Abstract
Men have more muscle than women, but most studies evaluating sex differences in muscle protein metabolism have been unable to discern sexual dimorphism in basal muscle protein turnover rates in young and middle-aged adults. We hypothesized that the anabolic response to nutritional stimuli (i.e., amino acids and insulin) would be greater in young/middle-aged men than women. We therefore measured the rates of muscle protein synthesis (MPS) in 16 healthy individuals [8 men and 8 women, matched for age (mean ± SE: 37.7 ± 1.5 yr) and body mass index (25.2 ± 0.7 kg/m2)] after an overnight fast (plasma insulin ∼5 μU/ml and plasma phenylalanine ∼60 μM) and during a hyperinsulinemic-hyperaminoacidemic-euglycemic clamp (plasma insulin ∼28 μU/ml; plasma phenylalanine ∼110 μM; plasma glucose ∼5.4 mM). The rates of MPS were not different between men and women (ANOVA main effect for sex; P = 0.49). During the clamp, the rate of MPS increased by ∼50% (P = 0.003) with no difference in the increases from basal values between men and women (+0.019 ± 0.004 vs. +0.018 ± 0.010%/h, respectively; P = 0.93). There were also no differences between men and women in the basal concentrations of muscle phosphorylated AktSer473, AktThr308, mTORSer2448, and p70s6kThr389 or in the hyperinsulinemia-hyperaminoacidemia-induced increases in phosphorylation of those signaling elements (P ≥ 0.25). We conclude that there are no major differences in the rate of MPS and its intracellular control during basal conditions and during hyperinsulinemia-hyperaminoacidema between young and middle-aged adult men and women.
Keywords: signal transduction, protein metabolism
women have less lean body (mostly muscle) mass and more body fat than men (9, 23). The differences in adult body composition are usually attributed to differences in the sex-hormone milieu. Testosterone is anabolic in muscle (15), and this is usually thought to be mediated by its transcriptional activity and eventual effect on muscle protein synthesis (MPS), although there are reports of inhibition of muscle protein breakdown by testosterone (5, 6, 35). Conversely, female sex steroids inhibit MPS and muscle growth in rodents (7, 34). Nonetheless, the rate of MPS has been reported to be the same during the follicular and luteal phase of the menstrual cycle in young women despite marked differences in plasma estradiol and progesterone concentrations (21), and the basal rate of MPS has been reported to be identical in men and women (11, 16, 26) or, contrary to the obvious assumption, slightly greater in women than in men (13, 32). Except for our recent study in older adults (32), previous studies focusing on sex differences in muscle protein metabolism evaluated only the basal rate of MPS. In 65- to 80-yr-old adults, we demonstrated significant sexual dimorphism in response to mixed meal ingestion (32) with a more pronounced anabolic response, both in terms of MPS and anabolic signaling, in men than in women. Consequently, we hypothesized that the anabolic response to nutritional stimuli (i.e., amino acids and insulin) would also be greater in younger men compared with younger women. We therefore measured the fractional rate of MPS during basal postabsorptive conditions and during hyperinsulinemia-hyperaminoacidemia-euglycemia by using stable isotope-labeled amino acid tracer techniques in young and middle-aged men and women; we also measured concentrations of total muscle RNA and protein to gauge the protein synthetic capacity. Furthermore, to gain some insight into the underlying cellular processes responsible for the anticipated differences, we measured the concentrations of activated (phosphorylated) elements of intracellular signaling pathways involved in the regulation of MPS [Akt; ribosomal protein S6 protein kinase (p70s6k); mammalian target of rapamycin (mTOR); extracellular signaling-regulated 1 and 2 mitogen-activated protein kinase (Erk 1/Erk 2 MAPK); and eukaryotic elongation factor 2 (eEF2)] (10, 29).
METHODS
Subjects.
Eight men and eight age- and body mass index (BMI)-matched premenopausal women participated in this study (Table 1). All subjects were considered to be in good health after completing a comprehensive medical evaluation, which included a history and physical examination and standard blood tests. None of the subjects engaged in regular physical activities (i.e., they exercised ≤1.5 h/wk) or took medications (including hormonal contraceptives) that could interfere with muscle protein metabolism, and none reported excessive alcohol intake or consumed tobacco products. Written informed consent was obtained from all subjects before their participation in the study, which was approved by the Human Research Protection Office and the Clinical Research Unit Advisory Committee at Washington University School of Medicine in St. Louis, MO.
Table 1.
Subject characteristics
| Men | Women | P Value | |
|---|---|---|---|
| Age, yr | 38±2 | 37±2 | 0.75 |
| Body mass index, kg/m2 | 25.5±1.0 | 25.0±0.9 | 0.75 |
| Body mass, kg* | 78.5±4.5 | 68.4±1.8 | 0.05 |
| Fat-free mass, kg* | 62.1±2.8 | 46.2±2.0 | <0.001 |
| Appendicular muscle mass, kg* | 27.1±1.3 | 17.8±1.1 | <0.001 |
| SHBG, nmol/l | 165±19 | 309±33 | 0.002 |
| Testosterone, ng/ml | 5.22±0.46 | 0.43±0.09 | <0.001 |
| Progesterone, ng/ml | 0.40±0.07 | 4.61±1.57 | 0.02 |
| 17β-Estradiol, pg/ml | 25.4±1.8 | 56.5±10.9 | 0.01 |
Values are means ± SE. SHBG: sex-hormone binding globulin.
Measured by dual-energy X-ray absorptiometry as described in Experimental protocol.
Experimental protocol.
Approximately 2 wk before the protein metabolism study, subjects' total body fat-free mass (FFM) and appendicular muscle mass (31) were measured by using dual-energy X-ray absorptiometry (Delphi-W densitometer, Hologic, Waltham, MA). Subjects were instructed to adhere to their usual diet and to refrain from vigorous physical activities for 3 days before the protein metabolism study. The evening before the study, subjects were admitted to the Clinical Research Unit at Washington University School of Medicine. At 2000, they consumed a standard meal providing 50.2 kJ/kg body weight (15% as protein, 55% as carbohydrates, 30% as fat). Subjects then rested in bed and fasted (except for water) until completion of the study the next day. At ∼0600 on the following morning, a cannula was inserted into an antecubital vein for the infusion of a stable isotope-labeled phenylalanine tracer; another cannula was inserted into a vein of the contralateral hand (which was warmed to 55°C) for blood sampling. At ∼0800, primed, constant infusions of [ring-2H5]phenylalanine (priming dose 2.8 μmol/kg FFM, infusion rate 0.08 μmol·kg FFM−1·min−1) and [6,6-2H2]glucose (priming dose 18 μmol/kg body wt, infusion rate 0.22 μmol·kg body wt−1·min−1), both purchased from Cambridge Isotope Laboratories (Andover, MA), were started and maintained for 7 h. Four hours after the start of the tracer infusions, a hyperinsulinemic-hyperaminoacidemic-euglycemic clamp was started and maintained for 3 h. Human insulin (Novolin R, Novo Nordisk, Princeton, NJ) was infused at a rate of 20 mU·m−2 body surface area (BSA)·min−1 (initiated with a priming dose of 80 mU·m−2 BSA·min−1 for 5 min and then 40 mU·m−2 BSA·min−1 for additional 5 min). Plasma amino acid availability was increased by providing an intravenous amino acid mixture (Travasol 10%, Baxter, Deerfield, IL) infused at a rate of 105 mg amino acids·kg FFM−1·h−1 (priming dose 35 mg amino acids/kg FFM). Euglycemia (blood glucose concentration of ∼5.5 mM) was maintained during the clamp procedure by variable rate infusion of 20% dextrose (Baxter) enriched to 2.5% with [6,6-2H2]glucose. To adjust for the increased plasma amino acid availability and reduced hepatic glucose production during the clamp procedure, the [ring-2H5]phenylalanine and [6,6-2H2]glucose infusion rates were increased to 0.12 μmol·kg FFM−1·min−1 (phenylalanine) and decreased to 0.11 μmol·kg body wt−1·min−1 (glucose), respectively.
Blood samples (4 ml) were obtained before beginning the tracer infusion and then at 30, 60, 90, 180, 210, 220, 230, 240, 270, 300, 330, 360, 390, 400, 410, and 420 min to determine the labeling of phenylalanine and glucose in plasma and plasma substrate and hormone concentrations. Additional blood (∼1 ml) was obtained every 10 min during the clamp procedure to monitor plasma glucose concentration. Muscle tissue (∼50–100 mg) was obtained under local anesthesia (lidocaine, 2%) from the quadriceps femoris using a Tilley-Henkel forceps (4) at 1 and 4 h to determine the basal rate of MPS (labeled amino acid incorporation into muscle protein; see Calculations) and the basal concentrations of phosphorylated elements of intramuscular signal transduction proteins (Akt; mTOR; p70s6k; Erk 1/Erk 2 MAPK; and eEF2) involved in the regulation of MPS. A third muscle biopsy was obtained at 7 h (i.e., 3 h after starting the clamp procedure) to determine both the rate of MPS and the intracellular signaling responses to hyperinsulinemia-hyperaminoacidemia. The second and third biopsies were obtained from the same incision on the leg contralateral to that biopsied first; the forceps was directed in proximal and distal direction so that the two biopsies were collected ∼5–10 cm apart.
Sample processing and analyses.
One milliliter of blood was collected in prechilled tubes containing heparin, plasma was separated immediately by centrifugation, and glucose concentration was measured immediately. The remaining blood (∼3 ml) was collected in prechilled tubes containing EDTA; plasma was separated by centrifugation within 30 min of collection and then stored at −80°C until final analyses. Muscle samples were rinsed in ice-cold saline immediately after collection, cleared of visible fat and connective tissue, frozen in liquid nitrogen, and stored at −80°C until final analysis.
Plasma glucose concentration was measured on an automated glucose analyzer (Yellow Spring Instruments, Yellow Springs, OH). Plasma insulin concentration was determined by radioimmunoassay (Linco Research, St. Louis, MO). ELISA kits purchased from Immuno-Biological Laboratories (IBL-America, Minneapolis, MN) were used to determine the concentrations of testosterone, progesterone, 17β-estradiol, and sex hormone binding globulin (SHBG) in plasma.
To determine the labeling of plasma glucose, plasma proteins were precipitated with ice-cold acetone, and hexane was used to extract plasma lipids. The aqueous phase, containing glucose, was dried by speed-vac centrifugation (Savant Instruments, Farmingdale, NY), glucose was derivatized with heptafluorobutyric acid, and the tracer-to-tracee ratio (TTR) was determined by using gas-chromatography/mass-spectrometry (GC-MS, Hewlett-Packard MSD 5973 system with capillary column) as previously described (24).
To determine plasma concentrations of leucine [thought to be a major regulator of MPS (30)] and phenylalanine and the labeling of plasma phenylalanine, known amounts of nor-leucine and [1-13C]phenylalanine were added to an aliquot of each plasma sample, plasma proteins were precipitated, and the supernatant, containing free amino acids, was collected to prepare the t-butyldimethylsilyl (t-BDMS) derivative of phenylalanine to determine their TTRs by GC-MS (MSD 5973 System, Hewlett-Packard) (27, 33). To determine phenylalanine labeling in muscle proteins and in tissue fluid, samples (∼20 mg) were homogenized in 1 ml trichloroacetic acid solution (3% wt/vol), proteins precipitated by centrifugation, and the supernatant, containing free amino acids, collected. The pellet containing muscle proteins was washed and then hydrolyzed in 6 N HCl at 110°C for 24 h. Amino acids in the protein hydrolysate and supernatant samples were purified on cation-exchange columns (Dowex 50W-X8–200, Bio-Rad Laboratories, Richmond, CA), and the t-BDMS derivative of phenylalanine prepared to determine its TTR by GC-MS (MSD 5973 System, Hewlett-Packard) analysis (27, 33). The extent of phenylalanine labeling in blood, muscle tissue fluid, and muscle protein was calculated based on the simultaneously measured TTR of standards of known isotope labeling.
Western analysis was used to measure the phosphorylation of Akt, mTOR, p70s6k, Erk 1/Erk 2 MAPK, and eEF2. Briefly, frozen muscle tissue (∼20 mg) was rapidly homogenized in ice-cold buffer [50 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 10 mM glycerophosphate, 50 mM NaF, 0.1% Triton X, 0.1% 2-mercaptoethanol, 1 complete protease inhibitor tablet (Roche Diagnostics, Burgess Hill, UK)] at 10 μl/mg tissue. Proteins were extracted by shaking for 15 min at 4°C, and samples were then centrifuged at 13,000 g for 10 min at 4°C and the supernatant containing the proteins was collected. The protein concentration in the supernatant was determined by the Bradford method with a commercial reagent (B6916, Sigma-Aldrich, St. Louis, MO) and adjusted to 3 mg/ml in 3× Laemmli buffer. Fifty micrograms of protein from each sample was loaded onto 12% XT-Bis Tris gels, separated by SDS PAGE, and transferred on ice at 100 V for 45 min to methanol-prewetted 0.2-μm PVDF membranes. Blots were then incubated sequentially with 5% (wt/vol) nonfat milk for 1 h, primary antibodies overnight at 4°C, and then secondary antibody (1:2,000 anti-rabbit; New England Biolabs, Ipswich, MA) for 1 h. The following primary antibodies were used at a concentration of 1:2,000: AktThr308, AktSer473; mTORSer2448, p70s6kThr389, eEF2Thr56, Erk 1/Erk 2 MAPKTyr202/204, and pan-actin (loading control), purchased from New England Biolabs. Membranes were developed using Immobilon Western Chemiluminescent HRP substrate (Millipore, Billerica, MA), and the protein bands were visualized and quantified by densitometry on a Chemidoc XRS (Bio-Rad Laboratories, Hercules, CA) ensuring no pixel saturation. Data were expressed in relation to GADPH.
To determine the total RNA-to-protein ratio in muscle, an index of the capacity for protein synthesis, an aliquot of the total RNA preparation prepared for RT-PCR was sequentially extracted (RNA > DNA > protein) according to the manufacturers (Sigma-Aldrich, St. Louis, MO) protocol. Total RNA was measured after complete removal of the upper phase following phase separation; protein concentration was measured after removal of the DNA interphase and precipitation of proteins with acetone from the bottom layer, which were then washed and resuspended in 1% SDS. Total RNA was quantified (in μg/g) spectrophotometrically at 260 nm, and protein (alkali soluble) was quantified (in mg/g) at 595 nm using Bradford reagents at a 1:10 dilution to reduce SDS interference.
Calculations.
Glucose rate of appearance (Ra) in plasma during basal conditions and the clamp procedure was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 30 min of the basal period and the last 30 min of the clamp. Glucose Ra during basal conditions represents endogenous glucose Ra and thus hepatic glucose production rate. During the clamp procedure, glucose Ra represents the sum of endogenous glucose Ra and the rate of infused glucose. Hepatic glucose production rate during the clamp was calculated by subtracting the glucose infusion rate from glucose Ra; glucose rate of disappearance (Rd) was assumed to be equal to glucose Ra plus the tracer infusion rate.
The fractional synthesis rate (FSR) of mixed muscle protein was calculated from the rate of incorporation of [ring-2H5]phenylalanine into muscle protein, using a standard precursor-product model as follows: FSR = ΔEp/Eic × 1/t × 100, where ΔEp is the change between two consecutive biopsies in extent of labeling (TTR) of protein-bound phenylalanine. Eic is the mean labeling over time of the precursor for protein synthesis, and t is the time between biopsies. The free phenylalanine labeling in muscle tissue fluid was chosen to represent the immediate precursor for MPS (i.e., aminoacyl-tRNA) (38). To allow comparison with the results presented by Henderson et al. (13) FSR values were also calculated using plasma phenylalanine TTR as a surrogate for the aminoacyl-tRNA. Values for FSR are expressed as percent per hour.
The translation efficiency (mg protein produced per μg RNA per h) was calculated by dividing the product of the muscle protein FSR (in %/h) and the muscle protein concentration (in mg/g wet tissue) by the muscle total RNA concentration (in μg/g wet tissue) (22, 36).
Statistical analysis.
All data sets were tested for normality. Differences between men and women in subject characteristics and single time-point measurements (e.g., plasma sex hormone concentrations) were evaluated by using Student's t-test; translation efficiency in men and women was compared by using the Mann-Whitney U-test. ANOVA was used to evaluate possible differences between men and women in plasma glucose, insulin, leucine, and phenylalanine concentrations, muscle protein FSR and muscle intracellular signaling elements during postabsorptive conditions and during the clamp procedure. Pearson product-moment correlation coefficients were determined to evaluate potential relationships between female sex steroid concentrations in plasma and muscle protein FSR. A P value of ≤0.05 was considered statistically significant. Data are presented as means ± SE or median with 25th and 75th percentiles in brackets for skewed data sets.
RESULTS
Subjects' age and body composition.
Men and women were matched for age and BMI (Table 1). As expected, total body weight, FFM, and muscle mass were less in women than in men; plasma SHBG, estradiol, and progesterone concentrations were ∼2-fold (SHBG and estradiol) to 10-fold (progesterone) greater (P ≤ 0.02) in women than in men and plasma testosterone concentration were ∼12-fold greater (P < 0.001) in men than in women (Table 1).
Plasma glucose, insulin, leucine, and phenylalanine concentrations.
Plasma glucose and insulin concentrations were not different between men and women in the basal state; during the clamp, plasma glucose was successfully maintained at ∼5.4 mM and insulin concentrations rose by ∼4-fold both in men and women (Table 2). There were no statistically significant differences in plasma leucine or phenylalanine concentrations between men and women; although plasma leucine concentration tended to be greater in men (Table 2). During the clamp, plasma leucine concentration increased by ∼30% from basal values (P < 0.001) and plasma phenylalanine concentration approximately doubled (P < 0.001) in each group (Table 2).
Table 2.
Plasma glucose, insulin, leucine, and phenylalanine concentrations during basal postabsorptive conditions, and during the hyperinsulinemic-hyperaminoacidemic-euglycemic clamp procedure
| Men |
Women |
|||
|---|---|---|---|---|
| Basal | Clamp | Basal | Clamp | |
| Glucose, mM | 4.9±0.1 | 5.4±0.1* | 4.8±0.1 | 5.4±0.1* |
| Insulin, μU/ml | 5.5±1.3 | 27.3±2.1* | 5.3±0.7 | 30.6±2.8* |
| Leucine, μM | 120±7 | 162±12* | 102±8 | 135±12* |
| Phenylalanine, μM | 57±7 | 100±13* | 64±3 | 123±7* |
Values are means ± SE
Value significantly different from corresponding value during basal conditions (P < 0.001).
Glucose kinetics.
Basal glucose Ra was not different in men and women (12.7 ± 0.7 vs. 14.2 ± 0.6 μmol·kg FFM−1·min−1, respectively; P = 0.12). During the clamp, glucose Ra decreased by ∼70% to 3.9 ± 0.3 μmol·kg FFM−1·min−1 in men and 4.1 ± 0.7 μmol·kg FFM−1·min−1 in women (P = 0.75). Glucose Rd during the clamp was ∼25% greater in women than in men (39.9 ± 3.7 vs. 32.1 ± 2.7 μmol·kg FFM−1·min−1, respectively; P = 0.057).
Free phenylalanine labeling in plasma and muscle tissue.
Plasma phenylalanine labeling was at steady state during evaluation of the muscle protein FSR, both in the basal state and during the hyperinsulinemic-hyperaminoacidemic clamp; i.e., the phenylalanine TTR (grand mean ± SE) was 0.092 ± 0.003 and 0.103 ± 0.002 at 60 and 240 min after the start of the tracer infusion, respectively, and 0.103 ± 0.003 and 0.098 ± 0.002 at 30 and 180 min after the start of the amino acid and insulin infusion, respectively. During basal conditions, the labeling of phenylalanine in plasma was greater (P < 0.01) in men than in women, whereas the labeling of free phenylalanine in muscle was greater (P < 0.01) in women than in men (Table 3). Consequently, the muscle-to-plasma free phenylalanine labeling ratio was greater (P < 0.01) in women than in men. These differences between the sexes disappeared during the amino acid insulin infusion (Table 3).
Table 3.
Free phenylalanine tracer-to-tracee ratios in plasma and muscle tissue water during basal postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic clamp procedure
| Men |
Women |
|||
|---|---|---|---|---|
| Basal | Clamp | Basal | Clamp | |
| Plasma | 0.1110±0.0026 | 0.0958±0.0013† | 0.1045±0.0018* | 0.0979±0.0027† |
| Muscle | 0.0615±0.0023 | 0.0741±0.0030† | 0.0759±0.0027* | 0.0757±0.0014 |
| Muscle/plasma | 0.5535±0.0122 | 0.7726±0.0244† | 0.7259±0.0217* | 0.7751±0.0153† |
Values are means ± SE
Value significantly different from corresponding value in men (P < 0.01).
Value significantly different from corresponding basal value (P < 0.05).
MPS.
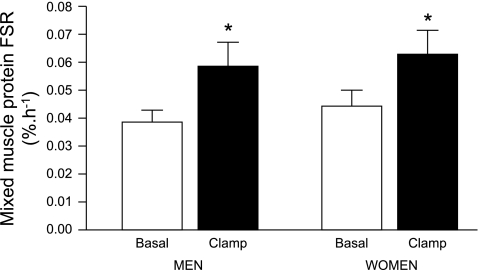
There were no differences between men and women in the muscle RNA concentration (0.34 ± 0.05 vs. 0.37 ± 0.05 μg RNA/mg muscle wet wt, respectively; P = 0.66), the muscle alkali soluble protein concentration (113 ± 24 vs. 101 ± 17 μg protein/mg muscle wet wt, respectively; P = 0.72), or the capacity for MPS (i.e., total RNA-to-protein ratio in muscle: 4.6 ± 1.3 vs. 4.5 ± 0.9 μg RNA/mg protein, respectively; P = 0.95). There were also no differences between men and women in the basal FSR when calculated with free muscle tissue phenylalanine labeling as the precursor pool labeling (Fig. 1); the minimum and maximum basal muscle protein FSR values in men and women were 0.021 and 0.025%/h, respectively (min) and 0.066 and 0.062%/h (max), respectively. During the amino acid/insulin infusion, the FSR increased by ∼50% (P = 0.003) in both men and women with no difference in the increase between them (+0.019 ± 0.004%/h vs. +0.018 ± 0.010%/h, respectively; P = 0.93). Hence, the translational efficiency was not different between men and women, either during basal conditions [0.010 (0.006, 0.019) vs. 0.008 (0.006, 0.021) mg protein·μg RNA−1·h−1, respectively; P = 0.82] or during the amino acid/insulin infusion [0.013 (0.008, 0.041) vs. 0.016 (0.008, 0.025) mg protein·μg RNA−1·h−1, respectively; P = 0.73].
Fig. 1.
Mixed skeletal muscle protein fractional synthesis rate (FSR) during basal postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic-euglycemic clamp procedure in men and women. Data are means ± SE. ANOVA revealed a significant main effect for feeding (P = 0.003) but no effect of sex (P = 0.49) and no interaction (P = 0.93).
Neither the plasma estradiol or progesterone concentrations nor the estradiol-to-progesterone concentration ratio in plasma correlated with the muscle protein FSR in women (all P ≥ 0.29).
Importantly, when muscle FSR values were calculated using plasma phenylalanine labeling as a surrogate for the precursor pool labeling [to allow a direct comparison of our results with those obtained by Henderson et al. (13), who unlike others (11, 16, 26) before reported a difference between men and women in the basal rate of muscle protein synthesis], the calculation yielded basal rates of MPS that were apparently greater in women than in men; the FSR values obtained (0.031 ± 0.004 vs. 0.021 ± 0.003%/h, respectively; P = 0.05) were very close to those reported by Henderson et al (13). The use of the plasma labeling as the precursor pool did not affect the magnitude of the anabolic responses or reveal any differences in the two groups during the insulin and amino acid infusion (P = 0.63).
Phosphorylation of signaling transduction proteins in muscle.
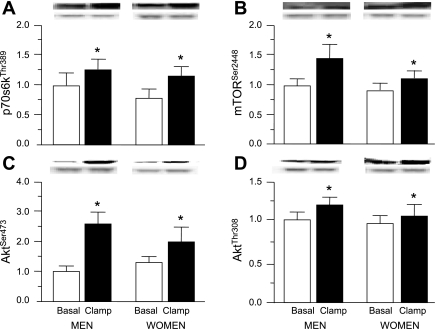
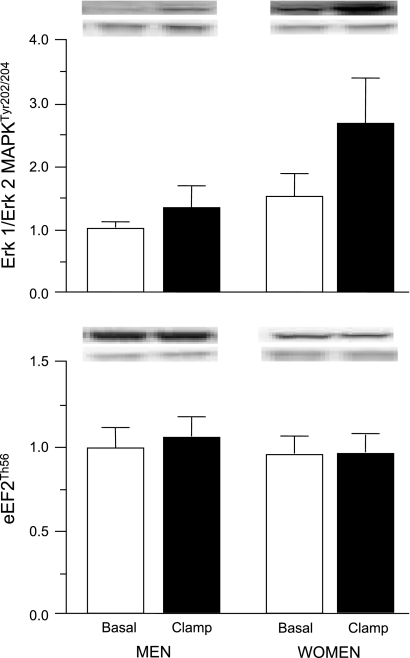
In the postabsorptive state, the concentrations of both phosphorylated AktThr308 and AktSer473, and phosphorylated mTORSer2448 and p70s6kThr389 in muscle were not different in men and women (Fig. 2). Hyperinsulinemia-hyperaminoacidemia increased the concentrations of these phosphorylated signaling elements (all P ≤ 0.05) in both men and women without a difference in the response (P ≥ 0.25) between men and women (Fig. 2). Phosphorylated Erk 1/Erk 2 MAPKTyr202/204 in muscle was ∼50–100% greater (P = 0.051) in women than in men both during postabsorptive conditions and during amino acid/insulin infusion; amino acid and insulin infusion had no effect (P = 0.14) on the concentration of phosphorylated Erk 1/Erk 2 MAPKTyr202/204 (Fig. 3). The concentration of phosphorylated eEF2Thr56 in muscle was ∼20% less in women than in men both during postabsorptive conditions and during amino acid/insulin infusion, but the difference did not reach statistical significance (P = 0.11); amino acid/insulin infusion had no effect (P = 0.60) on the concentration of phosphorylated eEF2Thr56 (Fig. 3).
Fig. 2.
Average concentrations (arbitrary units) and representative blots (top: protein of interest; bottom: GADPH) of phosphorylated p70s6kThr389 (A), mTORSer2448 (B), AktSer473 (C), and AktThr308 (D) during basal postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic-euglycemic clamp procedure in men and women. Values are means ± SE.*ANOVA revealed a significant main effect for amino acid/insulin infusion (P ' 0.05) but no effect of sex and no interaction.
Fig. 3.
Average concentrations (arbitrary units) and representative blots (top: protein of interest; bottom: GADPH) of phosphorylated Erk 1/Erk 2 MAPKTyr202/204 (top) and eukaryotic elongation factor 2 (eEF2Thr56; bottom) in muscle of men and women during basal postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic-euglycemic clamp procedure. Values are means ± SE. ANOVA revealed a trend for a main effect of sex (Erk 1/Erk 2 MAPK, P = 0.051; eEF2, P = 0.11) but no effect of amino acid/insulin infusion and no interaction.
DISCUSSION
In the present study we did not discover major differences between the rates of MPS in men and women, either during basal postabsorptive conditions or during hyperinsulinemia-hyperaminoacidemia. Furthermore we were unable to detect major differences in the capacity for MPS, as RNA-to-protein ratio, or in the concentration of phosphorylated elements of the Akt-mTOR pathway regulating translation.
Our results regarding the rates of MPS in men and women are in agreement with all previous studies (11, 13, 16, 26) in which sex differences in basal muscle protein turnover were evaluated in young to middle aged men and women. All (11, 16, 26) but one (13) of the previous studies failed to observe a difference in the basal rate of MPS between men and women. Moreover, in excellent agreement with the results we obtained by using plasma phenylalanine labeling in the calculation of the FSR, Henderson et al. (13) report similar basal FSR values as we did in the present study and a ∼15% greater basal rate of MPS in women than in men, although, unlike in the present study, the difference between men and women in the study by Henderson et al. (13) remained statistically significant when using the intracellular phenylalanine labeling as precursor. The reasons for this discrepancy are not entirely clear but most likely include both differences in sample size and subject characteristics. Henderson et al. (13) studied 62 young (30 men; 32 women) and 144 older (87 men; 57 women) adults during basal postabsorptive conditions and found a statistically significant main effect for sex with the FSR being greater in women than in men. However, they did not present the FSR values obtained using the intracellular phenylalanine labeling as precursor; therefore the magnitude of the difference using this analytical approach remains unknown. From the data presented, we cannot exclude the possibility that this effect was largely due to differences between older men and older women since we have previously observed a ∼30% greater basal FSR in older women than older men (32).
On the other hand, it is possible that we missed a difference in the rates of MPS between men and women due to a type II statistical error because the differences in the rates of MPS between our men and women were small. Overall (main effect), the average FSR was 13% greater in women than in men and the difference in the average anabolic response (i.e., the hyperaminoacidemia-hyperinsulinemia-induced increase in FSR from basal values) was 5%. To obtain an estimate of the sample size required to detect statistically significant differences of the biggest and smallest magnitude observed with sufficient power (≥ 0.80) to reject the null-hypothesis and a sufficiently small probability of Type I error (α < 0.05), we performed a power analysis on the basis of the following data. The mean ± SD basal FSR values in men and women were 0.038 ± 0.017 and 0.045 ± 0.014%/h, respectively. The difference between men and women in the average basal FSR was 0.0068%/h, the difference between men and women in the average FSR during the clamp was 0.0059%/h, and the difference in the average anabolic response (i.e., the hyperaminoacidemia-hyperinsulinemia-induced increase in FSR from basal values) in men and women was 0.0010%/h. The minimum sample size required is as follows: for basal FSR, ≥98 subjects per group assuming the larger of the two SD (i.e., that reported for our women) and ≥69 subjects per group assuming the smaller of the two SD (i.e., that reported for our men); for anabolic response, >1,220 subjects per group assuming the larger of the two SD and >605 subjects per group assuming the smaller of the two SD. Therefore it is conceivable that our study was underpowered to detect a difference in the absolute FSR between men and women; however, we consider it unlikely that the failure to observe a difference in the anabolic response in men and women was due to a lack of statistical power—more likely the difference, if it exists, is probably negligible.
Ideally, the muscle protein FSR is calculated by using the protein labeling as the numerator and the amino acyl-tRNA labeling as the denominator in the precursor-product equation. However, technical difficulties (38, 40) limit this approach, and the amino acid labeling in muscle fluid [or that of the venous plasma α-ketoisocaproic acid (KIC) for leucine (26)] has been shown to be a suitable surrogate (3, 20, 38). It has been demonstrated that the phenylalanine labeling in muscle tissue fluid is not different from the phenylalanine tRNA labeling, whereas the plasma phenylalanine labeling is ∼40–50% greater than the phenylalanine tRNA labeling (1, 3). Furthermore, it has been demonstrated that the relationship between the tissue fluid phenylalanine and the tRNA labeling remains constant during changing plasma phenylalanine availability when the phenylalanine labeling is maintained constant, whereas the relationship between plasma phenylalanine and tRNA labeling is not (3). In our view it is therefore valid to use the muscle tissue fluid phenylalanine labeling as the precursor labeling for the calculation of FSR, whereas the use of the plasma phenylalanine labeling may not be valid.
We observed differences between men and women in the labeling of phenylalanine in plasma and muscle free tissue fluid during basal conditions. Since the phenylalanine tracer was infused at the same rate relative to FFM in men and women in our study, this indicates that the rate of appearance of unlabeled phenylalanine into plasma relative to total body FFM is greater in women than men; however, the source of the “extra” phenylalanine is not known and cannot be determined from the data collected in our study. The muscle tissue fluid phenylalanine labeling is determined by the rate of appearance of unlabeled phenylalanine relative to the rate of appearance of labeled phenylalanine into the muscle tissue pool. The difference between men and women in muscle tissue fluid phenylalanine labeling cannot be interpreted conclusively with the data at hand. To our knowledge differences of this kind have previously either not been observed or not been reported. In any case, although interesting, these findings do not compromise the validity of our MPS measurements.
We were unable to discern substantial differences between the sexes in the capacity for protein synthesis (RNA/protein) or the concentration of a substantial number of the phosphorylated signaling proteins examined in muscle during postabsorptive conditions, except, and in agreement with our previous study in older men and women (32), a trend for decreased eEF2Thr56 and increased Erk 1/Erk 2 MAPKTyr202/204 in women. These results are novel and have not been reported previously. Furthermore, the anabolic response to hyperinsulinemia-hyperaminoacidemia in the present study, both with regard to signaling events as well as the change in muscle protein FSR (irrespective of the precursor enrichment; i.e., plasma phenylalanine vs. muscle free phenylalanine, used to calculate the FSR), was not different in men and women. We are not aware of any study that compared the muscle protein synthesis response to nutritional stimuli in men and women, except for a recent one we conducted in older adults in whom we found the anabolic response to liquid meal feeding was less in women than in men (32). The differences in experimental design, however, preclude us from drawing firm conclusions regarding potentially different age-associated changes in the anabolic response to nutritional stimuli.
The absence of a difference in the rates of MPS between young and middle-aged men and women or the greater rate of MPS in women than in men reported by us in the present study and others (11, 13, 16, 26) may at first seem counterintuitive, because testosterone is well known to be anabolic and increases the basal rate of MPS and muscle mass in both healthy and hypogonadal young men (2, 6, 12, 35); and there is evidence that ovariectomy increases the rate of MPS, and progesterone and estrogen reintroduction inhibit MPS in ovariectomized rats (34). However, it may be that although the steroid hormones may set the size of peak muscle mass attained during young adulthood, once that has been achieved and hormone levels remain within the normal physiological range they have no further influence on the relative rates of protein turnover. In fact, the difference in lean body mass, even when adjusted for differences in height between the sexes, is evident from infancy and becomes most marked after 15 years of age, due to continued growth in boys (37, 39), probably mediated by the surge in secretion of testosterone (8, 15). Before puberty and after the accelerated growth rate of lean body mass during puberty and early adulthood in boys, however, changes in lean body mass relative to longitudinal growth with time proceed largely in parallel in boys and girls/men and women; once adulthood is attained and sex hormone concentrations are relatively stable there is little change in fat-free/muscle mass in both men and women (9, 17, 18, 39). Furthermore, it has been demonstrated that the stimulatory effect of testosterone on the rate of MPS is lost after chronic administration when a new steady muscle mass is achieved (13, 25).
Although no difference between men and women was observed for the anabolic response of MPS to hyperinsulinemia-hyperaminoacidemia, glucose Rd during the clamp was greater in women than men, indicating greater sensitivity of glucose metabolism to insulin in women. This is consistent with the results of previous studies focused on glucose metabolism (28, 41) and potentially reveals a dissociation between the nutritional regulation of glucose and protein metabolism. Indeed, it has been demonstrated that muscle glucose uptake is many times more sensitive to the effect of insulin than MPS (14, 19). Since the response of relatively early events in the insulin signaling cascade (i.e., Akt and mTOR phosphorylation) were not different in men and women, divergent signaling events that could explain these findings on glucose metabolism may occur independent of or distal to Akt/mTOR.
In summary, we conclude that there are no major differences in the rate of MPS and its control during basal conditions and during hyperinsulinemia-hyperaminoacidema between young adult men and women.
GRANTS
This publication was made possible by Grant UL1-RR-024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); NIH Grants AR-49869, RR-00954 (Biomedical Mass Spectrometry Resource), and DK-56341 (Clinical Nutrition Research Unit); the University of Nottingham; UK Biotechnology and Biological Sciences Research Council Grants BB/XX510697/1 and BB/C516779/1; and a European Union EXEGENESIS program grant. D. N. Reeds was supported by an American Society of Nutrition Physician Nutrition Support Specialist Award. P. Atherton is a designated Research Councils UK fellow. G. I. Smith is supported by an Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Fellowship.
ACKNOWLEDGMENTS
We thank the staff of the Research Participant Registry for help in subject recruitment, the staff of the Center for Applied Research Services for technical assistance, and the study subjects for their participation.
REFERENCES
- 1. Baumann PQ, Stirewalt WS, O'Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol Endocrinol Metab 267: E203–E209, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab 81: 3469–3475, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Caso G, Ford GC, Nair KS, Vosswinkel JA, Garlick PJ, McNurlan MA. Increased concentration of tracee affects estimates of muscle protein synthesis. Am J Physiol Endocrinol Metab 280: E937–E946, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Dietrichson P, Coakley J, Smith PE, Griffiths RD, Helliwell TR, Edwards RH. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry 50: 1461–1467, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88: 358–362, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab 275: E864–E871, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Fisher JS, Hasser EM, Brown M. Effects of ovariectomy and hindlimb unloading on skeletal muscle. J Appl Physiol 85: 1316–1321, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Forbes GB. The effect of anabolic steroids on lean body mass: the dose response curve. Metabolism 34: 571–573, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism 19: 653–663, 1970 [DOI] [PubMed] [Google Scholar]
- 10. Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 103: 378–387, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab 292: E77–E83, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol 66: 498–503, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O'Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J 23: 631–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin's effect to stimulate protein synthesis in the human forearm. Am J Physiol Endocrinol Metab 274: E1067–E1074, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 63: 280–293, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab 84: 1007–1010, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89: 81–88, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr 55: 663–672, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Liu Z, Long W, Fryburg DA, Barrett EJ. The regulation of body and skeletal muscle protein metabolism by hormones and amino acids. J Nutr 136: 212S–217S, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Ljungqvist OH, Persson M, Ford GC, Nair KS. Functional heterogeneity of leucine pools in human skeletal muscle. Am J Physiol Endocrinol Metab 273: E564–E570, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Miller BF, Hansen M, Olesen JL, Flyvbjerg A, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol Endocrinol Metab 290: E163–E168, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle. Nature 241: 204–205, 1973 [DOI] [PubMed] [Google Scholar]
- 23. Mingrone G, Marino S, DeGaetano A, Capristo E, Heymsfield SB, Gasbarrini G, Greco AV. Different limit to the body's ability of increasing fat-free mass. Metabolism 50: 1004–1007, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Mittendorfer B, Klein S. Effect of aging on glucose and lipid metabolism during endurance exercise. Int J Sport Nutr Exerc Metab 11, Suppl: S86–S91, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ, Smith GE, 3rd, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355: 1647–1659, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol 91: 1041–1047, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism 46: 943–948, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Paula FJ, Pimenta WP, Saad MJ, Paccola GM, Piccinato CE, Foss MC. Sex-related differences in peripheral glucose metabolism in normal subjects. Diabetes Metab 16: 234–239, 1990 [PubMed] [Google Scholar]
- 29. Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J 403: 217–234, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol 575: 305–315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119: 526 e529–e517, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE 3: e1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith GI, Villareal DT, Mittendorfer B. Measurement of human mixed muscle protein fractional synthesis rate depends on the choice of amino acid tracer. Am J Physiol Endocrinol Metab 293: E666–E671, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am J Physiol Endocrinol Metab 280: E496–E501, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab 269: E820–E826, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am J Physiol Endocrinol Metab 277: E1077–E1086, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev 26: 114–146, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA 88: 5892–5896, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 21: 415–430, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Wolfe RR, Chinkes D. Isotope Tracers in Metabolic Research: Principles and Practices of Kinetic Analysis. Hoboken, NJ: Wiley, 2005 [Google Scholar]
- 41. Yki-Jarvinen H. Sex and insulin sensitivity. Metabolism 33: 1011–1015, 1984 [DOI] [PubMed] [Google Scholar]