Abstract
Breathing patterns in preterm infants consist of highly variable interbreath intervals (IBIs) that might originate from nonlinear properties of the respiratory oscillator and its input-output responses to peripheral and central signals. Here, we explore a property of nonlinear control, the potential for large improvement in the stability of breathing using low-level exogenous stochastic stimulation. Stimulation was administered to 10 preterm infants (postconceptional age: mean 33.3 wk, SD 1.7) using a mattress with embedded actuators that delivered small stochastic displacements (0.021 mm root mean square, 0.090 mm maximum, 30–60 Hz); this stimulus was subthreshold for causing arousal from sleep to wakefulness or other detectable changes in the behavioral state evaluated with polysomnography. We used a test-retest protocol with multiple 10-min intervals of stimulation, each paired with 10-min intervals of no stimulation. Stimulation induced an ∼50% reduction (P = 0.003) in the variance of IBIs and an ∼50% reduction (P = 0.002) in the incidence of IBIs > 5 s. The improved stability of eupneic breathing was associated with an ∼65% reduction (P = 0.04) in the duration of O2 desaturation. Our findings suggest that nonlinear properties of the immature respiratory control system can be harnessed using afferent stimuli to stabilize eupneic breathing, thereby potentially reducing the incidence of apnea and hypoxia.
Keywords: stochastic resonance, afferents, apnea, respiratory rhythm, wavelet transform
the breathing pattern of preterm infants often exhibits wide variations in the timing of breaths that are of clinical importance when associated with prolonged apnea and hypoxia (2, 43). Time series analyses have revealed that apneas can be associated with periodicities in breathing over broad time scales (68) or can follow irregular patterns with fractal-like dynamics of interbreath intervals (IBIs) (22). The physiological mechanisms underlying these patterns are not fully understood. Experimental and computational studies have suggested a number of factors that promote respiratory instabilities in neonates (5, 36, 48), including an increased loop gain of chemical feedback (6), abnormal interactions between control systems governing sleep and respiration (13, 39), and vulnerabilities related to intrinsic properties of the central respiratory oscillator (15, 22, 47). These studies support the idea that nonlinear input-output properties at many levels of organization could destabilize breathing in preterm infants.
Over the past decade, noise-enhanced stability of nonlinear control systems has been advanced as an important paradigm in biology and therapeutics (24, 46). In neural control systems, nonlinear mechanisms can be exploited for enhancing the stability of rhythm using small noisy (i.e., stochastically varying) perturbations. For example, an irregularly firing neuron can be transformed to a robust pacemaker by tiny noisy inputs that perturb the membrane potential at or near the neuron's resonance frequency (30, 52). The concept of noise-enhanced rhythmicity is also relevant to a population of poorly synchronized neurons in which the application of noisy stimuli promotes synchronized bursts of rhythmic activity (22a, 46a, 73). If we consider that central respiratory rhythm is generated by coordinated activities of groups of coupled oscillators (17), then small noisy inputs impinging upon the rhythm-generating neurons should stabilize the rhythmic output signal or even transform the system from subthreshold arrhythmic activity to robust rhythmic activity through a stochastic resonance-type mechanism (47). Enhancement of respiratory rhythmic drive could also reduce destabilizing effects of feedback and feedforward signals impinging upon the respiratory oscillator (5, 47, 48). The present study was motivated by the overall thesis that small noisy stimuli can enhance eupneic respiratory rhythm due to the nonlinear properties of the respiratory control system.
Experimental observations suggest that the stimulation of somatosensory afferents might be an appropriate method to promote the stability of breathing in neonates. Although there is limited information on how somatic afferents are processed by the neonatal respiratory control system, it is generally believed that somatic stimuli facilitate breathing and attenuate inhibitory reflexes (44, 66). The stimulation of cutaneous receptors can convert apnea to spontaneous regular breathing in the fetus (33, 59). Maternal licking of the newborn at birth is observed in many species and is postulated to provide an early stimulatory effect on breathing (16). Manual tactile stimulation applied to the limbs or the back is used routinely to abort apneic pauses in preterm infants (35). Vibratory stimuli applied to the chest wall of preterm infants can cause significant changes in the pattern of breathing (27), but there is a paucity of research on the effects of vibrotactile or other forms of somatosensory stimulation on the stability of breathing.
In the present study, we use stochastic vibrotactile stimulation, in frequency ranges previously shown to affect respiratory rhythm in neonates (27, 49), to evaluate the hypothesis that low-level noisy somatosensory stimulation can stabilize breathing in preterm infants. Oxygenation, pulse rate, sleep state, and gross body movements are evaluated concurrently. Our findings support the idea that nonlinear properties of the immature respiratory control system can be exploited using afferent stimuli to enhance the stability of breathing. Future studies are needed to investigate the potential for optimizing this effect and for determining whether this approach has relevance to clinical treatment of infant apnea.
METHODS
Human Subjects
This study was approved by the Committee for the Protection of Human Subjects in Research at the University of Massachusetts Medical School, which conforms to the standards set by the Declaration of Helsinki and the Office for Human Research Protections, United States Department of Health and Human Services. Infants were recruited for the study if they were 1) preterm with a gestational age of <36 wk and postconceptional age of >30 wk at the time of study and 2) spontaneously breathing room air or receiving supplemental O2 through nasal cannulae at a fixed flow rate. Infants treated with methylxanthines were included if the drug had reached a steady-state level. Exclusion criteria were as follows: evidence of pulmonary disease (e.g., bronchopulmonary dysplasia or hyaline membrane disease), congenital defect, infection, cord pH < 7, anatomic brain anomaly, hydrocephalus, or intraventricular hemorrhage > grade 2. Infants were studied in the neonatal intensive care unit after written informed consent was obtained from the mother of each infant. Nine infants were studied at our institution. One infant (infant 8) was studied at St. Elizabeth's Medical Center of Boston (with institutional approval and written informed consent), and an analysis of expiratory periods (without addressing IBI incidence or variance) in this infant was presented in a brief report (49).
The gender and pertinent ages and weights of the infants are listed in Table 1. Infants received routine clinical care, including standard resuscitative measures if an apnea exceeded 20 s [which occurred in only one infant (infant 2)]. One infant (infant 1) received nasal cannulae oxygen (35% O2 at 125 ml/min) throughout the study; all other subjects breathed room air. Three infants (infants 1, 2, and 6) had been receiving caffeine treatment of apnea with constant dosing for at least 3 days before the study. Six infants (infants 1–4, 6, and 7) were studied in an isolette required for thermal regulation, and four infants (infants 5 and 8–10) were studied in an open crib. Five infants (infants 2, 4, 7, 9, and 10) were studied in the prone position, one infant (infant 8) was studied in the supine position, and four infants (infants 1, 3, 5, and 6) were studied in the supine or lateral position during the morning session and in the prone position in the afternoon after the midday feeding.
Table 1.
Subject characteristics
| Gender | Birth Weight, g | Study Weight, g | Gestational Age, wk | Postconceptional Age at the Time of Study, wk | |
|---|---|---|---|---|---|
| Infant 1 | Male | 985 | 1,200 | 28.9 | 31.9 |
| Infant 2 | Male | 1,470 | 1,265 | 29.7 | 31.4 |
| Infant 3 | Male | 1,090 | 1,170 | 29.6 | 31.9 |
| Infant 4 | Female | 920 | 1,050 | 29.6 | 32.0 |
| Infant 5 | Male | 2,055 | 2,170 | 32.9 | 35.7 |
| Infant 6 | Female | 1,565 | 1,440 | 30.7 | 32.3 |
| Infant 7 | Female | 1,625 | 1,640 | 30.7 | 32.9 |
| Infant 8 | Female | 530 | 1,020 | 27.0 | 34.0 |
| Infant 9 | Male | 2,060 | 1,870 | 33.1 | 35.0 |
| Infant 10 | Female | 1,180 | 2,175 | 28.9 | 35.7 |
| Mean (SD) | 1,348 (497) | 1,500 (441) | 30.1 (1.9) | 33.3 (1.7) |
Physiological Measurements and Recordings
Respiratory inductance plethysmography (Somnostar PT, Viasys Healthcare, Yorbalinda, CA) was used to record thoracic and abdominal respiratory movements. Airflow was detected near the nares using a thermistor (Protech, Woodenville, WA) or cannulae attached to a pressure transducer (Braebon Ultra Pressure Sensor, Kanata, ON, Canada) or to an infrared capnometer (Novametrix Capnogard, Wallingord, CT) to detect expired CO2. Pulse rate and transcutaneous arterial blood O2 saturation (SaO2) were measured using a pulse oximeter attached to the infant's foot or wrist (Nellcor, Hayward, CA). Skin temperature was recorded continuously in four infants (infants 1, 2, 9, and 10) using a disposable adhesive temperature probe attached to the infants' axilla and an electronic monitoring thermometer (Physitemp TH-5, Clifton, NJ).
Polysomnographic activity was recorded in all but one subject (infant 8). Electroencephalographic (EEG) activity was recorded over at least one central lead (CZ, C3, or C4) and over Oz, referenced to the left mastoid or right mastoid in accordance with the International 10-20 System. Left and right electrooculographic (EOG) activities were recorded at the upper or lower outer canthus, referenced to the mastoid lead. Submental electromyographic (EMG) activity was used to record muscle tone. Surface EMGs were recorded over the quadriceps or gastrocnemius muscles to monitor limb movements. A forehead lead served as ground. Bandpass filters were 1–70 Hz for EEG and EOG, 5–35 Hz for submental EMG, and 0.3–70 Hz for limb EMG.
Vibrotactile Stimulation
The infant's mattress was replaced with a specially constructed mattress (TheraSound, Glenelg, MD). The mattress contained an actuator mounted to a sounding board imbedded within the mattress foam. The actuator was driven by a Gaussian white noise signal generator with adjustable low- and high-pass filters (Balance Engineering, Lexington, MA). Using a linear displacement transducer (Trans-Tek, Don Mills, ON, Canada), we determined that there were near-uniform (±2%) displacements across the surface of the mattress. Stimulation strength is reported as the root mean square (RMS) of displacement measured at the surface of the mattress.
The stimulation was filtered white noise in the 30- to 60-Hz band, which was confirmed by power analysis of the displacement signal at the mattress surface. This frequency range was selected based on previous observations in neonates showing that mechanosensory stimulation in this range changes respiratory rhythm (27, 49) and apnea reduction without causing arousal from sleep (49). We conducted preliminary investigations on sleeping preterm infants (unpublished observations) in which the strength of mattress stimulation was increased to a level that evoked behavioral awakenings in the infant (e.g., eye openings and body movements). On the basis of these observations, we selected a stimulus intensity of 0.021 mm RMS (0.090 mm maximum displacement) for the present study because this level was below the minimum threshold for behavioral arousal to wakefulness, as confirmed by formal polysomnographic analysis (see results). A sound meter (Extech Instruments, Waltham, MA) was used to record sound frequency and intensity [in dB(A)]; the sound sensor was place on top of the mattress bedding material adjacent to the infant's cranium.
To simulate the effect of the infant and bedding material on mattress vibration, we placed 1- to 1.5-kg saline-filled bags wrapped in a swaddle blanket on the mattress, which was covered with a sheet. Using thin accelerometers placed on the surface of the saline bags facing the mattress, we found that the presence of the bedding material and the saline bag caused an undetectable (<1%) shift in the power spectrum of accelerations over the 30- to 60-Hz band of frequencies.
Data Acquisition
All physiological signals, the ambient sound levels, and the analog output to the mattress were sampled at a rate of ≥200 Hz/channel. Data were displayed during the experiments and stored on hard disk using PC-compatible analog-to-digital data-acquisition systems. The data-acquisition system was AT-CODAS (DATAQ Instruments, Akron, OH) for eight infants and Embla N7000 (Broomfield, CO) for two infants. Observations by the investigators and nursing staff were recorded as time-stamped text comments along with the signals. Synchronized video recordings were achieved in two subjects with the Embla system (wide-angled lens Micro-Camera, Panasonic).
General Procedures
Experiments were conducted between 7 AM and 5 PM. All infants received feeding every 3–4 h; the exact time was at the discretion of the nursing staff. After the initial setup of the equipment and attachment of all sensors and electrodes, the infants were given their routine morning feeding, and there was an observation period of 30 min used to assure integrity of the recordings and to allow the infant to resume sleeping. Once this adjustment session was completed, each subject participated in an experimental session that lasted 1 h. Eight infants participated in a second (afternoon) experimental session. This followed the midday feeding, which involved removal from the crib in one infant (infant 5); all other infants received gavage feedings in their crib. A second adjustment period (30 min) followed the midday feeding and preceded the afternoon experimental session that lasted 1 h.
Experimental Protocol
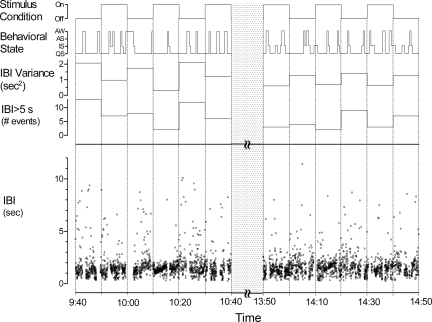
Throughout each experimental session, a computer controlled the mattress actuators with preset time intervals using a customized program (Labview version 6.1, National Instruments, Austin, TX). The mattress stimulation alternated in 10-min intervals between continuously vibrating (on interval) and not vibrating (off interval) through three on-off cycles, i.e., a total of 30 min of stimulation and 30 min of no stimulation for each 1-h experimental session. The order of on versus off intervals (i.e., trials with the on interval followed by the off interval vs. trials with the opposite order) was counterbalanced between the morning and afternoon sessions and randomized across subjects. Figure 1 (see also results) shows an illustration of the protocol in one infant (infant 4).
Fig. 1.
Example of protocol in one infant (infant 4) showing alternating stimulus condition (top), behavioral state [awake (AW), active sleep (AS), intermediate sleep (IS), and quiet sleep (QS)], and analysis of interbreath intervals [IBI variance, IBI > 5 s, and IBI (bottom)]. The dotted bar represents a period of feeding during which signals were not recorded.
Data Processing and Analyses
All data analyses involving manual measurements were completed while the investigators were masked with respect to the condition of mattress stimulation.
IBIs.
Inductance plethysmography of abdominal movements was used to generate a time series of IBIs in 10 subjects. To accomplish this, we excluded periods during which the respiratory movements were obscured by artifactual signals related to gross body movements. These exclusion periods were determined by examination of surface EMG activity and movement artifacts generated on the transcutaneous pulse signal. Text comments written at the time of the study were also used to confirm that signal artifacts were due to gross body movement. These sources of information on infant movement were used to corroborate that inductance plethysmographic signals included for analysis were respiratory in nature. Periods containing movement artifacts that exceeded 5 s were defined for each condition and removed from subsequent analyses (except sleep; see Sleep scoring and EEG power spectrum analyses). IBIs were determined using peak detection software (AT Codas, Dataq Instruments, Akron, OH). We empirically determined the software parameters that detected inductance changes in the abdominal movement recordings corresponding to the smallest eupneic breaths, using nasal airflow signals for corroboration.
Two types of analyses were conducted on IBIs: 1) the statistical properties of the IBI histogram and 2) the properties of the IBI time series. Statistical properties of the IBI histogram included the mean and variance of the IBI distribution. The latter is a measure of breathing stability. To quantify the incidence of IBIs over a specified range, the number of IBIs within the range of interest was calculated per unit of nonmovement time (in h).
IBIs of >5 s were defined as a pause in breathing rhythmicity. These pauses were always associated with a lack of airflow. Therefore, the pauses defined in this study were central (nonobstructive) apneas. Because the preterm infant has a highly compliant chest wall that often leads to paradoxical breathing, the accurate detection of true obstructive apnea is problematic using inductance plethysmography and requires simultaneous measurement of oral and nasal airflow. We could not confidently detect obstructive apneas in the present study and report only the central apneas.
Wavelet transform of the respiratory signal.
The respiratory inductance signal (with movement periods removed as detailed above) was subjected to wavelet analysis using standard methods (65). The wavelet transform computes the frequency content of a signal as a function of time. It incorporates the time scales of the signal within the window of analysis and thereby maintains the correct multitimescale properties of the signal. Using the algorithm of Torrence and Compo (65), we obtained the continuous wavelet transform of the discrete respiratory signal using a complex Morlet wavelet basis function. The smallest scale (so) was selected as twice the sampling period, and the other scales (sj) were selected according to the relation sj = so2jΔj, where Δj = 0.5 and j = 1, 2,…, J, where J is set at a value such that the highest scale obtained was 115.85 s. Scale-averaged power (SAP) was calculated as the weighted sum of the wavelet power spectrum in defined ranges. The SAP of the respiratory signal was calculated for specified ranges of periods (T): T ≤ 2 s includes the eupneic range of periodicities and T > 5 s includes the time scale of apneic pauses.
O2 destaturation and pulse rate.
O2 desaturation time was calculated as the percentage of time in which O2 saturation was below 85%. The beat-to-beat pulse rate was determined from the infrared pulse transducer. Mean pulse rate and pulse rate variance, a measure of heart rate stability, was calculated in nine subjects (the raw pulse signal was not available in infant 8). O2 desaturation time and pulse rate were analyzed with respect to valid recording time, in other words, the time that the respective recordings were not obscured by movement artifacts.
Sleep scoring.
The sleep stage was scored for 30-s epochs using EEG, EOG, and submental and limb EMGs. Scoring was based on the EEG waveforms and behavioral observation to define quiet sleep (QS), active sleep (AS), indeterminate sleep (IS), and wakefulness (W; defined by ≥1 min of movement artifacts), as detailed previously in studies of preterm neonates (10, 12). Time-stamped comments and observations from extracranial monitors were used secondarily (e.g., artifacts in the pulse oximeter waveform confirmed movement periods). The scorer of the behavioral sleep stage was masked to the respiratory channels and mattress status. The relative time in each stage was expressed as percentages of total condition time that included movement periods.
EEG power spectrum analyses.
To calculate the frequency content of EEG activities (60), discrete fast Fourier transforms were performed on a central lead (C3 or CZ) referenced to an anterior lead (A1 or A2) selected based on signal quality. For each infant, the mean power was computed for specified spectral bands [δ (0.5–4 Hz), θ (4–8 Hz), α (8–13 Hz), and β (13–22 Hz)] for the entire on condition, the entire off condition, and the 60-s epochs immediately before and immediately after the onsets and offsets of stimulation intervals. Movement periods were not excluded from these analyses. For each epoch of time, the percentage of the total power for each band was calculated.
Statistical analysis.
Statistical calculations were performed using commercially available software (SPSS version 11.5, Chicago, IL). Parametric tests were used for analyses of all continuous variables except for comparisons involving the incidences of specified ranges of IBIs, which is a skew distribution. Nonparametric tests were also used for analyses of ordinal datasets used to compare behavioral sleep states at stimulation transitions. The Kolmogorov-Smirnov (one-sample) and Friedman's and Wilcoxon signed-rank tests were used for the nonparametric analyses. For factorial analyses of parametric data, separate repeated-measures ANOVAs were used. For factors with more than two levels, the Greenhouse-Geisser correction was used, and ε with unadjusted degrees of freedom is reported. Where a main effect was observed for factors with more than two levels, post hoc tests using the Bonferroni adjustment are reported. For variables with two levels, pairwise t-tests were used to determine whether differences existed between stimulation on and stimulation off conditions. Two-tailed P values are reported. Pearson product-moment correlation coefficient analysis was used to establish the association between pathological pauses in breathing and breathing stability and between sound levels observed with mechanical vibration of the mattress and each of these variables. Values are expressed as means and SD. P values of <0.05 were considered statistically significant. For graphical depictions that summarize results across all subjects, we plotted the ratio of mean values in the on condition and off condition (a ratio of 1 is equivalent to no effect). The percent reduction of a value in the on condition compared with the off condition is 1 minus the ratio (×100). The range of likely mean values is shown using 95% confidence intervals.
RESULTS
Figure 1 is an example of the stimulation protocol in one infant (infant 4). There were three experimental trials in the morning; each trial consisted of a 10-min interval of no stimulation (off interval) and a 10-min interval of stimulation (on interval). This was followed by a feeding and an adjustment period (Fig. 1, dotted bar) that preceded the afternoon experiment, which consisted of another three experimental trials. Therefore, the total duration of each condition (on or off) was 60 min. Note that the order of the stimulus conditions at the onset of the morning and afternoon trials was counterbalanced; in this example, the morning experiment started with a 10-min stimulation off interval and the afternoon started with a 10-min stimulation on interval. Across the 10 subjects, the morning trials began between 8:30 and 10:00 AM and the afternoon trials began between 12:30 and 2:00 PM. Six infants received six on-off trials (3 in the morning and 3 in the afternoon), as shown in Fig. 1. The remaining four infants received fewer than six experimental trials, but the alternating stimulus on-off intervals and counterbalanced protocol were retained. One infant (infant 2) received five trials (2 in the morning and 3 in the afternoon), and the remaining three infants (infants 1, 6, and 8) received three trials (in the morning). The reasons for fewer than six trials were because the infant woke for a feeding before the end of an experimental period (infant 2) or technical problems related to sensors or recording equipment (infants 1, 6, and 8).
As noted in methods, gross body movements compromised the reliability of the respiratory inductance signal. In Fig. 1, this is shown as gaps in the IBI time series. In all experiments, infants exhibited gross body movements nearly 24% (SD 7.2) of the time throughout the study (see also Markers of Behavioral State Were Unaffected by Stimulation). The mean period of generalized movements in the stimulation on conditions was not significantly different from the stimulation off conditions (P = 0.36; see Table 2).
Table 2.
Respiratory, cardiac, behavioral, and EEG measurements
| Off Condition | On Condition | |
|---|---|---|
| IBI | ||
| Mean, s | 1.29 (0.24) | 1.22 (0.23) |
| σ2, s2 | 0.91 (0.55) | 0.46 (0.30) |
| IBI >5 s incidence, h−1 | 37.1 (25.60) | 18.6 (17.00) |
| O2 desaturation time, % total | 1.92 (1.61) | 0.68 (0.97) |
| Pulse rate | ||
| Mean, min−1 | 143.0 (11.00) | 142.4 (11.40) |
| σ, (min−1)2 | 121.2 (60.70) | 86.9 (45.30) |
| Movement, %total time | 23.4 (5.70) | 24.4 (8.70) |
| Quiet sleep, %total time | 61.1 (21.10) | 60.1 (20.00) |
| EEG δ, %total power | 81.1 (7.30) | 76.0 (10.50) |
Values are expressed as means (SD) in 10 subjects for interbreath interval (IBI) and O2 desaturation data and in 9 subjects for pulse rate, behavioral, and EEG data. σ2, variance.
Variance of IBIs Was Reduced During Stimulation
Compared with the off condition, the on condition was associated with significant reductions in the variance of IBIs. An example is shown in Fig. 1, which shows a reduction in IBI variance for each of the on conditions across all six experimental trials (third panel from the top). To test the effect on IBI variance of trial order (trials 1–6) and stimulus condition (on or off), IBI variance was examined with repeated-measures ANOVA in the six infants that had the full set of six trials. The on condition was associated with a significantly reduced variance of IBIs compared with the off condition; the main effect for condition (on or off) was significant (F1,5 = 10.235, P = 0.024). IBI variances were unaffected by trial order within each condition, as indicated in the analysis by no significant effect of trial order (P = 0.454) or reliable interaction term (P = 0.771).
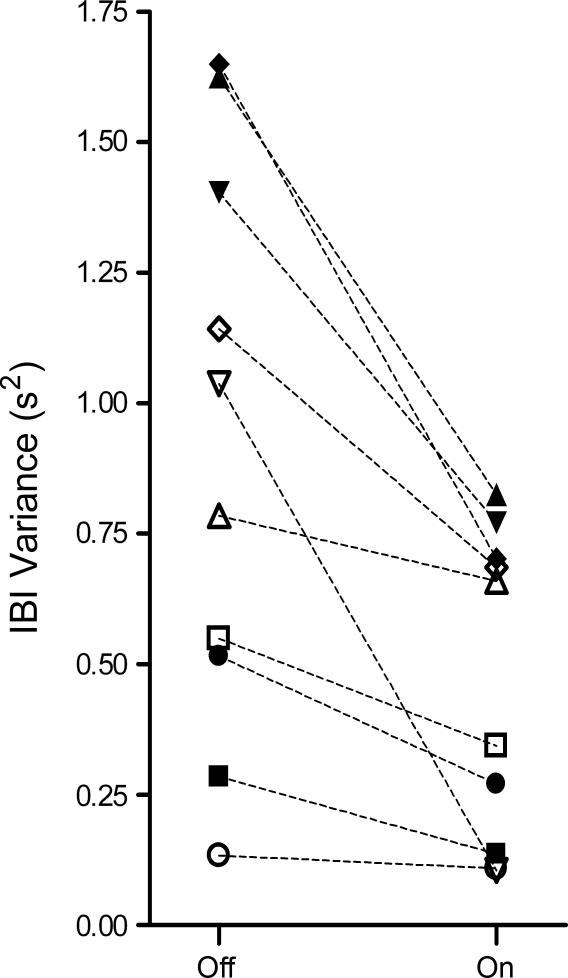
Figure 2 shows a plot of the mean IBI variance of all off intervals and all on intervals for each of the 10 infants. There was a wide range of IBI variances over nearly an order of magnitude among the 10 infants, but the IBI variance was consistently higher in the off condition (group mean of 0.91 s2, SD 0.55) compared with the on condition (group mean of 0.46 s2, SD 0.30). This ∼50% reduction in IBI variance associated with stimulation was significant (P = 0.003). Despite this large reduction in the variance of the IBI distribution, the mean IBI was not significantly affected by stimulus condition (Table 2).
Fig. 2.
Plot of IBI variance in the off condition and on condition for each infant. ■, Infant 1; ▲, infant 2; ▼, infant 3; ◆, infant 4; ●, infant 5; □, infant 6; △, infant 7; ▽, infant 8; ◇, infant 9; ○, infant 10. The mean IBI variance in the on condition (0.46 s2) was reduced by 50% (P = 0.003) compared with the off condition (0.91 s2).
Incidence of Prolonged IBIs Was Reduced During Stimulation
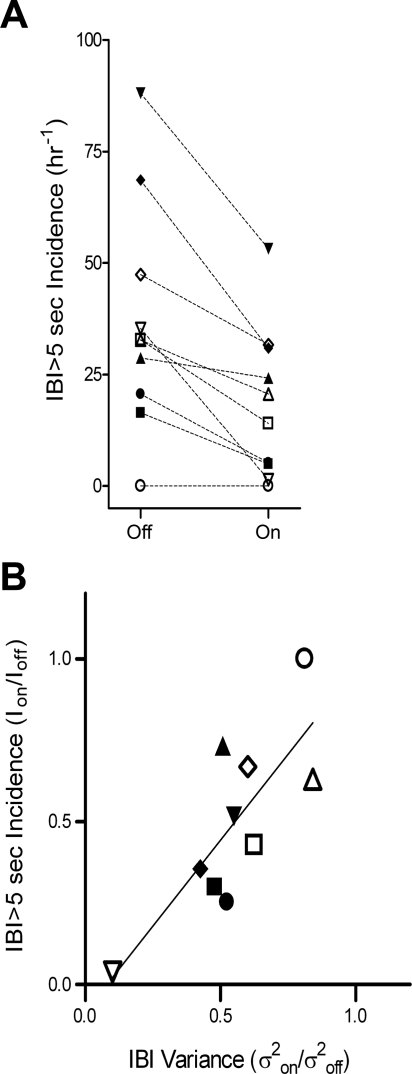
To obtain a more complete picture of how the breathing patterns are reconfigured during stimulation, we analyzed the IBI histograms, which were skewed distributions observed separately for both the on and off conditions for each infant (Kolmogorov-Smirnov one-sample tests, P < 0.001), with a preponderance of breaths occurring in the eupneic range (≤2-s IBIs). We were interested in how stimulation redistributes the incidences of IBIs that occur in the apneic range (IBIs > 5 s). Figure 1 shows an example in one infant of the reduction in pauses by plotting the number of times that IBIs of >5 s were observed for each 10-min period (fourth panel from the top). Note that in this infant, during each of the six trials, there was a reduction in the number of IBIs of >5 s in the on condition compared with the corresponding off condition. Figure 3A shows the mean incidence of IBIs of >5 s for all trials in the on and off conditions for each infant. There was a significant reduction in the incidence of IBIs of >5 s with stimulation (mean 18.62 h−1) compared with no stimulation (mean 37.07 h−1, P = 0.002). The reduction in the incidence of IBIs of >5 s observed during stimulation was also significantly correlated with the reduction in IBI variance, as shown in Fig. 3B (r = 0.733, P = 0.008).
Fig. 3.
A: plot of the incidence of IBI > 5 s in the off condition and on condition for each infant. ■, Infant 1; ▲, infant 2; ▼, infant 3; ◆, infant 4; ●, infant 5; □, infant 6; △, infant 7; ▽, infant 8; ◇, infant 9; ○, infant 10. The mean incidence of IBI > 5 s in the on condition (18.62 h−1) was reduced by 50% (P = 0.002) compared with the off condition (37.07 h−1). B: plot of the ratio of values in the on condition and off condition, showing a strong correlation between the stimulation-associated reductions in IBI variance and the incidence >5 s (r = 0.733, P = 0.008). The solid line is the linear regression.
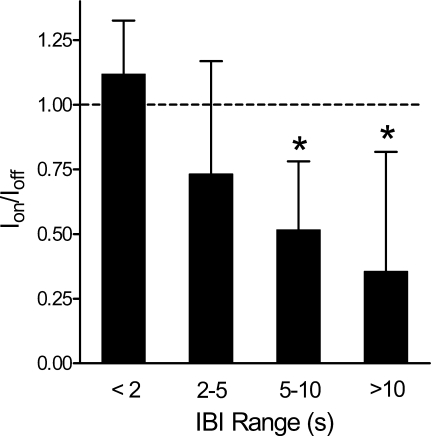
We further analyzed the incidences of IBIs within the following ranges: IBI ≤ 2 s, 2 s < IBI ≤ 5 s, 5 s < IBI ≤ 10 s, and IBI > 10 s. To determine whether stimulation caused a shift in these incidences, we calculated the ratio of mean values in the on condition and in the off condition. A ratio of 1 is equivalent to no effect of stimulation. Figure 4 shows a plot of these ratios for the IBIs of interest. Since the distribution of breaths among intervals was skewed, we applied nonparametric analysis using separate Friedman's ANOVA of ranks to determine the effect of stimulation (on vs. off) on the incidence of IBIs among each of the intervals and to determine if the effect varied between the ranges of IBIs defined in Fig. 4. Stimulation was associated with a significant redistribution of the incidences of IBIs across all intervals (χ2 = 67.08, P = 0.001) and between the intervals (χ2 = 9, P = 0.029), respectively. Separate post hoc paired comparisons (Wilcoxon with Bonferroni corrections for multiple comparisons) revealed that stimulation significantly reduced IBI incidence for IBI > 5 sec (i.e., 5 s < IBI ≤ 10 s, P = 0.013; and IBI > 10 s, P = 0.042).
Fig. 4.
Plot of incidences of IBIs within specified ranges, normalized as a ratio between the on condition and off condition (Ion/Ioff). A ratio of 1 (dotted line) reflects identical incidences in the two conditions. Error bars are SDs of the ratio across subjects. The reduction was significant (*P < 0.05) for IBI of 5–10 s and IBI >10 s. Mean (SD) values for incidences are as follows: IBI < 2 s, on condition 2,927 h−1 (SD 681) and off condition 2,616 h−1 (SD 625); IBI of 2–5 s, on condition 129 h−1 (SD 102) and off condition 176 h−1 (SD 98); IBI of 5–10 s, on condition 17.4 h−1 (SD 15.9) and off condition 33.8 h−1 (SD 25.0); and IBI > 10 s, on condition 1.18 h−1 (SD 2.00) and off condition 3.31 h−1 (SD 3.50). Apnea > 20 s was subjected to resuscitative measures by the medical staff and was recorded only twice in the entire study. Both instances occurred in infant 2 during the off condition.
Analysis of Respiratory Periodicities Using Wavelet Transform
Since the eupneic breathing period in preterm infants is ≤2 s, the variance of the time-dependent scale-averaged wavelet power for periods ≤2 s is an index of eupneic breathing stability (i.e., decreased variance reflects increased stability). For the group of 10 subjects, there was a significant correlation between the reduction in IBI variance induced by stimulation and the corresponding reduction in variance of wavelet power o≤2 s (r = 0.51, P = 0.021), with a 44% reduction in the variance of wavelet power ≤2 s in the on condition (mean 0.133 mV4, SD 0.144) compared with the off condition (mean 0.235 mV4, SD 0.261), although this reduction did not reach statistical significance (P = 0.076). Since apnea episodes should increase the wavelet power of periodicities, which include the apnea periods, we also analyzed wavelet power >5 s. We found no significant correlation (r = 0.14, P = 0.71) between the reduction of wavelet power >5 se and the reduction in IBI variance induced by stimulation. For the group of 10 infants, the wavelet power >5 s was 13.3 mV2 (SD 8.8) in the off condition and 15.4 mV2 (SD 17.4) in the on condition. The difference was not significant (P = 0.31). We found that wavelet analysis of the long periods (>5 s) was compromised by movement artifacts in the respiratory tracings, causing large increases in wavelet power >5 s that were unrelated to breathing. This artifact was demonstrable for periods on the order of the time scales of interest, causing shifts in SAP for up to 15 s before the onset of the movement artifact (i.e., removal of the artifactual movement signal did not correct the wavelet-derived power).
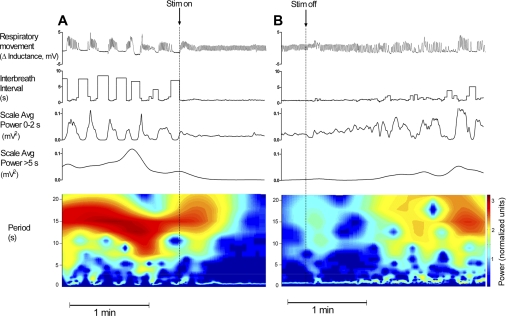
Figure 5 shows an example of tracings that were not contaminated by movement artifacts. In this case (infant 3), apneic pauses were periodically recurring, providing us with an opportunity to visualize and quantify the short-term dynamics of the responses to stimulation. The periodic breathing pattern converted to eupneic breathing after the onset of stimulation (Fig. 5A) and back to periodic breathing after the offset of stimulation (Fig. 5B). The corresponding IBI and wavelet power time series were computed along with the respiratory pattern. Note that the eupneic pattern after the onset of stimulation was associated with reduced variability in the wavelet power ≤2 s and a reduction in the wavelet power >5 s. The time course of the transition from periodic breathing to eupnea was rapid (<10 s) after the onset of stimulation. The time course for the transition back to periodic breathing after the offset of stimulation was longer (∼60 s). These changes were seen in the wavelet power time series in the eupneic range (<2 s) as well as the wavelet power for longer periodicities (>5 s) associated with periodic breathing. The contour plots (Fig. 5, A and B, bottom) show the normalized wavelet power over all relevant periodicities.
Fig. 5.
Example (infant 3) of temporal changes in breathing patterns after the onset of stimulation and offset of stimulation. A: onset of stimulation (at the end of the 10-min off interval) was associated with a rapid switch from periodic to regular breathing (top), with was associated with reduced variability in IBI and in wavelet-derived power ≤2 s and reduced power of periodicities >5 s. B: offset of stimulation (at the end of the 10-min on interval) was associated with a gradual (over ∼1 min) evolution in the breathing pattern from eupnea to a progressively less stable pattern with increasing variability in IBI and wavelet power <2 s and a rise in wavelet power >5 s, which corresponded to the emergence of periodic breathing and apnea pattern. The bottom images show the contour plot of wavelet power. The color scale was mapped to log of normalized power using the normalized energy function (65). Note the complex temporal evolution of a range of periodicities, induced by the onset and offset of stimulation.
O2 Desaturation and Pulse Rate Variance Were Reduced During Stimulation
O2 saturation levels were recorded in all infants. Stimulation was associated with a significant reduction in the amount of time the infants' O2 levels desaturated below 85% (P = 0.04). On average, 1.92% (SD 1.6) of the time (i.e., 7 min/h) SaO2 was <85% without stimulation compared with 0.68% (SD 0.97) of the time (i.e., 2.4 min/h) with stimulation. This represents nearly a threefold reduction in O2 desaturation associated with stimulation.
The pulse signal from the oximeter was recorded in all infants except for infant 8, in which the pulse signal was displayed but the analog output signal was unavailable for recording. The mean pulse rate in the nine infants was 142 min−1 (SD 11) and was unaffected by stimulation (P = 0.14). The mean pulse rate variance was reduced in the stimulation on condition [mean 87 (min−1)2, SD 45] relative to the stimulation off condition [mean 121 (min−1)2, SD 61], but this reduction did not reach significance (P = 0.086).
Skin Temperature and Ambient Sound Levels
The infants' skin temperature, recorded continuously in four infants (infants 1, 2, 9, and 10), remained constant throughout the study. There were no significant differences in skin temperature between stimulation off (mean 36.11°C, SD 0.31) and stimulation on (mean 36.09°C, SD 0.17) conditions (P = 0.13). Sound levels were recorded in all infants near the cranium (see methods). There was a 2-dB(A) difference in the sound level, measured adjacent infants' cranium, between conditions. The mean sound level during the off condition was 50.9 dB(A) (SD 2.8), which was not significantly different than the sound level during the on condition [52.9 dB(A), SD 3.9, P = 0.13]. There was no correlation among the 10 infants between the change in sound level (between off and on conditions) and either the reduction of IBI variance (P = 0.40) or the reduction in the incidence of IBI > 5 s (P = 0.30) associated with stimulation.
Markers of Behavioral State Were Unaffected by Stimulation
Sleep scores and EEG spectra throughout the experimental periods.
There were no differences between conditions in either the relative amount of sleep or wakefulness observed (P = 0.35 and P = 0.32, respectively). The mean percentage of total sleep was 81.8% (SD 10.4); the mean percentage of wakefulness was 17.6% (SD 9.9).
Two separate repeated-measures ANOVAs were performed to test effects due to stimulation condition (on or off) and either sleep state (QS, AS, IS, or W) or EEG power (δ, θ, α, or β). The stimulation condition had no significant effect on either the percentage of time spent in any particular sleep state (P = 0.17) or on the EEG frequencies examined (P = 0.59). There was a main effect for sleep state (F3,24 = 23.96, P < 0.001, ε = 0.477) and for EEG power (F3,24 = 361.8, P < 0.001, ε = 0.388). Separate post hoc comparisons were performed to compare the amount of QS with the other levels of sleep and to compare the amount of δ power with that of the other EEG frequency bands. Regardless of condition, there was significantly more QS compared with either AS, IS, or W (P < 0.003 with Bonferroni corrections) and significantly more δ EEG than each of the other three frequency bands (P < 0.003 with Bonferroni corrections). There were no significant interaction terms for either of these analyses.
Sleep state and EEG frequency content at stimulation transition.
Wilcoxon signed-rank tests were used to compute whether the sleep state for the 30-s epoch immediately before a stimulation transition was the same as the sleep state for the 30-s epoch immediately after a stimulation transition. Sleep and wake states were not significantly affected by stimulation transitions going from either off to on (P = 0.45) or on to off (P = 0.25). Out of 90 transitional periods, regardless of the direction of the transition, 80% of the sleep/wake states remained the same for the epoch immediately before and after the transition, 10% resulted in changes between sleep states, 3.3% resulted in a change from wake to sleep, and 6.6% resulted in a change from sleep to wake. Notably, the change to wake occurred three times for the on to off transition and three times for the off to on transition.
Discrete fast Fourier transforms were also performed on the 1-min epoch immediately before and after the onset and offset of stimulation. For each stimulation condition, the mean percentage of the total power was computed for each EEG frequency band (δ, θ, α, and β) separately for the off and on stimulation transitions. Repeated-measures ANOVA revealed no main effect for transition (before or after, P = 0.18) or for direction (on to off or off to on, P = 0.80). The effect for EEG frequency band (δ, θ, α, and β) was significant (F3,24 = 301, P < 0.001, ε = 0.412). This was due to significantly more power in the δ band than any other EEG frequency band (P < 0.006 with Bonferroni corrections), more θ than α or β (P < 0.006 with Bonferroni corrections), and more α than β (P = 0.018 with Bonferroni corrections). There were no significant interaction terms. Together, these finding support that regardless of whether the stimulation transition went from on to off or from off to on, there were no measurable changes either in the infants' behavioral state or in the EEG power spectra.
DISCUSSION
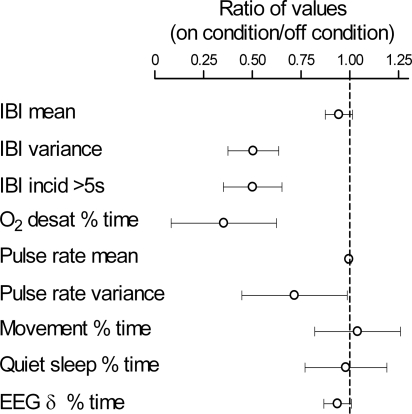
The major finding of this study is that immature patterns of breathing in preterm infants can be shifted toward greater stability of eupneic patterns by stochastic mechanosensory stimulation. The stabilizing effect was associated with reduced apnea and oxygen desaturations. Our results were achieved with stimulus intensities that were subthreshold for causing behavioral arousal to wakefulness or for causing shifts in the power spectrum of EEG activities. Figure 6 and Table 2 show a summary of the study results.
Fig. 6.
Summary of findings with the stimulation on condition compared with the off condition. See Table 2 for values in each condition. Error bars represent intervals of 95% confidence around the mean values.
The apparent enhancement of respiratory rhythm supports the idea that nonlinearities in the immature respiratory control system can be harnessed to stabilize eupneic breathing patterns using small noisy inputs (47–49). Our work is an addition to the accumulating body of evidence documenting enhanced performance of physiological control systems by stochastic stimulus inputs having specified intensities and spectral properties (7–9, 30, 40, 45–47, 49, 52, 56, 57, 62, 72, 73). Improvement in function using noisy external stimulation might seem counterintuitive. One explanation is based on theory of stochastic resonance (23, 24), a universal property of nonlinear systems with thresholds, in which information transmission can be enhanced by raising the rate of threshold crossing. This effect of noise is maximal for an optimal intensity of stimulation and drops with further increases in stimulus intensity. If central apnea represents a state of inspiratory neuronal quiescence, noisy inputs having sufficiently low intensity could increase the likelihood of threshold crossing for inspiratory neuronal burst generation without disrupting the proper sequencing of the respiratory phases. The presence during apnea of subthreshold oscillations in neuronal excitability might facilitate this prorhythmic effect of noise but is not necessary; even neurons at a steady potential can be induced to fire rhythmically via a stochastic resonance-type mechanism (47, 52, 53a). Other hypothetical mechanisms include stochastic phase-locking or stochastic synchronization of populations of rhythm-generating neurons (46a, 73) that exhibit bursting during discrete phases of eupneic breathing (17). Our findings point to the need for further experimental work in animal models of infant apnea (e.g., Ref. 11) to address these possible mechanisms and other basic questions on how stochastic inputs might enhance the function of respiratory rhythm-generating circuits. For example, is rhythm most readily stabilized by pink (1/f) noise, Gaussian noise, or some other type of distribution? Are there particular stimulus frequencies and phase relationships (relative to the respiratory rhythm) that optimally stabilize the normal rhythmic state over pathological states? Can noisy perturbations have rhythm-destabilizing effects, in which certain stimuli cause a switch from eupneic to dysrhythmic states (21, 47–52, 71)?
Our analysis also complements previous observations suggesting that diverse physiological stimuli are capable of reducing apnea in infants (3, 32, 35, 37, 42, 53, 67) and in immature animals models of apnea (33, 47, 59, 61, 64). The novelty of our contribution is to quantify the effect of a defined mechanosensory stimulus on breathing patterns using physiological time series analyses and to provide a link between stability of rhythm and the incidence of apneic pauses. Our experiments were not designed to address clinical outcomes; longitudinal studies in more infants would be required to address the implications of our approach for treatment of infant apnea (19, 29). It is also important to point out that while unstable breathing patterns associated with apnea and hypoxia arouse clinical concern, the precise features of highly variable breathing in infants that distinguish normal from pathological states are not well understood (5, 22, 36, 48, 68). This underscores the point that certain types of variability can be biomarkers of healthy physiological control (see, e.g., Refs. 25 and 54).
Mechanosensory Stimulation
Mechanosensory stimulation was achieved using actuators embedded within the infant's mattress. Our use of displacement frequencies in the range of 30–60 Hz was based on previous observations of mechanosensory-induced changes in respiratory rhythm in premature infants (27, 49). The optimal displacement frequencies that stimulate non-nociceptive mechanoreceptors in infants are uncertain. In newborn animal models, there are important maturational changes in the mechanoresponsiveness of nerve endings and central processing of afferent impulses, with a shift from lower frequency responsivity in the newborn to a much broader frequency response in adults (18, 20). Our choice of the lower range of displacement frequencies is supported by these developmental considerations.
There are some caveats regarding our method of stimulation. We quantified the displacements and spectral properties of the mechanical stimulus at the surface of the mattress. The bedding material and presence of the infant did not cause significant shifts in the power spectrum of mattress vibration. However, despite the reproducibility of the mechanical stimulus, it is important to recognize that the somatotopic receptive field of stimulation must have varied according to the position of the infant on the mattress. Most of the trials took place while the infant was prone; however, some infants were placed in the supine and lateral positions. The respiratory responses were qualitatively similar in all infants (increased stability of breathing during stimulation irrespective of position), but a larger study would be required to address whether there are quantitative effects of position.
Another consideration is whether our findings could have resulted at least in part from auditory stimulation. Changes in the breathing pattern in response to sound has been used as a test of auditory perception in the newborn (4, 70), and auditory evoked responses are recorded in the neonate with frequencies as low as 500 Hz (58). The lower limit of frequency responsiveness of the neonatal auditory system is not known, but to our knowledge a measurable auditory response is not established for the 30- to 60-Hz frequency range used in our study. Furthermore, the increase over background sound levels associated with stimulation was very small [∼2 dB(A)] and did not reach statistical significance. Neonates have a higher auditory threshold compared with adults (38). We therefore suggest that the effective stimulus in our study was predominantly if not exclusively of somatic mechanoreceptors. An interesting question for future study is whether auditory stimulation per se might have similar stabilizing effects on respiratory rhythm.
Respiratory Response
Our finding of a respiratory stabilizing effect of stimulation was robust using different measures. Reductions in the variance of IBIs were well correlated with reductions in the incidence of apneic pauses and the variance of wavelet-derived power of respiratory rhythm for periodicitites in the eupneic range. While analysis of the wavelet power of eupneic periods (<2 s) provides an index of respiratory stability, our analysis of longer time scales was compromised by artifacts related to gross (nonrespiratory) movements. Epochs with no movement artifacts (e.g., Fig. 5) provide interesting preliminary evidence that changes in wavelet-derived periodicities at the onset and offset of stimulation exhibit temporal changes that are consistent with previous work on short-term potentiation of the respiratory control system (69). These preliminary observations suggest a richness in the dynamics of the mechanosensory response of the immature respiratory control that merits further study.
We used a test-retest protocol in which infants were studied during off-on stimulus periods over up to six trials throughout the day. The initial stimulus condition was randomized across infants, and the order of on versus off stimulus conditions was counterbalanced between morning and afternoon experimental sessions. Although there are no known periodicities in breathing over 20-min time periods, counterbalancing the stimulus condition excludes any potential confounds that might emerge from variations in breathing that coincide with the paired stimulus trials.
The improvement in breathing stability associated with mechanosensory stimulation was consistent across trial periods throughout the day. We found no evidence of cumulative enhancements or reductions in breathing stability across trials. These conclusions are based on our measurement of variance of IBIs during each of the 10-min periods across the trials.
The increase in stability of breathing was associated with a reduction in O2 destauration. This finding is not surprising because the incidences of apneic pauses across all ranges were reduced by mechanosensory stimulation. The relationship between apnea duration and transcutaneous O2 desaturation is highly variable across infants; the level of O2 stores, metabolic rate, and hemoglobin properties are important factors. A generally accepted view is that respiratory pauses lasting beyond 20 s result in dangerously low tissue O2 levels, which can cause damage to the heart, lungs, and brain (2, 43). But, it is important to note that tissue O2 can desaturate precipitously with apnea pauses as brief as 8–10 s (55). A reduction in the incidence of apneic pauses therefore is the most parsimonious explanation for the concurrent reduction in hypoxia. Our experiments do not exclude other mechanisms, for example, improvement in alveolar gas exchange due to mechanical perturbations of the lower airways (26, 62). The incidence of O2 desaturation and of prolonged apnea were relatively low in our study, and correlation analyses between breathing patterns and O2 saturation levels were further confounded by the presence of movement artifacts in both the respiratory and pulse oximeter tracings. Further study is needed in more infants for establishing a direct correlation between these events with sufficient power to address the mechanism of improved oxygenation during mechanical stimulation.
In premature infants, the onset of apnea is usually nonobstructive, and there is no inspiratory effort throughout the episode or an obstructed effort near the end of the apnea (31). Airway obstruction at apnea onset is uncommon in premature infants, especially with the careful head positioning and airway suctioning practices used in the neonatal intensive care unit. Our focus on IBIs is sufficient for defining nonobstructive apneic pauses. The detection of obstructive apnea requires the monitoring of nasal and oral airflows. While preterm infants are thought to breathe predominantly through the nose, the transition to mouth breathing occurs frequently during nasal occlusion (14), and, therefore, the attenuation of nasal airflow with continuing respiratory movement is not pathognomonic for obstructive apnea.
We used inductance plethysmography to define breathing patterns. However, during gross body movements, which typically occur during wakefulness, this method cannot reliably distinguished respiratory movement from other movements. Apnea episodes can occur during a variety of vigorous motor activities, which have been demonstrated using special methods for quantifying minute ventilation and lower esophageal pressure during locomotive bouts in preterm infants (1). Therefore, further studies would be needed using such methods to determine if mechanosensory input has an effect on breathing during these locomotive periods associated with wakefulness.
Behavioral State and EEG Responses
Our results were achieved with stimulus intensities that were subthreshold for causing behavioral arousal to wakefulness or for causing shifts in the power spectrum of EEG activities. Most of the infants in our study were <33 wk postconception, and they exhibited a preponderance of QS with EEG patterns consisting of prominent discontinuous patterns. Infants >33 wk postconception had more active sleep. The respiratory stabilizing effect and the lack of arousal to wakefulness with stimulation were found for all infants irrespective of postconceptional age. Furthermore, we found no evidence that mechanosensory stimulation induces shifts from one stage of sleep to another.
Our findings raise the question as to whether mechanosensory stimulation enhances breathing by causing “subcortical arousal.” This state is defined as an increase in gross body movements and concurrent changes in heart rate or respiratory rate during sleep without a change in EEG activity (63). In our subjects, mechanosensory stimulation was associated with a state of reduced variability in respiratory and pulse rates without changing their mean levels and without increasing gross body movement. Mechanosensory stimulation therefore appears to induce a stabilizing influence on subcortical autonomic activities, distinct from the state of subcortical arousal that has been associated with autonomic instability (28) and sudden death (34).
GRANTS
This work was funded in part by National Heart, Lung, and Blood Institute Grants R01-HL-49848 and R01-HL-071884 (to D. Paydarfar), an American Heart Association Scientist Development Grant (to E. Bloch-Salisbury), and intramural Pilot Project Programs of the University of Massachusetts Medical School (Department of Clinical and Translational Science and the Mental Retardation Developmental Disabilities Research Center).
DISCLAIMER
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or American Heart Association.
ACKNOWLEDGMENTS
The authors thank Rebecca Byrne, Nicole Raptis, Priya Sharma, and Xuanxuan Gan for providing invaluable assistance in recruiting subjects, conducting experiments, and analyzing data; Daniel Robichaud II and Mehran Dehpanah for programming the computer-controlled stimulation system; Donald Chin for assistance in scoring sleep data; Stephen Baker and Kevin Kane for advice on statistical analyses; and Riccardo Barbieri for comments on a previous version of the manuscript. The authors are also grateful to the neonatal intensive care unit physicians and nursing staff for assistance in identifying subjects and integrating the study into the routine care of the preterm infants as well as to the infants' families for volunteering to participate in the study.
REFERENCES
- 1. Abu-Osba YK, Brouillette RT, Wilson SL, Thach BT. Breathing pattern and transcutaneous oxygen tension during motor activity in preterm infants. Am Rev Respir Dis 125: 382–387, 1982 [DOI] [PubMed] [Google Scholar]
- 2. Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what's new? Pediatr Pulmonol 43: 937–944, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Al-Saif S, Alvaro R, Manfreda J, Kwiatkowski K, Cates D, Qurashi M, Rigatto H. A randomized controlled trial of theophylline versus CO2 inhalation for treating apnea of prematurity. J Pediatr 153: 513–518, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bennett MJ, Lawrence RJ. Trials with the auditory response cradle. II. The neonatal respiratory response to an auditory stimulus. Br J Audiol 14: 1–6, 1980 [DOI] [PubMed] [Google Scholar]
- 5. Bruce EN. Temporal variations in the pattern of breathing. J Appl Physiol 80: 1079–1087, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Carley DW, Shannon DC. Relative stability of human respiration during progressive hypoxia. J Appl Physiol 65: 1389–1399, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Collins JJ, Imhoff TT, Grigg P. Noise-enhanced tactile sensation. Nature 383: 770, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Collins JJ, Imhoff TT, Grigg P. Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J Neurophysiol 76: 642–645, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Cordo P, Inglis JT, Verschueren S, Collins JJ, Merfeld DM, Rosenblum S, Buckley S, Moss F. Noise in human muscle spindles. Nature 383: 769–770, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Crowell DH, Chime Study Group (editors). An Atlas of Infant Polysomnography. New York: Parthenon, 2003 [Google Scholar]
- 11. Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruption in rat pups with fewer brain stem 5-HT neurons. Am J Physiol Regul Integr Comp Physiol 296: R1783–R1796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curzi-Dascalova L, Mirmiran M. Manual of Methods for Recording and Analyzing Sleep-Wakefulness States in Preterm and Full-Term Infant. Paris: Les Editions INSERM, 1996 [Google Scholar]
- 13. Darnall RA, Ariagno R, Kinney HC. The late preterm infant and the control of breathing, sleep, and brainstem development: a review. Clin Perinatol 33: 883–914, 2006 [DOI] [PubMed] [Google Scholar]
- 14. deAlmeida VL, Alvaro RA, Haider Z, Rehan V, Nowaczyk B, Cates D, Kwiatkowski K, Rigatto H. The effect of nasal occlusion on the initiation of oral breathing in preterm infants. Pediatr Pulmonol 18: 374–378, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Del Negro CA, Wilson CG, Butera RJ, Rigatto H, Smith JC. Periodicity, mixed-mode oscillations, and quasiperiodicity in a rhythm-generating neural network. Biophys J 82: 206–214, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fariday EE. Instinctive resuscitation of the newborn rat. Respir Physiol 51: 1–19, 1983 [DOI] [PubMed] [Google Scholar]
- 17. Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrington DG, Hora MO, Rowe MJ. Functional maturation of tactile sensory fibers in the kitten. J Neurophysiol 52: 74–85, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics 117: S47–S51, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J Physiol 383: 79–92, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forger DB, Paydarfar D. Starting, stopping, and resetting biological oscillators: in search of optimum perturbations. J Theor Biol 230: 521–532, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Frey U, Silverman M, Barabási AL, Suki B. Irregularities and power law distributions in the breathing pattern in preterm and term infants. J Appl Physiol 85: 789–797, 1998 [DOI] [PubMed] [Google Scholar]
- 22a. Galán RF, Fourcaud-Trocmé N, Ermentrout GB, Urban NN. Correlation-induced synchronization of oscillations in olfactory bulb neurons. J Neurosci 26: 3646–3655, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gammaitoni L, Hänggi P, Jung P, Marchsoni F. Stochastic resonance. Rev Mod Phys 70: 223–288, 1998 [Google Scholar]
- 24. Glass L. Synchronization and rhythmic processes in physiology. Nature 410: 277–284, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PCh, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci USA 99, Suppl 1: 2466–2472, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gross D, Zidulka A, O'Brien C, Wight D, Fraser R, Rosenthal L, King M. Peripheral mucociliary clearance with high-frequency chest wall compression. J Appl Physiol 58: 1157–1163, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Hagan R, Bryan AC, Bryan MH, Gulston G. Neonatal chest wall afferents and regulation of respiration. J Appl Physiol 42: 362–367, 1977 [DOI] [PubMed] [Google Scholar]
- 28. Hanzer M, Kerbl R, Urlesberger B, Mueller W, Pichler G, Zotter H. Comparison of heart rate responses during cortical and subcortical arousals in term and preterm infants. Early Hum Dev 83: 511–515, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Henderson-Smart DJ, Osborn DA. Kinesthetic stimulation for preventing apnea in preterm infants. Cochrane Database Systematic Rev: CD000373, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Hunter JD, Milton JG, Thomas PJ, Cowan JD. Resonance effect for neural spike time reliability. J Neurophysiol 80: 1427–1438, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Idiong N, Lemke RP, Lin YJ, Kwiatkowski K, Cates DB, Rigatto H. Airway closure during mixed apneas in preterm infants: is respiratory effort necessary? J Pediatr 133: 509–512, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Ingersoll EW, Thoman EB. The breathing bear: effects on respiration in premature infants. Physiol Behav 56: 855–859, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Ioffe S, Jansen AH, Russell BJ, Chernick V. Respiratory response to somatic stimulation in fetal lambs during sleep and wakefulness. Pflügers Arch 388: 143–148, 1980 [DOI] [PubMed] [Google Scholar]
- 34. Kato I, Franco P, Groswasser J, Scaillet S, Kelmanson I, Togari H, Kahn A. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med 168: 1298–1303, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Kattwinkel J, Nearman HS, Fanaroff AA, Katona PG, Klaus MH. Apnea of prematurity. Comparative therapeutic effects of cutaneous stimulation and nasal continuous positive airway pressure. J Pediatr 86: 588–592, 1975 [DOI] [PubMed] [Google Scholar]
- 36. Khoo MCK. Determinants of ventilatory instability and variability. Respir Physiol 122: 167–182, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Korner AF, Guilleminault C, van den Hoed J, Baldwin RB. Reduction of sleep apnea and bradycardia in preterm infants on oscillating waterbeds: a controlled polygraphic study. Pediatrics 61: 528–533, 1978 [PubMed] [Google Scholar]
- 38. Lary S, Briassoulis G, de Vries L, Dubowitz LM, Dubowitz V. Hearing threshold in preterm and term infants by auditory brainstem response. J Pediatr 107: 593–599, 1985 [DOI] [PubMed] [Google Scholar]
- 39. Lehtonen L, Martin RJ. Ontogeny of sleep and awake states in relation to breathing in preterm infants. Semin Neonatol 9: 229–238, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Liu W, Lipsitz LA, Montero-Odasso M, Bean J, Kerrigan DC, Collins JJ. Noise-enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy. Arch Phys Med Rehabil 83: 171–176, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Longtin A, Bulsara A, Moss F. Time-interval sequences in bistable systems and the noise-induced transmission of information by sensory neurons. Phys Rev Lett 67: 656–659, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Marlier L, Gaugler C, Messer J. Olfactory stimulation prevents apnea in premature newborns. Pediatrics 115: 83–88, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Miller MJ, Martin RJ. Apnea of prematurity. Clinics Perinatol 19: 789–808, 1992 [PubMed] [Google Scholar]
- 44. Mortola JP. Respiratory Physiology of Newborn Mammals: a Comparative Perspective. Baltimore: The Johns Hopkins Press, 2001, chapt. 4 [Google Scholar]
- 45. Moss F, Milton JG. Medical technology: balancing the unbalanced. Nature 425: 911–912, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol 27: 677–682, 2004 [DOI] [PubMed] [Google Scholar]
- 46a. Neiman A, Schimansky-Geier L, Cornell-Bell A, Moss F. Noise-enhanced phase synchronization in excitable media. Phys Rev Lett 83: 4896–4899, 1999 [Google Scholar]
- 47. Paydarfar D, Buerkel DM. Dysrhythmias of the respiratory oscillator. Chaos 5: 18–29, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Paydarfar D, Buerkel DM. Collapse of homeostasis during sleep. In: Sleep Science: IntegratingBasic Research and Clinical Practice, edited by Schwartz WJ. Basel: Karger, 1997, p. 60–85 [Google Scholar]
- 49. Paydarfar D, Buerkel DM. Sporadic apnea: paradoxical transformation to eupnea by perturbations that inhibit inspiration. Med Hypotheses 49: 19–26, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Paydarfar D, Eldridge FL. Phase resetting and dysrhythmic responses of the respiratory oscillator. Am J Physiol Regul Integr Comp Physiol 252: R55–R62, 1987 [DOI] [PubMed] [Google Scholar]
- 51. Paydarfar D, Eldridge FL, Kiley JP. Resetting of mammalian respiratory rhythm: existence of a phase singularity. Am J Physiol Regul Integr Comp Physiol 250: R721–R727, 1986 [DOI] [PubMed] [Google Scholar]
- 52. Paydarfar D, Forger DB, Clay JR. Noisy inputs and the induction of on-off switching behavior in a neuronal pacemaker. J Neurophysiol 96: 3338–3348, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Pichardo R, Adam JS, Rosow E, Bronzino J, Eisenfeld L. Vibrotactile stimulation system to treat apnea of prematurity. Biomed Instrum Technol 37: 34–40, 2003 [DOI] [PubMed] [Google Scholar]
- 53a. Pikovsky AS, Kurths J. Coherence resonance in a noise-driven excitable system. Phys Rev Lett 78: 775–778, 1997 [Google Scholar]
- 54. Pincus SM. Assessing serial irregularity and its implications for health. Ann NY Acad Sci 954: 245–267, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Poets CF, Southall DP. Patterns of oxygenation during periodic breathing in preterm infants. Early Hum Dev 26: 1–12, 1991 [DOI] [PubMed] [Google Scholar]
- 56. Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. Lancet 362: 1123–1124, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol 59: 4–12, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Ribeiro FM, Carvallo RM. Tone-evoked ABR in full-term and preterm neonates with normal hearing. Int J Audiol 47: 21–29, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Scarpelli EM, Condorelli S, Cosmi EV. Cutaneous stimulation and generation of breathing in the fetus. Pediatr Res 11: 24–28, 1977 [PubMed] [Google Scholar]
- 60. Scher MS, Steppe DA, Banks DL, Guthrie RD, Sclabassi RJ. Maturational trends of EEG-sleep measures in the healthy preterm neonate. Pediatr Neurol 12: 314–322, 1995 [DOI] [PubMed] [Google Scholar]
- 61. Stewart MW, Stewart LA. Modification of sleep respiratory patterns by auditory stimulation: indications of a technique for preventing sudden infant death syndrome? Sleep 14: 241–248, 1991 [DOI] [PubMed] [Google Scholar]
- 62. Suki B, Alencar AM, Sujeer MK, Lutchen KR, Collins JJ, Andrade JS, Jr, Ingenito EP, Zapperi S, Stanley HE. Life support system benefits from noise. Nature 393: 127–128, 1998 [DOI] [PubMed] [Google Scholar]
- 63. The International Paediatric Work Group on Arousals The scoring of arousals in healthy term infants (between the ages of 1 and 6 months). J Sleep Res 14: 37–41, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Thomas AJ, Friedman L, MacKenzie CN, Strohl KP. Modification of conditioned apneas in rats: evidence for cortical involvement. J Appl Physiol 78: 1215–1218, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Torrence C, Compo GP. A practical guide to wavelet analysis. B Am Meteorol Soc 79: 61–78, 1998 [Google Scholar]
- 66. Trippenbach T, Flanders D. Interaction between somatic and vagal afferent inputs in control of ventilation in 2-week-old rabbits. Respir Physiol 116: 25–33, 1999 [DOI] [PubMed] [Google Scholar]
- 67. Tuck SJ, Monin P, Duvivier C, May T, Vert P. Effect of a rocking bed on apnoea of prematurity. Arch Dis Child 57: 475–477, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Waggener TB, Frantz ID, 3rd, Stark AR, Kronauer RE. Oscillatory breathing patterns leading to apneic spells in infants. J Appl Physiol 52: 1288–1295, 1982 [DOI] [PubMed] [Google Scholar]
- 69. Wagner PG, Eldridge FL. Development of short-term potentiation of respiration. Respir Physiol 83: 129–139, 1991 [DOI] [PubMed] [Google Scholar]
- 70. Wharrad HJ, Davis AC. Behavioural and autonomic responses to sound in pre-term and full-term babies. Br J Audiol 31: 315–329, 1997 [DOI] [PubMed] [Google Scholar]
- 71. Winfree AT. The Geometry of Biological Time (2nd ed.). New York: Springer, 2001 [Google Scholar]
- 72. Yamamoto Y, Struzik ZR, Soma R, Ohashi K, Kwak S. Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Ann Neurol 58: 175–181, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Zhou C, Kurths J. Noise induced synchronization and coherence resonance of a Hodgkin-Huxley model of thermally sensitive neurons. Chaos 13: 401–409, 2003 [DOI] [PubMed] [Google Scholar]