a single SCG cell from a normoxic fetus in the absence and presence of ryanodine. Ryanodine clearly decreased the magnitude of EFS-evoked [Ca2+]i transients. Figure 1B shows a representative [Ca2+]i recording of a SCG cell from a normoxic fetus in the absence and presence of caffeine. When cells were exposed to 5 mM caffeine, there was a clear caffeine-evoked [Ca2+]i transient, which returned to baseline. This effect is consistent with previous studies (1, 50) in isolated sensory and sympathetic neurons. While in the continued presence of caffeine, EFS-evoked [Ca2+]i transients clearly increased compared with controls.
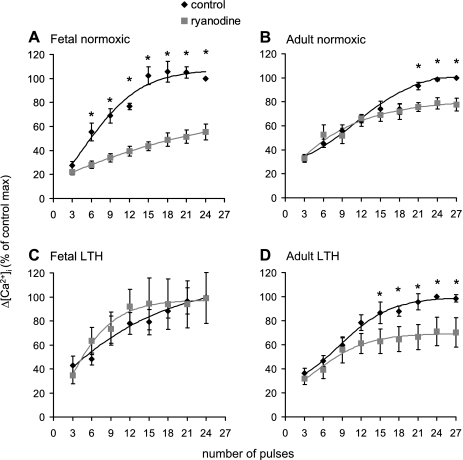
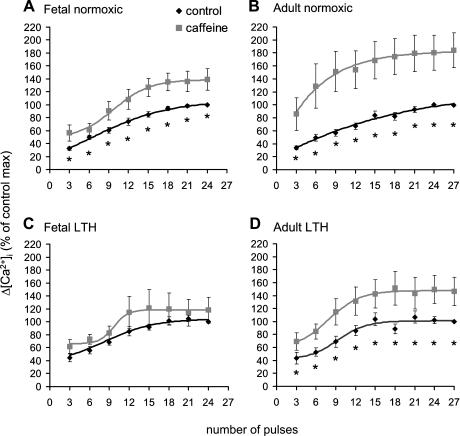
Figure 2 shows the impact of maturation and LTH on the contribution of CICR to EFS-evoked [Ca2+]i transients in isolated SCG cells. In SCG cells from normoxic fetuses (Fig. 2A), ryanodine significantly decreased EFS-evoked [Ca2+]i transients, from 6 to 24 pulses. In SCG cells from normoxic adults, ryanodine also decreased the efficacy of EFS to evoke [Ca2+]i transients, but, in contrast to the normoxic fetus, over a higher range of 21–27 pulses (Fig. 2B). Thus, development from the near-term fetus to the adult appears to decrease the CICR mechanism in ovine SCG neurons.
Fig. 2.
Impact of maturation and long-term hypoxia (LTH) on EFS-evoked [Ca2+]i transients in the absence and presence of the RyR antagonist ryanodine in isolated ovine SCG cells. Cells were activated by EFS as per protocol 1 in the absence and presence of 100 μM ryanodine (see Fig. 1A and materials and methods). A: normoxic fetal cells; B: normoxic adult cells; C: LTH fetal cells; D: LTH adult cells. Data are means ± SE; n = 12–19 cells from 6–8 animals/groups. *Significantly greater than ryanodine-treated cells (P < 0.05 by paired t-test).
Compared with SCG cells from the normoxic fetus, in cells from LTH fetuses, ryanodine failed to decrease EFS-evoked [Ca2+]i transients over the entire stimulation range (Fig. 2C). However, in SCG cells from LTH adults, ryanodine decreased EFS-evoked [Ca2+]i transients beginning at 15 pulses (Fig. 2D) compared with 21 pulses in the normoxic group (Fig. 2B). Overall, the impact of LTH treatment on the CICR was as follows: 1) a decrease in CICR in SCG neurons from the fetus and 2) maintained CICR in SCG neurons in adults compared with their respective normoxic counterparts.
Caffeine has been noted to sensitize CICR to EFS in sensory and sympathetic neurons (1, 50). Thus, we tested the hypothesis that the application of caffeine may reclaim some CICR in fetal LTH SCG cells. The application of caffeine significantly enhanced EFS-evoked [Ca2+]i transients at all stimulation trains in normoxic fetal and adult SCG cells and in SCG cells from LTH adults (Fig. 3, A, B, and D). In stark contrast, caffeine failed to enhance EFS-evoked [Ca2+]i transients in SCG cells from LTH near-term fetuses (Fig. 3C).
Fig. 3.
Impact of maturation and LTH on EFS-evoked [Ca2+]i transients in the absence and presence of the RyR agonist caffeine in isolated ovine SCG cells. Cells were activated by EFS as per protocol 2 in the absence and presence of 5 mM caffeine (see Fig. 1B and materials and methods). A: normoxic fetal cells; B: normoxic adult cells; C: LTH fetal cells; D: LTH adult cells. Data are means ± SE; n = 14–24 cells from 6–8 animals/groups. *Significantly less than caffeine-treated cells (P < 0.05 by paired t-test).
Effect of Maturation and LTH on Maximal EFS-Evoked [Ca2+]i Transients in the Absence and Presence of Ryanodine and on Caffeine-Evoked [Ca2+]i Transients
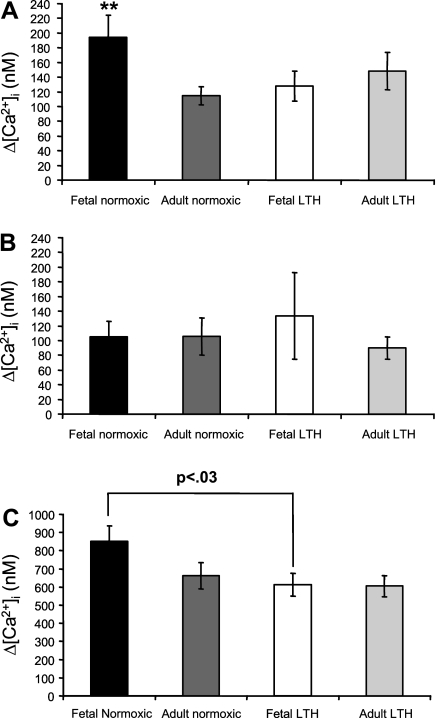
We compared the magnitude (Δ[Ca2+]i) of maximal EFS-evoked [Ca2+]i transients at 24 pulses between all four treatment groups (Fig. 4A). Maximal control EFS-evoked [Ca2+]i transients from all cells that preceded either caffeine or ryanodine treatment were greatest in SCG cells from normoxic near-term fetuses, and they significantly declined with postnatal maturation from the fetus to the adult. Furthermore, after acclimatization to LTH, maximal EFS-evoked [Ca2+]i transients significantly declined in fetal SCG cells but not in adult SCG cells.
Fig. 4.
Impact of maturation and LTH on maximal EFS-evoked [Ca2+]i transients in the absence and presence of ryanodine and on caffeine-evoked [Ca2+]i transients in isolated ovine SCG cells. The maximal response to EFS was defined as Δ[Ca2+]i at 24 pulses at 3 Hz and 300 mA. A: magnitude of EFS-evoked [Ca2+]i transients in SCG cells at the maximal response (24 pulses) in the absence of ryanodine. B: magnitude of EFS-evoked [Ca2+]i transients in SCG cells at the maximal response (24 pulses) in the presence of 100 μM ryanodine. C: measurements of 5 mM caffeine-evoked [Ca2+]i transients. Data are means ± SE; n = 26–43 cells from 12–16 animals/treatment group in A, 12–19 cells from 6–8 animals/treatment group in B, and 14–24 cells from 6–8 animals/treatment group in C. **Significantly different from normoxic adult cells and LTH fetal cells [P < 0.05 by ANOVA with Fisher's protected least-significant difference (PLSD) test].
Maximal EFS-evoked [Ca2+]i transients are due to the combined influence of extracellular Ca2+ influx and CICR (15). When the contribution of CICR to EFS-evoked [Ca2+]i is blocked with ryanodine, the remaining [Ca2+]i transient reflects Ca2+ influx, which we presume to be mainly due to the activation of voltage-gated Ca2+ channels. In the presence of ryanodine, maximal EFS-evoked [Ca2+]i transients were not significantly different in SCG cells from all four treatment groups (Fig. 4B).
Caffeine (5 mM) application induces a global [Ca2+]i transient due to uniform activation of all RyRs in the cell, and thus this transient reflects the capacity of the SER to release Ca2+ (14, 52). The [Ca2+]i transient evoked by the application of 5 mM caffeine in SCG cells from all four treatment groups is shown in Fig. 4C. Caffeine-evoked [Ca2+]i transients in SCG cells from near-term fetuses tended to be greater compared with SCG cells from normoxic adults. In addition, caffeine-evoked [Ca2+]i transients in SCG cells from LTH fetuses were similar in magnitude to those of adult SCG cells and significantly less than their normoxic counterparts. In SCG cells from adult animals, LTH did not alter the magnitude of caffeine-evoked [Ca2+]i transients.
Impact of Maturation and LTH on Cellular Levels of RyRs
RyRs are the channels that mediate CICR, a process that functionally alters with LTH and maturation. Therefore, we quantified relative RyR levels in SCG cells from each of the treatment groups (Fig. 5). RyR levels were normalized to GAPDH levels in each ELISA assay. GAPDH protein levels in SCG from normoxic and LTH fetuses and adults were virtually identical, which validates the normalization of relative RyR isoform levels to this marker (Fig. 5, inset). RyR1 was the dominant isoform in normoxic and LTH near-term fetal and adult SCG cells, and LTH did not alter the levels of RyR1 in either group. RyR2 and RyR3 levels significantly declined with maturation from the near-term fetus to adult, and LTH did not alter the levels of these receptors in either group.
Impact of Maturation and LTH on Cellular Levels of nNOS and cADPr
Our studies and those of others have shown that the SCG as well as the peripheral and cerebral vasculature contains adrenergic and nNOS-containing nerves. In addition, nNOS neurons augment the function of adrenergic neurons, and these effects may be mediated through the modulation of cADPr levels (10, 12, 31, 32, 53). Table 1 shows the impact of development and LTH on nNOS and cADPr levels normalized to cellular DNA levels. The abundance of nNOS in normoxic and LTH adult SCGs was significantly greater than in the fetal normoxic and LTH SCGs. Acclimatization to high-altitude LTH did not alter nNOS abundance in adult SCGs. However, LTH significantly increased the abundance of nNOS in fetal SCGs.
Table 1.
Levels of nNOS protein and cADPr with maturation and LTH in ovine SGC cells
| Group | nNOS, ng/μg protein | cADPr, pmol/mg DNA | DNA content, mg DNA/mg tissue |
|---|---|---|---|
| Normoxia | |||
| Fetal cells | 2.56±0.18 | 254.79±29.19 | 2.45±0.17 |
| Adult cells | 4.65±0.30* | 267.66±52.18 | 2.21±0.13 |
| LTH | |||
| Fetal cells | 3.30±0.25† | 254.79±50.70 | 2.48±0.16 |
| Adult cells | 4.51±0.32* | 298.33±76.39 | 2.25±0.17 |
Values are means ± SE; n = 10 ganglia from 10 normoxic adults, 10 ganglia from 10 long-term hypoxic (LTH) adults, 20 ganglia (2 ganglia pooled for each experiment) from 10 normoxic fetuses, and 20 ganglia (2 ganglia pooled for each experiment) from 10 LTH fetuses. Each experiment was performed in triplicate. Neuronal nitric oxide synthase (nNOS) was quantified by ELISA assay using a recombinant nNOS standard (see materials and methods). cADP-ribose (cADPr) was extracted from superior cervical ganglia (SCG) homogenates and quantified using a fluorimetric cycling assay and standard curve (r = 0.99; data not shown; see materials and methods). cADPr values were normalized to total genomic DNA content.
Significantly different from fetal cells [P < 0.005 by ANOVA and Fisher's protected least-significant difference (PLSD) test];
significantly different from normoxic fetal cells (P < 0.001 by ANOVA and Fisher's PLSD test).
nNOS modulates the activity of ribosyl cyclase, which synthesizes cADPr, which, in turn, modulates the sensitivity of RyRs to elevations in [Ca2+]i (12, 26). Therefore, we quantified the impact of development and LTH on tissue levels of cADPr. The tissue levels of cADPr normalized to total DNA content were not significantly different in fetal or adult normoxic or LTH SCGs (Table 1). Furthermore, DNA content per mass of tissue was not significantly different in SCGs from any of the study groups (Table 1).
Impact of Maturation and LTH on SERCA Function in SCG Cells
To maintain repetitive CICR during normal neuronal function, SER Ca2+ stores must be refilled. The refilling of SER Ca2+ stores is in part maintained by the function of SERCA. SERCA function can be assessed by the application of SERCA antagonists, which block the uptake of Ca2+ into the SER (38, 48). Two key features of SERCA antagonists allow for this type of surrogate measure: 1) there is a small transient reflecting a progressive release of Ca2+ from the SER and 2) there is a prolonged rate of recovery of EFS-evoked [Ca2+]i transients, as reflected by a broadening of the EFS-evoked [Ca2+]i transient and an increase in τ. Thus, by measuring τ, we evaluated the role of SERCA during EFS-evoked (5 Hz, 50 pulses, 300 mA) [Ca2+]i transients before and after the application of a SERCA blocker [CPA (10 μM)] in SCG cells from each treatment group (Fig. 6). Figure 6, A and B, shows representative EFS-evoked [Ca2+]i transients in SCG cells from a fetal normoxic and fetal LTH animal, respectively. In the fetal normoxic SCG cell, CPA application led to a distinct broadening of the EFS-evoked [Ca2+]i transient (Fig. 6A), an effect that was compromised in the SCG cell from a LTH fetus (Fig. 6B).
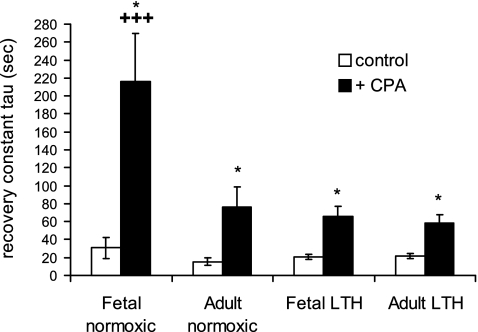
Figure 7 shows data obtained from the protocol shown in Fig. 6. In the presence of CPA, there was a significant increase in τ in all four treatment groups (Fig. 7). However, this increase was greatest in SCG cells from normoxic near-term fetuses. The increase in τ declined with maturation from the normoxic near-term fetus to normoxic adult. Furthermore, during LTH, τ declined in fetal SCG cells only.
Fig. 7.
Impact of maturation and LTH on the recovery of maximal EFS-evoked [Ca2+]i transients in isolated ovine SCG cells. Supramaximal trains were applied in the absence and presence of 10 μM CPA (see Fig. 6 and materials and methods). τ was obtained for all [Ca2+]i transients as a first-order exponential decay fit from the peak [Ca2+]i to 95% basal [Ca2+]i. Data are means ± SE; n = 10–14 cells from 6–8 animals. *Significantly different from control (P < 0.01 by paired t-test); +++significantly different from three other treatment groups (P < 0.05 by ANOVA and Fisher's PLSD test).
DISCUSSION
The Ovine Model and High-Altitude LTH Treatment
The ovine model has been recognized as an ideal model in translational research from animal to human studies with respect to many topics including reproductive physiology, development, and chronic hypoxia (44). The temperament and size of the ovine model allows for chronic instrumentation and monitoring of the vasculature within the fetus, mother, or nonpregnant adult to obtain data regarding blood gases, pH, and hemoglobin levels. With respect to development alone, the gestational period of the ovine fetus (138–142 days) is more similar to that of the human than most other animal models. The sequence of development of the ovine fetus may better reflect that of a human than the commonly used rodent or mouse models.
Our group has also observed that the ovine model is ideal for investigating the acclimatization of animals to LTH in combination with the process of development. Adaptation to LTH at higher altitudes is, in part, dependent on proper functioning of the sympathetic nervous system (23). The model of maternal and fetal hypoxia in this study (sheep maintained at 3,820 m) is thought to be one of moderate and well-adapted hypoxia. There is a threshold level of oxygen and duration of exposure where, if exceeded, detrimental effects can occur (27). In this study, adult and fetal arterial Po2 values declined significantly, whereas arterial pH remained unchanged and hemoglobin rose to increase oxygen-carrying capacity (25). The large decrease in the adult Po2 (∼40%) compared with the decrease in fetal Po2 (∼15%) in response to LTH is a reflection of the differential capacity of oxygen-hemoglobin binding kinetics and saturation between each age group (2, 25). Near-term fetal LTH weights were comparable with control fetuses maintained at 718 m, and fetal mortality and morbidity did not increase during LTH exposure. Thus, this ovine model is ideal for observing adaptive responses to high-altitude LTH.
SCG Innervation Targets
While the SCG mostly innervates the cerebrovasculature, it also provides sympathetic neural input into other organs such as the eye and heart to modulate pupillary diameter and heart rate, respectively (3, 8). The SCG provides sympathetic innervation to cerebral blood vessels, where these nerves are recognized as playing a vital role in modulating cerebral blood flow and protection of blood vessels from environmental stress such as high-altitude LTH (24).
Contribution of CICR to EFS-Evoked [Ca2+]i Transients During Maturation and LTH
Changes in [Ca2+]i levels in sympathetic nerves are a necessary signaling mechanism for proper neuronal function, and CICR contributes to stimulation-evoked increases in [Ca2+]i (16, 18, 45). Therefore, we assessed, for the first time, the contribution of CICR to EFS-evoked [Ca2+]i transients in the ovine SCG cell model. We also assessed the combined impact of maturation and LTH on the function of CICR. The most significant findings in this study are that CICR contributes to EFS-evoked [Ca2+]i transients in fetal and adult SCG cells and that CICR is greater in normoxic fetuses compared with normoxic adults (Fig. 2, A and B). Furthermore, CICR appears to be completely abolished in SCG cells from fetuses during LTH (Fig. 2C). These data suggest that CICR is a greater component in Ca2+ signaling in normoxic sympathetic neurons and may ultimately aid in the regulation of the cardiovascular system before birth. Furthermore, adaptation to LTH results in the loss of CICR as a signaling mechanism in fetal SCG cells, which may possibly reduce the protective function of these neurons during LTH stress.
Transmitter release is determined by the magnitude and duration of [Ca2+]i transients as well as the sensitivity of the release mechanism to changes in [Ca2+]i (38). CICR mediated by RyRs, in part, determines the size and duration of neuronal [Ca2+]i transients (49, 51) and is important to the magnitude of stimulation-evoked neurotransmitter release (18, 36, 45). The robust CICR in normoxic fetal compared with adult SCG cells correlates well with our previous findings demonstrating that stimulation-evoked NE release from sympathetic nerve endings in the fetal middle cerebral artery (MCA) is twofold greater than in the adult (5, 39). These studies are consistent with others that showed sympathetic nerve activity rises before birth in both sheep and rat models (11, 55). One possible mechanism that is supported by our evidence is that the rapid rise in sympathetic nerve activity before birth may be due to a greater contribution of CICR to transmitter release.
The capacity of stimulation-evoked NE release declines with LTH in fetal but not adult sheep MCAs (6). However, the mechanism(s) underlying this difference is unknown. The loss of the contribution of CICR to EFS-evoked [Ca2+]i in fetal sympathetic neurons due to LTH (Fig. 2C) may, in part, account for the decline in NE release in our previous study (6). As this study focused on CICR in the SCG, which is the cell body, the implications for function at the level of NE release in the nerve ending are correlative. However, Smith and Cunnane (45) showed that ryanodine applied presynaptically decreased the excitatory postsynaptic response using an intact sympathetic neuronal model. These data suggest that altered CICR, presynaptically, alters a postsynaptic response. Thus, the data presented in this study showing that CICR is abolished in fetal SCG neurons obtained from LTH animals may indeed have functional consequences of sympathetic neurons innervating the cerebrovasculature that originate in the SCG.
In contrast to the fetus, in adult SCG cells, LTH appears to sensitize CICR to EFS, and CICR is maintained (Fig. 2D). Thus, it is possible that the stable contribution of CICR to nerve function in the adult may possibly provide for maintained function of these sympathetic nerves during LTH stress. Indeed, in our previous study (6), the capacity of stimulation-evoked NE release in the adult MCA during LTH is maintained. Our previous and current studies are entirely congruent with one another and show that fetal and adult sympathetic nerves adapt differently to LTH stress.
Caffeine Sensitization of EFS-Evoked [Ca2+]i Transients During Maturation and LTH
Caffeine, a RyR agonist, is a well-known CICR-sensitizing agent potentiating EFS-evoked [Ca2+]i transients in sensory and sympathetic neurons (1, 50, 51). In a recent study (1), we showed that the contribution of CICR to EFS-evoked [Ca2+]i transients significantly declines in SCG cells from senescent rats and that caffeine can reclaim a portion of the EFS-induced CICR response to that of the middle-aged adult. As LTH abolishes CICR in fetal sheep SCG cells, much like aging, leading to reduced CICR in rat SCG neurons, we measured EFS-evoked [Ca2+]i in the presence of caffeine to see if CICR could be reclaimed. Caffeine clearly enhanced EFS-evoked [Ca2+]i transients in SCG cells from normoxic fetuses (Figs. 1B and 3A) and in neurons from normoxic and LTH adults (Fig. 3, B and D). However, caffeine failed to enhance EFS-evoked [Ca2+]i transients in SCG cells from LTH fetuses (Fig. 3C). These data suggest that there may be a fundamental alteration in the regulation of RyRs in fetal neurons during LTH, which completely decouples CICR from membrane depolarization, something that does not occur in adult SCG neurons. Furthermore, as RyR abundance does not decline with LTH in fetal sympathetic neurons, this suggests that there is a fundamental alteration in the interaction between RyRs and the known agonist caffeine.
Magnitude of EFS and Caffeine-Evoked [Ca2+]i Transients and Coupling of Ca2+ Influx and CICR During Maturation and LTH
The magnitude and duration of stimulation-evoked [Ca2+]i transients are dependent on the coupling of Ca2+ influx to CICR (14, 49, 52). The decline of EFS-evoked [Ca2+]i transients that occurs in SCG cells from the normoxic fetus to adult and in the fetus exposed to LTH (Fig. 4A) appears to be mostly due to loss of CICR, as opposed to a decline in Ca2+ influx through voltage-gated Ca2+ channels. This conclusion is supported by the comparison of EFS-evoked [Ca2+]i transients in the presence of ryanodine (Fig. 4B). When ryanodine is present, CICR is blocked, and the remaining EFS-evoked [Ca2+]i transient reflects Ca2+ influx; we found that there were no significant differences between the groups examined. These data suggest that Ca2+ influx in SCG neurons is maintained during LTH in both the fetus and adult.
The RyR agonist caffeine has been used to estimate the capacity of SCG neurons to release Ca2+ from SER stores (52). The application of 5 mM caffeine to SCG cells evoked robust Ca2+ responses in the neurons we studied. However, LTH depressed caffeine-evoked [Ca2+]i transients in fetal SCG neurons (Fig. 4C). Interestingly, while the contribution of CICR to EFS-evoked [Ca2+]i transients is lost in SCG cells from fetuses exposed to LTH, the response to caffeine significantly declined but was not eliminated, suggesting that SER stores still contain releasable Ca2+. These data reinforce the hypothesis that the coupling between Ca2+ influx and CICR is lost in fetal SCG cells during LTH, possibly as a result of the decline in the sensitivity of RyRs to changes in [Ca2+]i. In contrast, CICR in adult SCG cells appears to be sustained in response to EFS, suggesting that the coupling between Ca2+ influx and CICR is maintained during LTH in the adult.
Impact of Maturation and LTH on RyR Levels
The RyRs are the prime mediators of CICR; thus, we measured RyR levels in SCG cells in all study groups (Fig. 5). In this study, RyR1 was the dominant subtype of RyR in SCG cells from fetal and adult normoxic and LTH sheep. There is a significant postmaturational decrease in the levels of RyR2 and RyR3; however, LTH does not influence RyR expression in fetal or adult SCG neurons. These data are consistent with a long-term hypoxic study (56 days) using cardiomyocytes from rats (40), which showed that RyRs did not decline over the duration of hypoxia. As RyR1, RyR2, and RyR3 are all expressed in sheep SCG cells, it is reasonable to conclude that they all contribute to CICR in the ovine SCG. However, these data do not support the hypothesis that the loss of CICR in fetal sympathetic neurons during LTH is due to a change in the levels of RyRs. Thus, the alteration in CICR appears to be due to fundamental changes in the response of RyR channels to EFS-evoked increases in [Ca2+]i.
The impact of postnatal maturation on the levels of the three RyR subtypes we studied is comparable with other animal models with some notable differences. In the mouse brain, the genetic expression of RyR1 mRNA predominates during the embryonic stage with a progressive increase in RyR2 mRNA after postnatal day 7 (P7) and RyR3 mRNA after P7 (34). In addition, immunodetection of RyR2 in the mouse cerebral cortex occurs by embryonic day 12 and continues to increase during development, and caffeine elicits [Ca2+]i release in developing neurons (13). In near-term fetal sheep SCG, all three RyR subtypes are robustly expressed, whereas the levels of RyR2 and RyR3 decrease sometime after birth. These data contrast that of the mouse model. Overall, the data suggest that RyRs are expressed in central nervous system and SCG neurons over the course of development and are important to CICR in maturing neurons. Furthermore, as CICR contributes to EFS-evoked [Ca2+]i in fetal neurons at much lower numbers of pulses, this suggests that the RyRs in these neurons are more sensitive to changes in [Ca2+]i levels, where fetal neurons have greater coupling between Ca2+ influx and CICR.
Cellular nNOS and cADPr Levels During Maturation and LTH
NO in mammalian biology appears to be ubiquitous and regulates a number of cellular processes, including blood vessel contractility and neuronal excitability (7, 10, 31). We have quantified nNOS levels within the sympathetic nerve endings in the sheep MCA and rat SCG, and the function of nNOS appears to augment stimulation-evoked NE release from these nerve endings (31, 32). One proposed mechanism that accounts for the augmentation of NE release via nNOS nerves is the enhancement of Ca2+ influx and/or Ca2+ release from internal stores. In this case, nNOS increases the synthesis cADPr via increased activity of ribosyl cyclase, which in turn facilitates CICR (10, 12). Given the importance of nNOS as a modulator of NE release and cADPr levels, we quantified the abundance of nNOS and the ribosyl cyclase end product cADPr in fetal and adult sheep SCG (Table 1). nNOS abundance in the SCG more than doubles with development from the fetus to adult; however, LTH only increased nNOS abundance in the fetal SCG. Despite these changes in nNOS expression, cADPr levels were well maintained across the groups we studied. Thus, as cADPr sensitizes the coupling between Ca2+ influx and CICR in different cellular models (12, 26, 57), the decline in CICR in SCG cells from the fetus to adult, the abolition of CICR in the LTH fetus, and the sensitization of CICR in the LTH adult cannot be explained via this pathway. Furthermore, despite the differences in nNOS levels found between the groups, overall the data suggest that nNOS function in terms of cADPr synthesis is not altered with postnatal maturation or LTH in the ovine SCG. The regulation of RyRs also occurs by proteins such as calmodulin, FKBPs, and phosphorylation (14, 30). Thus, the measurement of the level of these modulators and their function is a logical avenue for future studies.
Our results for nNOS abundance in SCG cells from each treatment group are in direct contrast to those observed in our previous study in nNOS-containing neurons within the MCA (32). In that study, nNOS abundance was nearly identical in fetal and adult MCAs, and, during LTH, nNOS levels declined in both age groups. In addition, nNOS levels in the fetal SCG are ∼30-fold greater compared with nNOS levels in the fetal MCA, and, in the adult SCG, nNOS levels are ∼100-fold greater compared with nNOS levels in the adult MCA. These data demonstrate that there is great heterogeneity in nNOS levels between the point of origination of nNOS-containing neurons and termination of the neurons in the blood vessels themselves.
Impact of Maturation and LTH on SERCA Function in the SCG
The molecular data derived in this study do not provide a clear mechanism that can account for the profound changes in CICR that occur with LTH. Another modulator of the coupling between Ca2+ influx and CICR is the luminal SER Ca2+ level. Indeed, the magnitude of stimulation-evoked [Ca2+]i can be altered by the manipulation of SER Ca2+ levels, demonstrating that the contribution of CICR to these transients is in part dependent on the Ca2+ levels within the SER (15, 16, 51, 52). Furthermore, the refilling of SER Ca2+ stores is dependent on SERCA function, which simultaneously buffers [Ca2+]i transients and refills SER Ca2+ stores (48, 52). Thus, in this study, we blocked SERCA function with CPA and measured the rate of recovery of EFS-evoked [Ca2+]i transients as an index of SERCA function (Figs. 6 and 7). In fetal SCG cells, SERCA function declined profoundly with LTH. Furthermore, compared with fetal SCG cells, SERCA function was clearly less evident in neurons from adult sheep. These data suggest that the SER filling levels are greatest in SCG cells from near-term normoxic fetuses, consistent with the indication of a robust CICR. The LTH-induced depression in SERCA function in fetal SCG neurons may therefore account for a portion of the loss of CICR that occurs. However, LTH fetal SCG cells can still release some Ca2+ (Fig. 4C). Thus, the loss of CICR appears to be a combination of lowered luminal SER Ca2+ levels and a decoupling between extracellular Ca2+ influx and intracellular Ca2+ release.
The maturational decrease in SERCA function in SCG neurons from normoxic animals may explain why CICR contributes less to EFS-evoked [Ca2+]i transients in adult SCG cells. However, it is important to note that RyR2 and RyR3 levels also decline during postnatal maturation from the fetus to adult (Fig. 5). Thus, the lowered CICR in the adult may be a combination of lowered SER Ca2+ and RyR levels. In contrast to the fetus, SERCA function does not significantly change during LTH in adult SCG cells, and CICR appears to be sensitized to EFS as CICR occurs at lower trains of EFS. However, the modulation of CICR is dependent on other modulators of RyR function, such as FKBP proteins (30), and changes in the levels of these modulators may possibly account for the maintenance of CICR in adult SCG cells during LTH.
While SERCA function is important to the refilling of SER Ca2+ stores, Ca2+ entry through SOCCs also plays an indirect role in maintaining SER Ca2+ levels. The role of SOCCs in the maintenance of SER Ca2+ stores (4) is an immediate topic in progress in our laboratory. It is possible that differential SOCC activity between the groups in this study may offer an additional explanation of varying SER filling levels and, hence, CICR in these experiments.
Summary and Conclusions
To our knowledge, this is the first investigation to use the ovine SCG model to study the impact of maturation and LTH on Ca2+ signaling, specifically the contribution of CICR to EFS-evoked [Ca2+]i transients. In this study, we found that the contribution of CICR to EFS-evoked [Ca2+]i transients is greatest in SCG cells from normoxic fetuses and is abolished during LTH. These data cannot be accounted for by alterations in RyR or cADPr levels, which are critical to CICR. However, a decline in SERCA function in fetal SCG cells during LTH may reduce SER Ca2+ levels to a threshold that reduces the coupling between Ca2+ influx and CICR. During development from the fetus to adult, the decrease in CICR may reflect both a reduction in RyR2 and RyR3 levels and SERCA function. In contrast to the fetus, CICR function in adult SCG cells is maintained during LTH and may reflect alterations in other mechanisms that modulate the CICR process.
As CICR is necessary for the function of sympathetic neurons in the cerebrovasculature, the loss of this signaling mechanism in the fetus may have consequences for the adaptation to LTH and may leave the fetus more susceptible to vascular insults during LTH stress.
GRANTS
L. Leite was supported by Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior Research Fellowship 23080.008182/2005-32. This work was supported in part by National Institutes of Health Grants R01-HL-69078, P01-HD-31226, and R01-HL-54210.
ACKNOWLEDGMENTS
The authors thank Charles Hewitt for the technical expertise with the assays used in these experiments. The authors gratefully acknowledge Dr. Richard Graeff (University of Minnesota) for the assistance with the development of the assay for measuring cADPr in this study. In addition, the authors acknowledge the generous gift of purified ribosyl cyclase from the laboratory of Dr. Hon Cheung Lee (University of Minnesota).
REFERENCES
- 1. Behringer EJ, Vanterpool CK, Pearce WJ, Wilson SM, Buchholz JN. Advancing age alters the contribution of calcium release from smooth endoplasmic reticulum stores in superior cervical ganglion. J Gerontol Biol Sci Med Sci 64A: 34–44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG. Increased nitrite reductase activity of fetal versus adult ovine hemoglobin. Am J Physiol Heart Circ Physiol 296: H237–H246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bowers CW, Zigmond RE. Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J Comp Neurol 185: 381–391, 1979 [DOI] [PubMed] [Google Scholar]
- 4. Brueggemann LI, Markun DR, Henderson KK, Cribs LL, Byron KL. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther 317: 488–499, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Buchholz J, Teunissen KE, Duckles SP. Impact of development and chronic hypoxia on NE release from adrenergic nerves in sheep arteries. Am J Physiol Regul Integr Comp Physiol 276: R799–R808, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Buchholz J, Duckles SP. Chronic hypoxia alter prejunctional α2-adrenoceptor function in vascular adrenergic nerves of adult and fetal sheep. Am J Physiol Regul Integr Comp Physiol 281: R926–R934, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Buchholz JN, Behringer EJ, Pottorf WJ, Pearce WJ, Vanterpool CK. Age-dependent changes in Ca2+ homeostasis in peripheral neurones: Implications for changes in function. Aging Cell 6: 285–296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cardinali DP, Vacas MI, Gejman PV, Pisarev MA, Barontini M, Boado RJ, Juvenal GJ. The sympathetic superior ganglia as “little neuroendocrine brains”. Acta Physiol Latinoam 33: 205–221, 1983 [PubMed] [Google Scholar]
- 9. Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve acitivity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 294: R1255–R1261, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Chen C, Schofield GG. Nitric oxide donors enhanced Ca2+ currents and blocked noradrenaline-induced Ca2+ current inhibition in rat sympathetic neurons. J Physiol 482: 521–531, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi S, Weisenberg SN, Kellogg CK. Control of endogenous norepinephrine release in the hypothalamus of male rats changes over adolescent development. Brain Res Dev Brain Res 98: 134–141, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Clementi E, Riccio M, Sciorati C, Nistico G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. J Biol Chem 271: 17739–17745, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Faure AV, Grunwald D, Moutin MJ, Hilly M, Mauger JP, Marty I, De WM, Villaz M, Albrieux M. Developmental expression of the calcium release channels during early neurogenesis of the mouse cerebral cortex. Eur J Neurosci 14: 1613–1622, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Friel DD, Chiel HJ. Calcium dynamics: analyzing the Ca2+ regulatory network in intact cells. Trends Neurosci 31: 8–19, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Friel DD, Tsien RW. A caffeine and ryanodine-sensitive Ca2+ store in bull frog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol 450: 217–246, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furuichi S, Endo S, Haji A, Takeda R, Nisijima M, Takaku A. Related changes in sympathetic nerve activity, cerebral blood flow and intracranial pressure, and effect of an α-blocker in experimental subarachnoid hemmorhage. Acta Neurochir (Wien) 141: 415–424, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell purkinje cell synapse. J Neurosci 23: 11229–11234, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gleed RD, Poore ER, Figueroa JP, Nathanielsz PW. Modification of maternal and fetal oxygenation with the use of tracheal gas infusion. Am J Obstet Gynecol 155: 429–435, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Graeff R, Lee HC. A novel cycling assay for cellular cADP-ribose with nanomolar sensitivity. Biochem J 361: 379–284, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grunwald R, Meissner G. Lumenal sites and C terminus accessibility of the skeletal muscle calcium release channel (ryanodine receptor). J Biol Chem 270: 11338–11247, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 23. Hainsworth R, Drinkhill MJ, Rivera-Chira M. The autonomic nervous system at high altitude. Clin Auton Res 17: 13–19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Kamitomo M, Longo LD, Gilbert RD. Right and left ventricular function in fetal sheep exposed to long-term high-altitude hypoxemia. Am J Physiol Heart Circ Physiol 262: H399–H405, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev 37: 1133–1164, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 264: R65–R72, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Longo LD, Pearce WJ. Fetal cerebrovascular acclimitization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol 288: R16–R24, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Lu S, Gu X, Hoestje S, Epner DE. Identification of an additional hypoxia responsive element in the glyceraldehyde-3-phosphate dehydrogenase gene promoter. Biochim Biophys Acta 1574: 152–156, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Marks AR. Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. J Mol Cell Cardiol 33: 615–624, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Mbaku EN, Zhang L, Duckles SP, Buchholz JN. Nitric oxide synthase-containing nerves facilitate adrenergic transmitter release in sheep middle cerebral arteries. J Pharmacol Exp Ther 293: 397–402, 2000 [PubMed] [Google Scholar]
- 32. Mbaku EN, Zhang L, Pearce WJ, Duckles SP, Buchholz J. Chronic hypoxia alters the function of NOS nerves in cerebral arteries of near-term fetal and adult sheep. J Appl Physiol 94: 724–732, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Messutat S, Heine M, Wicher D. Calcium-induced calcium release in neurosecretory insect neurons: fast and slow responses. Cell Calcium 30: 199–211, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Mori F, Fukaya M, Abe H, Wakabayashi K, Watanabe M. Developmental changes in expression of the three ryanodine receptor mRNAs in the mouse brain. Neurosci Lett 285: 57–60, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Murchison D, Griffith WH. Increased calcium buffering in basal forebrain neurons during aging. J Neurophysiol 80: 350–364, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Narita K, Akita T, Hachisuka J, Huang SM, Ochi K, Kuba K. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals. Amplification of exocytosis and plasticity. J Gen Physiol 115: 519–532, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Negulescu PA, Machen TE. Intracellular ion activities and membrane transport in parietal cells measured with fluorescent dyes. Methods Enzymol 192: 38–81, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20: 389–399, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Pearce WJ, Duckles SP, Buchholz J. Effects of maturation on adrenergic neurotransmission in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 277: R931–R937, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Pei JM, Kravtsov GM, Wu S, Das R, Fung ML, Wong TM. Calcium homeostasis in rat cardiomyocytes during chronic hypoxia: a time course study. Am J Physiol Cell Physiol 285: C1420–C1428, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Pottorf WJ, Duckles SP, Buchholz JN. Adrenergic nerves compensate for a decline in calcium buffering during ageing. J Auton Pharmacol 20: 1–13, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Rittenhouse AR, Zigmond RE. Role of N- and L-type calcium channels in depolarization-induced activation of tyrosine hydroxylase and release of norepinephrine by sympathetic cell bodies and nerve terminals. J Neurobiol 40: 137–148, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Said HM, Hagemann C, Stojic J, Schoemig B, Vince GH, Flentje M, Roosen K, Vordermark D. GAPDH is not regulated in human glioblastoma under hypoxic conditions. BMC Mol Biol 8: 55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scheerlinck JP, Snibson KJ, Bowles VM, Sutton P. Biomedical applications of sheep models: from asthma to vaccines. Trends Biotechnol 26: 259–266, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Smith AB, Cunnane TC. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J Physiol 497: 657–664, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith PK, Krohn RI, Harmanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk D. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985 [DOI] [PubMed] [Google Scholar]
- 47. Teschemacher AG. Real-time measurements of noradrenaline release in periphery and central nervous system. Autonom Neurosci Basic Clin 117: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Tsai H, Pottorf WJ, Buchholz JN, Duckles SP. Adrenergic nerve smooth endoplasmic reticulum calcium buffering declines with age. Neurobiol Aging 19: 89–96, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Tully K, Treistman SN. Distinct intracellular calcium profiles following influx through N- versus L-type calcium channels: role of Ca2+-induced Ca2+ release. J Neurophysiol 92: 135–143, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Usachev YM, Thayer SA. All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J Neurosci 17: 7404–7414, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Usachev YM, Thayer SA. Controlling the urge to surge: all or none Ca2+ release in neurons. Bioessays 21: 743–750, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Vanterpool CK, Pearce WJ, Buchholz JN. Advancing age alters rapid and spontaneous refilling of caffeine-sensitive calcium stores in sympathetic superior cervical ganglion cells. J Appl Physiol 99: 963–971, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vanterpool CK, Vanterpool EA, Pearce WJ, Buchholz JN. Advancing age alters the expression of ryanodine receptor 3 isoform in adult rat superior cervical ganglia. J Appl Physiol 101: 392–400, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wagerle LC, Heffernan TM, Sacks LM, Delivoria-Papadopoulos M. Sympathetic effect on cerebral blood flow regulation in hypoxic newborn lambs. Am J Physiol Heart Circ Physiol 245: H487–H494, 1983 [DOI] [PubMed] [Google Scholar]
- 55. Wagerle LC, Kurth CD, Roth RA. Sympathetic reactivity of cerebral arteries in the developing fetal lamb and adult sheep. Am J Physiol Heart Circ Physiol 258: H1432–H1438, 1990 [DOI] [PubMed] [Google Scholar]
- 56. Wilfinger WW, Mackey K, Chomcynski P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques 22: 474–481, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Zhang G, Teggatz EG, Zhang AY, Koeberl MJ, Yi F, Chen L, Li PL. Cyclic ADP ribose-mediated Ca2+ signaling in mediating endothelial nitric oxide production in bovine coronary arteries. Am J Physiol Heart Circ Physiol 290: H1172–H1181, 2006 [DOI] [PubMed] [Google Scholar]