Abstract
Porphyromonas gingivalis is a Gram-negative anaerobic bacterium that is one of the causative agents of chronic adult periodontal disease. Among the potential virulence factors of P. gingivalis are the hemagglutinins. Recombinant Hemagglutinin B (rHagB) from P. gingivalis has been shown to activate the immune system by inducing specific antibodies that protect against experimental periodontal bone loss following P. gingivalis infection. Since different microbial products can stimulate dendritic cells (DC) through Toll-like receptors (TLRs), subsequently leading to T cell activation and antibody production, we wanted to investigate the immunostimulatory effect of rHagB on DC and the role of TLR signaling in this process. Using an endotoxin free rHagB preparation, our results show that stimulation of murine bone marrow-derived DC with rHagB leads to upregulation of the costimulatory molecules CD86 and CD40, activation of p38 and ERK MAP kinases, transcription factors NF-κB, CREB and IRF-3 and the production of IL-6, TNF-α, IL-12p40 and to a lesser extent IL-10 and IFN-β. This activation process was absolutely dependent on TLR4 and CD14. While upregulation of CD86 was independent of the adaptor molecule MyD88, CD40 upregulation and optimal cytokine (IL-6, TNF-α, IL-12p40, IL-10 and IFN-β) production required both MyD88 and TRIF molecules. These results are of importance since they are the first to provide insights into the interaction of rHagB with DC and TLRs. The information from this study will aid in the design of effective vaccines strategies against chronic adult periodontal disease.
Keywords: Hemagglutinin B, Dendritic cells, Toll-like receptors, Porphyromonas gingivalis
1. Introduction
Porphyromonas gingivalis, a Gram-negative anaerobic coccobacilli, has been implicated in the etiology of adult periodontitis (Lamont and Jenkinson, 1998; Slots et al., 1986; Socransky et al., 1998). This disease is characterized by a chronic inflammatory process of the tissues supporting the teeth, which ultimately causes resorption of alveolar bone. In addition to periodontal disease, P. gingivalis infection has been associated with a number of systemic disorders such as cardiovascular disease, atherosclerotic complications in hemodialysis patients and preterm low birth weight babies (Beck et al., 1996; Craig, 2004; Craig et al., 2007; Offenbacher et al., 1996). Thus, an understanding of the immune interactions between P. gingivalis or its components with the host is of outmost importance for the development of means to protect against P. gingivalis infection.
Several virulence factors have been described for P. gingivalis, including fimbriae, hemagglutinins, lipopolysacchride (LPS) and cysteine proteases known as gingipains (Duncan et al., 1993; Hirose et al., 1996; Katz et al., 2000; Kuramitsu, 1998; Lamont and Jenkinson, 1998; Oleksy et al., 2002; Potempa et al., 2000; Zhang et al., 1999). Hemagglutinins are non-fimbrial surface expressed adhesins that are thought to mediate the attachment of the bacteria to the host tissue, as well as to agglutinate erythrocytes (Nelson and Cox, 2005). Currently, hemagglutinins A, B, C, D and E (HagA, B, C, D and E, respectively) have been cloned and described (Lepine and Progulske-Fox, 1996; Progulske-Fox et al., 1989, 1995), and much focus in the last few years has been on HagB due to its potential as a vaccine candidate. The basis for this idea comes from several studies that show that HagB can induce an immune response that is protective against P. gingivalis infection and alveolar bone loss (Dusek et al., 1993, 1994; Katz et al., 1999; Yang et al., 2002; Zhang et al., 2003, 2004, 2005a,b). The exact mechanism of how HagB exerts its protective effect has not yet been determined.
Dendritic cells (DC) are the antigen-presenting cells per excellence as they link the innate and adaptive arms of the immune system (Reis e Sousa, 2001). Although both macrophages and DC play similar roles in participating in an innate immune response, each cell type has distinct functions. Macrophages are more programmed to recruit other cell types to the inflammatory site, while DC are more effective in developing T cell response (Jang et al., 2008). DC are the only cell type that can take up antigens at inflammatory sites and migrate to the secondary lymphoid tissue to active naive T cells. The activation of DC is characterized by an upregulation in the expression of costimulatory molecules and by the production of inflammatory cytokines (Banchereau and Steinman, 1998; Mellman and Steinman, 2001; Revy et al., 2001; Steinman and Hemmi, 2006). Both of these signals as well as antigen presentation are required for an optimal T cell response.
Toll-like receptors (TLRs) are pattern recognition receptors that act as sensors for conserved microbial components. TLRs are part of the innate immune system but their involvement exerts important consequences on the ensuing adaptive host response (Barton and Medzhitov, 2002; O'Neill, 2006; O'Neill et al., 2003; Takeda and Akira, 2005). Expression of TLRs is detected in different host cells, including DC. Several microbial components have been identified as ligands for specific TLRs. The lipopolysaccharide (LPS) from enteric bacteria is a well-characterized TLR4 agonist. TLR2 can heterodimerize with TLR1 or TLR6 and recognize triacylated or diacylated lipoproteins, respectively (Hajjar et al., 2001). Double and single stranded RNA and DNA are agonists of TLR3, 7 or 8 and 9, respectively. Once DC are activated via TLRs, recruitment of adaptor molecules and phosphorylation events take place, leading to the activation of several signaling pathways that results in the expression of gene products encoding costimulatory molecules, inflammatory mediators and cytokines. Two independent TLR signaling pathways have been well characterized, a MyD88 dependent pathway, which is utilized by all TLRs known except TLR3 and a MyD88 independent pathway (TRIF dependent), which is utilized by TLR3 and TLR4. Studies have suggested that signaling via the MyD88 dependent pathway usually mediates a T helper type 1 (Th-1) response, while a Th-2 response is induced when the adaptor molecule MyD88 is absent, although this is not always the case (Kaisho et al., 2002; Schnare et al., 2001).
Both LPS and fimbriae from P. gingivalis have been shown to signal through TLR2 (Asai et al., 2005; Asai et al., 2001; Hajishengallis et al., 2006; Hirschfeld et al., 2000; Pulendran et al., 2001); however, no studies have determined whether TLRs are involved in HagB stimulation of DC. Since some bacterial and viral hemagglutinins have been shown to induce an immune response by stimulation through TLR signaling pathway (Banus et al., 2008; Bieback et al., 2002) and because HagB is a major virulence factor of P. gingivalis, it is important to determine its effect on DC and to understand the immune interaction between TLR signaling and HagB as this process can influence the outcome of the immune response. In the current study, we set out to dissect the requirement for TLRs and adaptor molecules as well as the signaling pathways influencing the activation of DC by HagB. The acquired knowledge will be valuable in the future development of protective vaccines or therapeutics against infection with the periodontal pathogen P. gingivalis.
2. Material and Methods
2.1. Mice
C57BL/6 wild type (WT), TLR2−/−, TLR4−/−, MyD88−/− and TRIF Lps2 mice were bred and maintained in an environmentally controlled, pathogen-free animal facility at the University of Alabama at Birmingham. The original TLR2−/− , TLR4−/− and MyD88−/−breeding pairs were obtained under a Materials Transfer Agreement from Dr. Shizuo Akira (Osaka University, Osaka, Japan). The original TRIFps breeding pairs were obtained from the Jackson Laboratories (Bar Harbor, ME). The CD14−/− mice were a kind gift from Dr. John Kearney at this same institution. Female mice were 7–10 weeks of age when used in the studies. All experiments were done according to the guidelines of the National Institutes of Health. Protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
2.2. Purification of rHagB
rHagB was purified as previously described (Yang et al., 2002; Zhang et al., 2003, 2004) with some modifications. Briefly, rHagB was purified from the soluble fraction of the lysate of Eschericia coli JM109 expressing the hagB gene under the control of a lac Z promotor. The cultures were induced with 1 mM isopropyl β-D-thiogalactoside (IPTG) for 4 h. The soluble fraction of the bacterial lysate was initially denatured using 6 M urea prior to application to a His-bind resin column (Novagen, Madison, WI), according to the manufacturer’s instructions. However, prior to elution, the bound rHagB was washed with 0.5% sodium deoxycholate in binding buffer in order to eliminate any contaminating LPS bound to the protein. This method reduced the endotoxin level by > 90% as determined by the Limulus amebocyte Lysate (LAL) assay (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) (data not shown). The eluted rHagB was then dialyzed against 0.5 M NaCl plus 20 mM HEPES solution, concentrated using a centriprep spin filtration system (Amicon, Millipore Corporation, Bedford, MA) and then passed through 0.2 µ,M HT Tuffryn® low protein binding, non-pyrogenic membrane Acrodisc® syringe filters (Pall corporation, Ann Arbor, MI) to sterilize. The purity of rHagB was confirmed by SDS-PAGE and by Western blot probed with Penta.His™ HRP Conjugate kit (Qiagen Inc., Valencia, CA, USA) or rabbit anti-HagB antibody (a kind gift from Dr. Ann Progulske-Fox at University of Florida, Gainesville). The concentration of rHagB was determined using the bicinchoinic acid (BCA) protein determination assay (Pierce, Rockford, IL). The final preparation of rHagB contained a negligible amount of endotoxin (0.0016 ng/µg rHagB protein), as determined by the LAL assay.
2.3. Generation of dendritic cells
Bone marrow-derived dendritic cells (DC) were generated as previously described (Inaba et al., 1992, 1998). Briefly, the femurs and tibias of mice were flushed with ice cold PBS to remove the bone marrow. Erythrocytes were lysed using M-Lyse buffer (R&D Systems, Minneapolis, MN) and washed cells were suspended in RMPI-1640 media supplemented with 10% heat-inactivated fetal calf serum, pencillin (50 U/ml), streptomycin (50 µg/ml), L-glutamine (2 mM), β-mercaptoethanol (50 µM), sodium pyruvate (1 mM), sodium bicarbonate (1.5 mg/ml) and HEPES (25 mM). The bone marrow cells were cultured in 24-well plates at a density of 106 cells/ml/well and incubated at 37°C in a 7.5% CO2 environment. Recombinant GM-CSF (R&D Systems, Minneapolis, MN) was added to the cultures at a final concentration of 20 ng/ml. Culture media and GM-CSF were replaced on days 2 and 4. Additional culture media with GM-CSF were added on day 6 and cells were harvested on day 7. This protocol routinely yielded > 80% CD11c+ cells as determined by flow cytometry.
2.4. Dendritic cells stimulation
DC (2×105/well) were cultured in 96-well plates in supplemented RPMI-1640 culture media at 37°C in a humidified 7.5% CO2 incubator. Cultures were stimulated with various concentrations of rHagB (10, 20 or 40 µg/ml, see Results) or with E. coli K12 LPS (100 ng/ml) (InvivoGen, San Diego, CA) as a positive control. Unstimulated cultures served as the negative control. DC were harvested from one set of cultures following 16 h of stimulation for assessment of the level of expression of costimulatory molecules by flow cytometry. To determine cytokine production by ELISA, culture supernatants were harvested from a second set of cultures at 24 h post stimulation.
To assess the involvement of different signaling pathways in cell activation, DC were cultured in 24-well plates (2.5×106 /well) and incubated with rHagB (40 µg/ml) for 10, 30, 60 or 120 min or with E. coli LPS (100 ng/ml) for 60 min in a humidified 7.5% CO2 incubator at 37°C.
The role of specific cell signaling molecules involved in the cytokine response to rHagB was determined by culturing DC (2×105/well) in 96-well plates for 2 h at 37°C with one of the following inhibitor (10 µM); U0126 [an inhibitor for ERK1/2 phosphorylation that acts by inhibiting the kinase activity of MEK (Favata et al., 1998)], InSolution™ SB 203580 [an inhibitor that blocks p38 kinase activity, but not its phosphorylation (Tong et al., 1997)], SN50 Cell-Permeable Inhibitor Peptide [an inhibitor of the nuclear translocation of NF-κB (Lin et al., 1995)], InSolution™ GSK3 Inhibitor IX [a reversible ATP competitive inhibitor of GSK3α/β (Meijer et al., 2003)] (Calbiochem Biosciences Inc., LaJolla, CA) followed by stimulation with rHagB (40 µg/ml). Both non-stimulated and non-treated cells served as controls. Culture supernatants were harvested 24 h after stimulation and the levels of cytokines were analyzed as described below.
To ensure that trace amount of endotoxin did not contribute to observed responses, our rHagB preparation was further subjected to the effects of boiling for 30 min, proteinase K (5µg/ml) digestion at 37°C for 2 h (Fermentas Inc., Glen Burnie, MD, USA) and then boiled for 5 min to ensure proteinase K degradation, or polymyxin B sulphate (PMB) (10 µg/ml) (Sigma-Aldrich, St. Louis, MO, USA) for 15 min at RT. These preparations and appropriate controls were then used to stimulate DC and the culture supernatants were harvested after 24 h and assessed for TNF-α levels by ELISA. To determine whether these treatments caused degradation of rHagB, equivalent amounts of untreated and treated rHagB samples were analyzed by SDS-PAGE on a 12.5% Tris-HCl gel followed by (a) staining the gel with Gelcode® blue stain reagent (Pierce, Rockford, IL) or (b) electrotransferring the proteins onto immobilon-P transfer membranes (Millipore, Bedford, MA) that were incubated with Penta.HisTM HRP Conjugate kit (Qiagen Inc., Valencia, CA, USA), followed by detection of the bands using ECL Western blotting detection reagents (Amersham Bioscience, England).
2.5. Cytokine ELISA and flow cytometry
Culture supernatants were assessed for the levels of TNF-α, IL-6 and IL-23 (p19/p40) (eBioscience, San Diego, CA), IL-10 and IL-12p40 (R&D Systems), and IFN-β (PBL InterferonSource, Piscataway, NJ) by ELISA, according to the manufacturers’ instructions. For detection of the expression of costimulatory molecules, DC were harvested and stained with fluorescent-labeled antibodies against CD11c, CD80, CD86 and CD40 or appropriate isotype controls (eBioscience) in PBS buffer supplemented with 2% bovine serum albumin and 0.1% sodium azide for 40 min on ice. Cells were washed twice with buffer. Samples were acquired using a FACSCaliber (BD Bioscience, San Jose, CA) and analyzed using CellQuest software (BD Bioscience).
2.6. Preparation of whole cell lysates and Western blot analysis
Following stimulation, DC were harvested at various times (see Results), washed twice with cold PBS, and then lysed for 10 min on ice in radioimmunoprecipitation assay lysis buffer (Upstate Biotechnology, Lake Placid, NY) supplemented with 1 mM phenylmethylsulphonyl flouride, 1 mM Na3VO4, 1 mM NaF, protease inhibitor cocktail tablets (Complete, Mini, EDTA-free, Roche Applied Science, Indianapolis, IN, USA) and 5 µM microcystin-LR (Alexis Biochemicals, San Diego, USA). The cell lysates were then transferred to tubes, incubated for an additional 20 min on ice, and then centrifuged (15,000 rpm) for 15 min at 4ºC. The supernatants were collected and stored at −20°C until analyzed.
For Western blot analysis, equivalent amounts of protein from whole cell lysates were analyzed by SDS-PAGE on a 12.5% Tris-HCl gel followed by electrotransfer onto immobilon-P transfer membranes (Millipore, Bedford, MA). The membranes were then incubated with specific antibodies to the phosphorylated form of p38 (Thr180/Tyr182), ERK1/2 (p44/42, Thr202/Tyr204), SAPK/JNK (Thr183/Tyr185), CREB (Ser133), NF-κB p65 (Ser536), GSK3α/β (Ser21/9), Akt (Ser473), IκBα (Ser32) or IRF-3 (Ser396) (Cell Signaling Technology, Beverly, MA). To detect equal loading of samples, blots were also probed with antibodies to either total p38, GSK3β or IRF-3 (Cell signaling Technology, Beverly, MA). Bands were detected by using a HRP-linked anti-rabbit IgG antibody followed by ECL Western blotting detection reagents (Amersham Bioscience, England).
2.7. Statistical analysis
The data were subjected to an unpaired ANOVA, followed by post-hoc analysis with the Tukey-Kramer multiple comparison test using the GraphPad InStat Version 3.0a (GraphPad Software, San Diego, CA). Differences between groups were considered significant at the level of P < 0.05.
3. Results
3.1. Activation of dendritic cells by rHagB
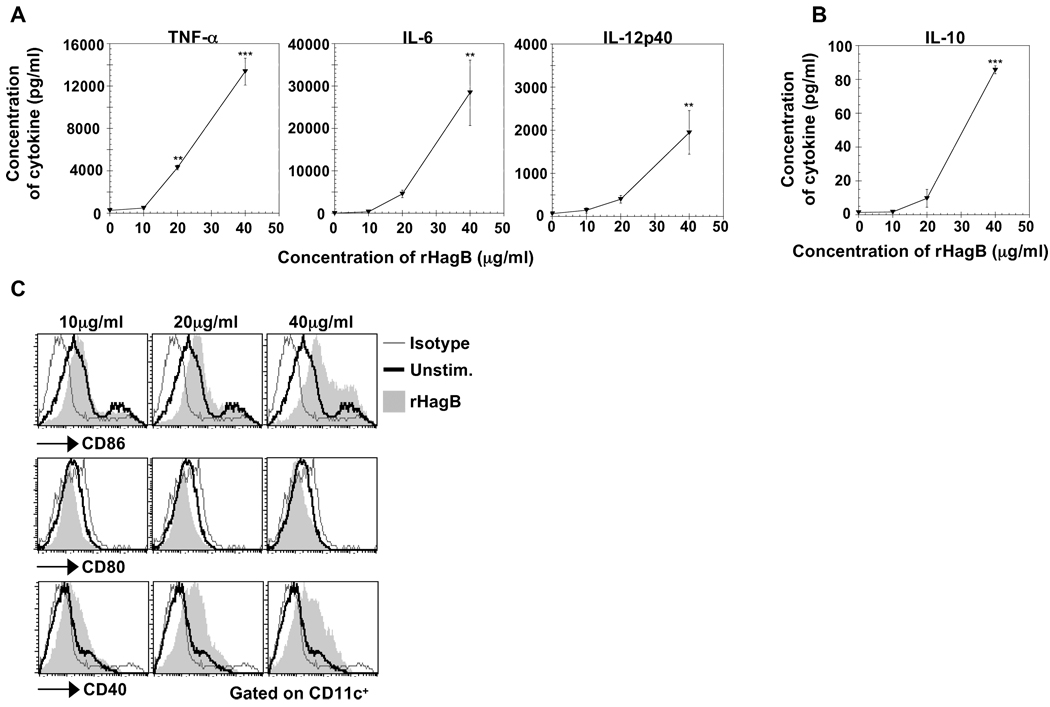
Activation of DC is the initial step in mediating an adaptive immune response since it provides signals for naive T cells to proliferate and differentiate. Therefore, to determine if rHagB can activate DC, bone marrow-derived DC from C57BL/6 mice (WT) were stimulated with 10, 20 or 40 µg/ml of rHagB for 24 h, and culture supernatants were assessed for cytokine production by ELISA. A dose-dependent production of the proinflammatory cytokines TNF-α, IL-6 and IL-12p40 was observed following stimulation of DC with rHagB (Fig. 1A). Since the p40 subunit is common to both IL-12 and IL-23 cytokines (Oppmann et al., 2000), we next assessed the supernatants for the presence of IL-23p19. No IL-23p19 was observed (data not shown) indicating that the p40 was likely due to IL-12. The anti-inflammatory cytokine IL-10 was also induced in a dose-dependent manner (Fig. 1B); however, the quantity of IL-10 produced was relatively low compared to the pro-inflammatory cytokines, indicating that rHagB induced mainly a pro-inflammatory response by DC.
Fig. 1.
Stimulation of DC with rHagB results in the production of cytokines and the upregulation of costimulatory molecules. Bone marrow-derived DC (2×105) from WT mice were either unstimulated (0 µg/ml; negative control) or stimulated with 10, 20 or 40 µg/ml rHagB. Culture supernatants were harvested 24 h post-stimulation and assessed for the levels of the pro-inflammatory cytokines TNF-α, IL-6 and IL-12p40 (A) and the anti-inflammatory cytokine IL-10 (B) by ELISA. Results are expressed as the mean ± standard error of triplicate cultures from one of three independent experiments. ***, ** Significant differences at P < 0.001 and P < 0.01, respectively, compared to unstimulated cultures. (C) DC (2×105) from WT mice were stimulated with 10, 20 and 40 µg/ml rHagB for 16 h (shaded histograms) or left unstimulated as negative controls (thick lines). Cells were harvested and stained with fluorescent-labeled antibodies against CD11c, CD80, CD86, CD40 or matched isotype controls (thin lines). Histogram plots were gated on CD11c+ cells. Results represent one of three independent experiments.
In addition to cytokine production, rHagB induced the upregulation of the costimulatory molecules CD86 and CD40 in a dose-dependent fashion (Fig. 1C). No upregulation of CD80 was detected, which was in agreement with previous findings showing that the HagB specific antibody response is not altered in CD80−/− mice (Zhang et al., 2004). These results suggested that CD80 does not play a role in responses towards rHagB. Since 40 µg/ml of rHagB induced the optimal DC activation, this concentration was used in the subsequent experiments.
3.2. Signaling pathways involved in rHagB activation of DC
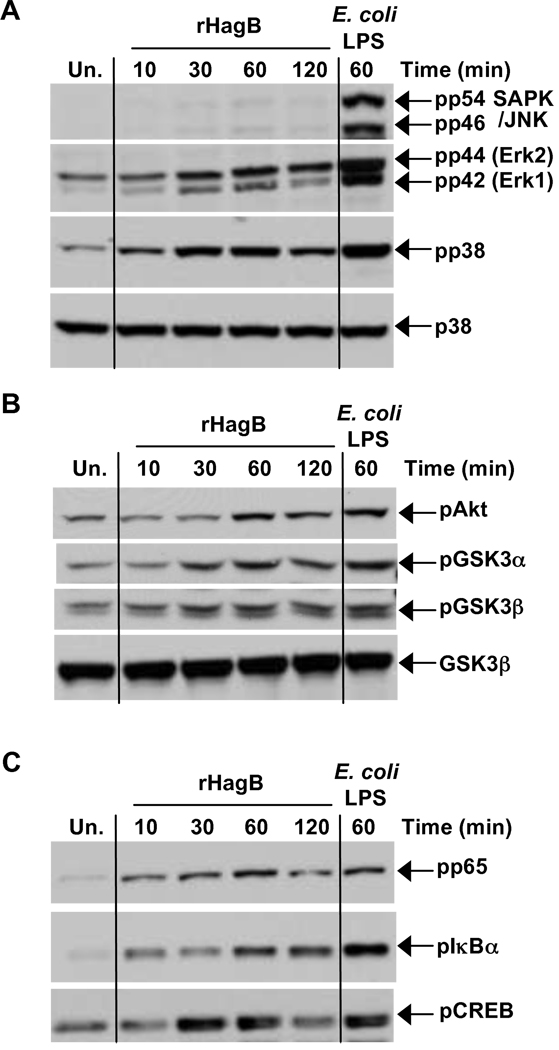
Activation of DC by microbial products involves the participation of signaling molecules and transcription factors. Therefore, to delineate the signaling pathways involved in rHagB activation of DC, we determined whether this activation involved MAP kinases and/or Akt/GSK3 pathways. We found that rHagB phosphorylates p38, ERK1/2, Akt and GSK3α/β but not JNK MAP kinase (Fig. 2A and 2B). We also observed phosphorylation of the transcription factor CREB (Fig. 2C), a downstream target of p38, ERK1/2 or Akt (Deak et al., 1998; Du and Montminy, 1998; Kato et al., 2007; Xing et al., 1998) and of NF-κB p65 and IκBα (Fig. 2C). While phosphorylation of p38, ERK1/2 and CREB peaked at 30 min, Akt, GSKα/β, NF-κB p65 and IκBα phosphorylation was more pronounced at 60 min, indicating that following rHagB stimulation of DC, MAP kinase activation occurs earlier than activation of Akt/GSK3 or NF-κB.
Fig. 2.
Signaling pathways activated following rHagB stimulation of DC. DC were stimulated with 40 µg/ml rHagB for 10, 30, 60 or 120 min. Following stimulation, cells were lysed and whole cell lysates were assessed for phosphorylation of (A) JNK, ERK1/2 and p38, (B) Akt and GSK3α/β and (C) NF-κBp65, IκBα and CREB by Western blot. Total p38 (A) and GSK3β (B) were used as loading controls. Unstimulated DC (far left lane) or DC stimulated with 100 ng/ml E. coli K12 LPS for 60 min (far right lane) were used as controls. Results represent one of three independent experiments.
3.3. Signaling pathways involved in rHagB induced cytokine response
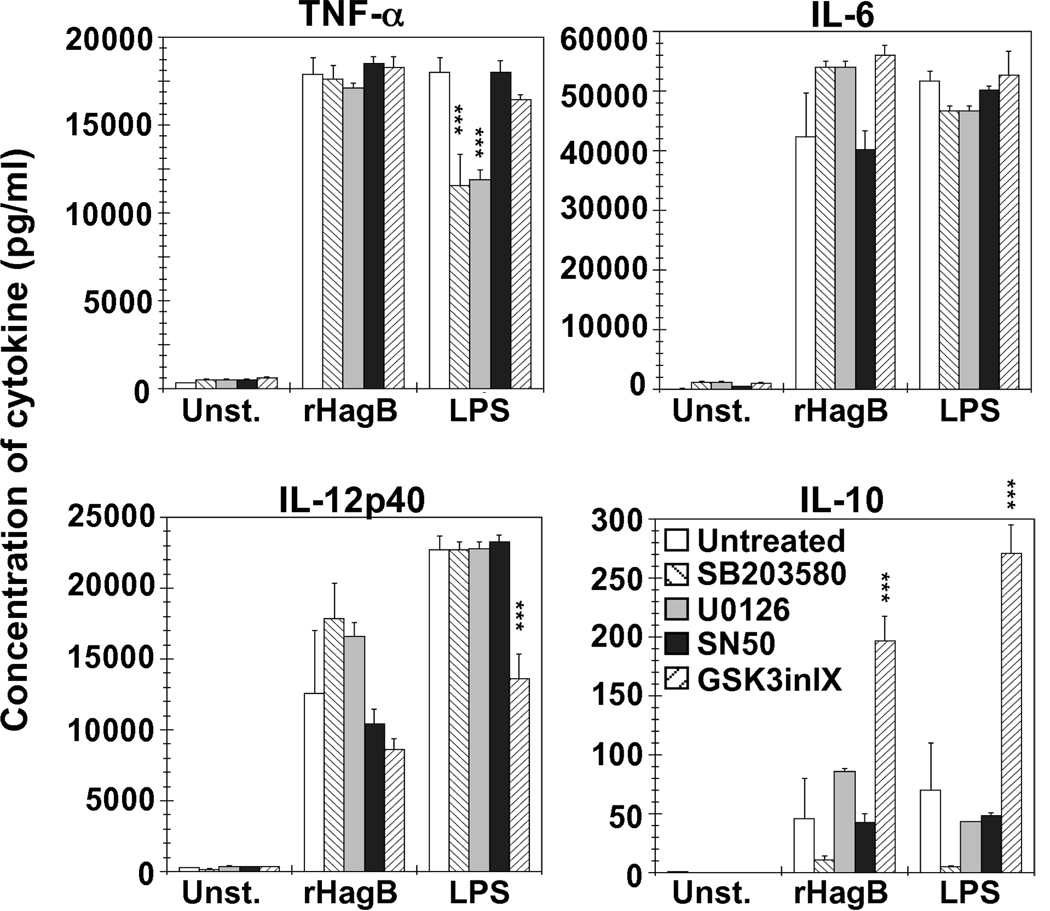
We next determined the involvement of various signaling molecules in cytokine production by rHagB stimulated DC using specific inhibitors of p38, ERK1/2, NF-κB and GSK3. DC were pre-incubated with each specific inhibitor, stimulated with rHagB and supernatants were then assessed for cytokine production.
Pre-incubation of DC with SB203580, an inhibitor of p38, had no effect on the production of TNF-α or IL-6, but showed a slight increase in IL-12p40 and a decrease in IL-10, although the differences were not significant (Fig. 3). Conversely, when DC were stimulated with LPS, inhibition of p38 activity resulted in a significant decrease in TNF-α production (~ 35.9%). Inhibition of ERK1/2 by U0126 did not have a significant effect on the cytokines induced by rHagB stimulation, whereas a 33% decrease was seen in the amount of TNF-α produced by LPS stimulated DC as compared to non-inhibited cells.
Fig. 3.
Cytokine production by DC stimulated with rHagB is dependent on more than one signaling pathway, while GSK3 signaling regulates IL-10. DC (2×105) from WT mice were treated with 10 µM of U0126 (left striped bar), SB203580 (grey bar), NF-κB SN50 (black bar), or GSK-3 Inhibitor IX (right striped bar) (ERK1/2, p38, NF-κB, or GSK3 specific inhibitors, respectively) for 2 h. Untreated cells were used as a negative control (white bars). The cultures were then stimulated with 40 µg/ml rHagB, 100 ng/ml E. coli K12 LPS, or left unstimulated. Culture supernatants were harvested 24 h post-stimulation and assessed for the production of TNF-α, IL-6, IL-12p40 and IL-10 by ELISA. Results are expressed as the mean ± standard error of triplicate cultures from one of two independent experiments. *** Significant differences at P < 0.001, compared to untreated cultures stimulated with rHagB or LPS.
Blocking nuclear translocation of NF-κB with 10 µM SN50 peptide did not affect cytokine production by rHagB or LPS stimulated DC (Fig. 3). However, a severe abrogation of cytokine production was observed, ranging from ~ 81 to 95% depending on the cytokine assessed, with 25 µM of the SN50 peptide (data not shown). Abrogation of cytokine production was also seen with SN50 peptide in LPS stimulated DC cultures with a reduction ranging from ~ 25 and 53% (data not shown).
While inhibition of GSK3α/β did not affect TNF-α or IL-6 production by either rHagB or LPS stimulated DC, it strongly influenced IL-10 production, resulting in a ~ 400% (~ 3.3 fold) and ~ 390% (~ 2.9 fold) increase in rHagB and LPS stimulated DC, respectively (Fig. 3). The increase in IL-10 was accompanied by ~ 31% and 40% decrease in IL-12p40 production in cultures stimulated with rHagB and LPS, respectively. These results are in agreement with those of others demonstrating that inhibition of GSK3 signaling results in an increase in IL-10 and a decrease in IL-12p40 production (Martin et al., 2005; Ohtani et al., 2008; Rodionova et al., 2007). Overall, the differential regulation of cytokine production by rHagB and LPS suggests that each antigen exerts its unique influence on the different signaling pathways that ultimately lead to cytokine production.
3.4. Activity of rHagB and LPS contamination
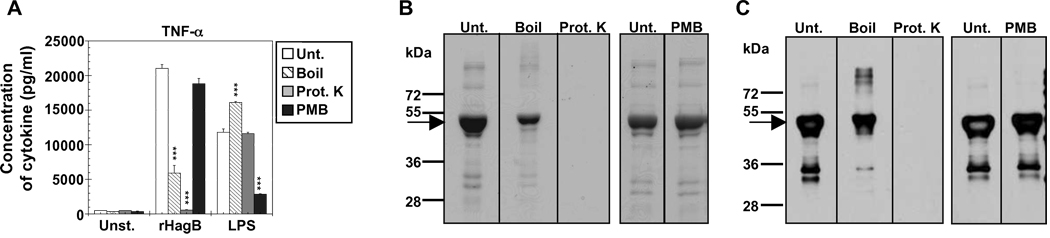
Since we purified rHagB from a lysate of E. coli expressing the hagB gene, it was imperative to rule out that any stimulatory effect seen was not due to LPS contamination. The results of the Limulus amebocyte lysate reaction assay revealed a concentration of approximately 0.0016 ng of endotoxin/µg of rHagB protein. When this amount of LPS was used to stimulate DC cultures, no cytokine production was seen (data not shown). To further ensure that the amount of endotoxin detected had no effect, rHagB was boiled for 30 min, degraded with proteinase K for 2 h or treated with polymyxin B (PMB) for 15 min. A significant decrease (~ 71%) in TNF- α cytokine production was detected when rHagB was boiled prior to stimulation (Fig. 4A). Treatment of rHagB with proteinase K resulted in a complete abolishment of the cytokine response, whereas treatment of rHagB with PMB had no effect on the response (Fig. 4A). Conversely, pretreatment of LPS with PMB prior to stimulation of DC abrogated the cytokine response (~ 75% decrease), whereas boiling or proteinase K treatment resulted in an increase or no effect on the stimulatory activity of LPS, respectively (Fig. 4A). Analysis of the rHagB preparation by SDS-PAGE and Western blot after the various treatments revealed partial degradation and/or dimerization and complete degradation of rHagB (running at ~ 49 kDa) by boiling or proteinase K treatment, respectively (Fig. 4B and 4C). As expected, PMB treatment had no effect on rHagB as compared to the untreated rHagB preparation. These results demonstrate that the stimulatory activity of rHagB was not due to LPS contamination, but rather due to the activity of the protein itself.
Fig. 4.
Activity of rHagB is not due to endotoxin contamination. (A) DC from WT mice (2×105 ) were stimulated with 40 µg/ml rHagB, 100 ng/ml E. coli K12 LPS, or left unstimulated. Prior to stimulation, samples were untreated (white bars), boiled for 30 min (striped bars) or treated with proteinase K (grey bars) or polymyxin B (PMB) (black bars). Culture supernatants were harvested 24 h post-stimulation with rHagB or LPS and assessed for TNF-α production. Results are expressed as the mean ± standard error of triplicate cultures from one of three independent experiments. *** Significant differences at P < 0.001 compared to untreated cultures stimulated with rHagB or LPS. (B) Stained SDS-PAGE and (C) Western blot probed with specific HRP conjugated antibody against Penta.His for equivalent amounts of untreated and treated rHagB protein samples. Arrows represent rHagB protein band that runs at ~ 49 kDa. Results represent one of three independent experiments.
3.5. Requirement of TLR4, MyD88 and TRIF for cytokine production by rHagB
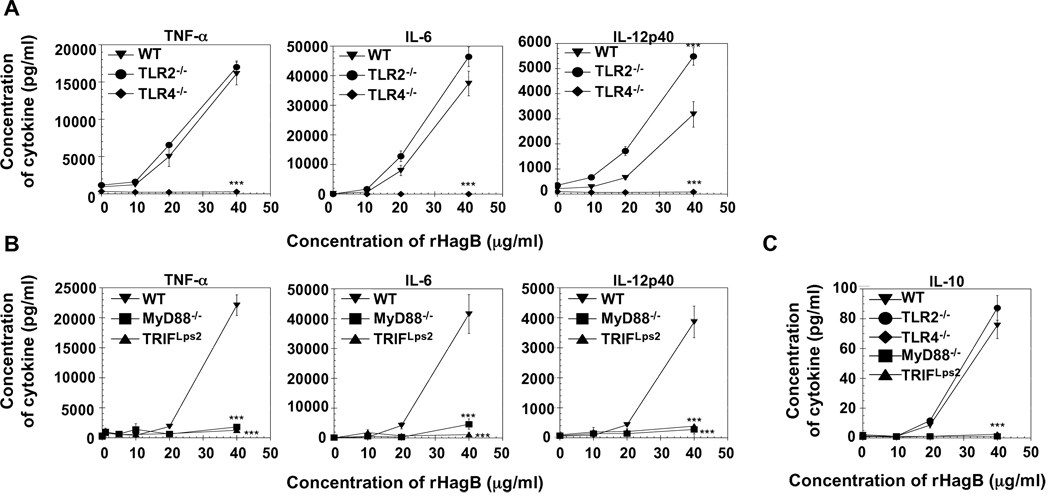
Next, we determined whether the activation induced by rHagB is mediated via TLR signaling. The requirement of TLR2 and TLR4 was assessed by determining the production of cytokines by DC derived from WT, TLR2−/− and TLR4−/− mice following stimulation with rHagB. DC from TLR2−/− and WT mice produced similar levels of TNF-α and IL-6 (Fig. 5A). However, no cytokine production was detected in cultures derived from TLR4−/− mice, indicating that rHagB is a TLR4 agonist. Since TLR4 signaling can occur through the MyD88 and TRIF pathways, we examined cytokine production by MyD88−/− and TRIFLps2 DC. DC lacking either MyD88 or TRIF showed a significant reduction in pro-inflammatory cytokine production (Fig. 5B), thus indicating HagB signals through both the MyD88 and TRIF pathways.
Fig. 5.
Cytokine production by rHagB activated DC is mediated through TLR4 signaling and requires both MyD88 and TRIF. DC (2×105) from WT, TLR2−/− and TLR4−/− mice (A, C) or WT, MyD88−/− and TRIF Lps2 mice (B, C) were stimulated with 10, 20 or 40 µg/ml rHagB for 24 h. Culture supernatants were then harvested and assessed for TNF-α, IL-6, IL-12p40 (A, B) and IL-10 production (C) by ELISA. Results are expressed as the mean ± standard error of triplicate cultures from one of four independent experiments. *** Significant differences at P < 0.001 compared to WT cultures stimulated with rHagB.
Interestingly, DC from TLR2−/− mice produced significantly higher levels of IL-12p40 than WT DC following stimulation with rHagB (Fig. 5A). Since IL-10 and IL-12 are counter-regulatory cytokines, we compared the levels of IL-10 in DC derived from all deficient mice examined to determine if a difference in IL-10 levels could explain the observed differences in the levels of IL-12p40. Our results show that the levels of IL-10 produced by WT and TLR2−/− cells were not significantly different (Fig. 5C). Therefore, the increase in IL-12p40 was not due to a decrease in IL-10 production by TLR2−/− DC. No IL-10 was detected in rHagB stimulated DC cultures derived from TLR4−/−, MyD88−/−and TRIFLps2 mice.
3.6. Activation of p38, ERK1/2 MAPK, the Akt/GSK3 pathway and NF-κB by rHagB stimulated DC is dependent on TLR4
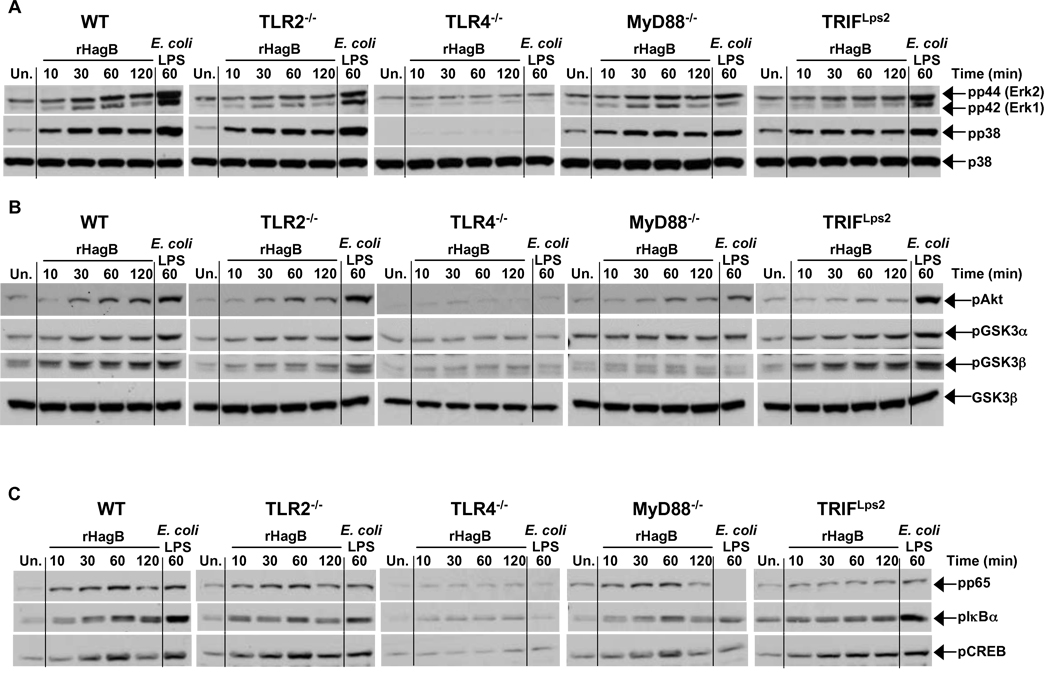
Since activation of MAP kinase, GSK3 and NF-κB results in the expression of numerous genes involved in the activation of DC and optimal cytokine responses, we next assessed if the abolished cytokine response observed in TLR4−/−, MyD88−/− and TRIFLps2 DC was due to a lack in the activation of one of the above signaling molecules following rHagB stimulation. Therefore, we first compared the phosphorylation of p38 and ERK1/2 from lysates of TLR2−/−, TLR4−/−, MyD88−/− and TRIFLps2 DC to that of WT. The phosphorylation kinetics for p38 and ERK1/2 in TLR2−/− and WT DC lysates were similar and reached maximum at 30 min post rHagB stimulation (Fig. 6A). No phosphorylation was detected in the lysates of DC from TLR4−/− mice. Interestingly, lysates from MyD88−/− and TRIFLps2 DC showed similar kinetics to lysates from WT DC. Similar results were seen with the phosphorylation of Akt (Fig. 6B) and CREB (Fig. 6C). Taken together, these results indicate that a defect in the activation of p38, ERK1/2, Akt or CREB did not account for the diminished cytokine response observed by rHagB stimulated MyD88 and TRIF deficient DC.
Fig. 6.
Phosphorylation of p38, ERK1/2, Akt/GSK3 and activation of NF-κB and CREB by rHagB stimulated DC is dependent on TLR4. DC from WT, TLR2−/−, TLR4−/−, MyD88−/− and TRIFLps2 mice were stimulated with 40 µg/ml rHagB for 10, 30, 60 or 120 min. Following stimulation, cells were lysed and whole cell lysates were assessed for (A) ERK1/2 and p38 and (B) Akt and GSK3α/β (C) p65 NF-κB (Ser 536), IκBα and CREB phosphorylation by Western blot. Total p38 (A) and GSK3β (B) were used as loading controls. Unstimulated DC (far left lanes) or DC stimulated with 100 ng/ml E. coli K12 LPS for 60 min (far right lanes) were used as controls. Results are representative of two independent experiments.
However, upon examining GSK3α/β, no phosphorylation was detected in lysates from TLR4−/− and MyD88−/− DC, while phosphorylation kinetics in lysates from TRIFLps2 DC were comparable to that from WT DC, suggesting that rHagB mediated inhibition of GSK3 was dependent on MyD88 pathway rather than the TRIF pathway (Fig. 6B). In addition, a decrease in the phosphorylation of GSK3β was also seen in lysates from TLR2−/− DC, suggesting that the increase in IL-12p40 production detected in cultures from TLR2−/− DC may be due to an increase in the activity of GSK3, as it has been previously shown that GSK3 induces IL-12 production (Martin et al., 2005; Ohtani et al., 2008; Rodionova et al., 2007).
No activation of NF-κB was observed in lysates of TLR4−/− DC, whereas activation kinetics of NF-κB were similar in DC lysates derived from WT, TLR2−/− and MyD88−/− mice, where maximum phosphorylation of IkBα and p65 occurred at 60 min following stimulation with rHagB (Fig. 6C). However, the TRIFLps2 cell lysates showed a weak phosphorylation at 10 min following stimulation, which was sustained for the 2 h experimental period (Fig. 6C). Moreover, no degradation of IκBα was observed (data not shown). These results suggest that TRIF and MyD88 may have differential roles in the activation of NF-κB in HagB stimulated DC. These results further prove that the suboptimal cytokine response in MyD88−/− DC was not due to a defect in the activation of NF-κB.
3.7. Upregulation of CD86 is dependent on TLR4 and TRIF signaling, while CD40 expression requires both MyD88 and TRIF in rHagB activated DC
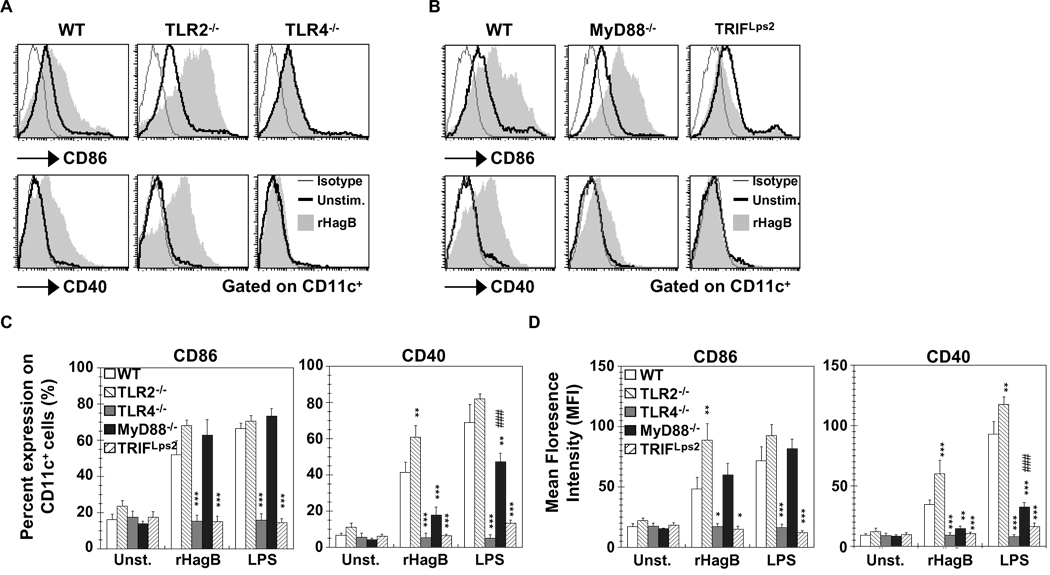
Since upregulation of costimulatory molecules is a necessary secondary signal for an effective DC-T cell response, we next assessed the upregulation of CD86 and CD40 on rHagB stimulated DC derived from TLR2−/− and TLR4−/− mice. Upregulation of CD86 and CD40 was seen with TLR2−/− DC, while no upregulation was noticed with TLR4−/−DC (Fig. 7A). Similar to TLR4−/− DC, TRIFLps2 DC showed no upregulation of CD86 and CD40 (Fig. 7B, C and D). This finding was in agreement with previous data showing that the adaptor molecule TRIF is required for upregulation of costimulatory molecules (Hoebe et al., 2003b; Kaisho et al., 2001; Yamamoto et al., 2003). Stimulation of DC with LPS resulted in a similar response. Interestingly, although MyD88−/− DC stimulated with rHagB showed a similar level of CD86 expression as WT DC, the expression of CD40 following stimulation was markedly reduced and was not significantly different from unstimulated MyD88−/− DC (Fig. 7B, C and D). Moreover, we also noticed a reduction in the level of CD40 expression when MyD88−/− DC were stimulated with LPS, although the level of expression was still significantly higher than that seen with unstimulated MyD88−/− DC. These results suggest that the MyD88 adaptor molecule, or a molecule downstream of MyD88, might be playing a major role in regulating CD40, but not CD86 expression.
Fig. 7.
Upregulation of CD86 and CD40 with rHagB stimulation is mediated through TLR4 and TRIF signaling. DC (2×105) from WT, TLR2−/− and TLR4−/− mice (A, C and D), or WT, MyD88−/− and TRIF Lps2 mice (B, C and D) were stimulated with 40 µg/ml rHagB (shaded histograms), 100 ng/ml E. coli K12 LPS or left unstimulated (thick lines) for 16 h. Cells were harvested and stained with fluorescent-labeled antibodies to CD11c, CD86, CD40 or matched isotype controls (thin lines). Histogram plots were gated on CD11c+ cells. Data in (C) and (D) are expressed as the percentage of CD86 and CD40 positive cells and the mean florescence on CD11c+, respectively. Results are expressed as the mean ± standard error of four independent experiments. ***, ** and * Significant differences at P < 0.001, P < 0.01 and P < 0.05, respectively, compared to WT cultures stimulated with rHagB or LPS. ### Significant differences at P < 0.001 compared to unstimulated MyD88−/− cultures.
3.8. Activation of IRF-3 requires TLR4 and TRIF, while production of IFN-β is also dependent on MyD88 in rHagB activated DC
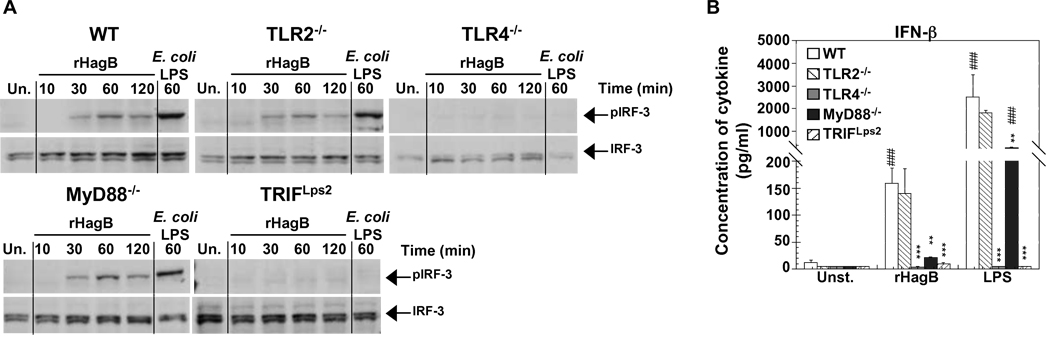
In the case of TLR4 signaling, the activation of IRF-3, production of IFN-β and upregulation of costimulatory molecules occur downstream of the TRIF dependent pathway (Hoebe et al., 2003a,b; Hoshino et al., 2002; Weighardt et al., 2004; Yamamoto et al., 2003). Since TLR4 and TRIF are involved in rHagB activation of DC, we next determined the role of the IRF-3 transcription factor and of IFN-β in the activation of DC, and whether the reduced expression of CD40 in MyD88−/− DC was in part due to a defect in the production of IFN-β. rHagB induced IRF-3 activation in lysates from DC derived from TLR2−/− and MyD88−/− mice, but not from those derived from TLR4−/− or TRIFLps2 mice (Fig. 8A). This was consistent with the results from others (Hoebe et al., 2003a,b; Hoshino et al., 2002; Weighardt et al., 2004; Yamamoto et al., 2003), as well as to our own data with LPS stimulated DC (Fig. 8A). Furthermore, this response correlated with IFN-β production, as no IFN-β was detected in cultures of DC derived from TLR4−/− or TRIFLps2 mice (Fig. 8B). However, IFN-β production was almost abolished in MyD88−/− DC cultures stimulated with rHagB and was not significantly different from that seen with unstimulated MyD88−/− DC cultures. This decrease in IFN-β production correlated with the low upregulation of CD40 in MyD88−/− DC following rHagB stimulation (Fig. 7C and 7D). Since LPS stimulated MyD88−/− DC cultures expressed higher levels of IFN- β production (Fig. 8B) and of CD40 expression than unstimulated MyD88−/− DC cultures (Fig. 7C and 7D), these results suggest that an insufficient production of IFN-β may account for the low CD40 upregulation seen in MyD88−/− DC.
Fig. 8.
Phosphorylation of IRF-3 and production of IFN-β by rHagB stimulated DC is dependent on TLR4 signaling and the adaptor molecule TRIF. (A) DC from WT, TLR2−/−, TLR4−/−, MyD88−/− and TRIF Lps2 mice were stimulated with 40 µg/ml rHagB for 10, 30, 60 or 120 min. Following stimulation, cells were lysed and whole cell lysates were tested for phosphorylation of IRF-3 by Western blot. Total IRF-3 was used as a loading control. Unstimulated DC (far left lanes) or DC stimulated with 100 ng/ml E. coli K12 LPS for 60 min (far right lanes) were used as controls. Results are representative of two independent experiments. (B) DC (2×105) were stimulated with 40 µg/ml rHagB, 100 ng/ml of E. coli K12 LPS or left untreated. Culture supernatants were harvested 24 h post-stimulation and assayed for IFN-β production by ELISA. Results are expressed as the mean ± standard error of duplicate cultures from three independent experiments. *** and ** Significant differences at P < 0.001 and P < 0.01, respectively, compared to WT cultures stimulated with rHagB or LPS. ### Significant differences at P < 0.001 compared to control unstimulated cultures.
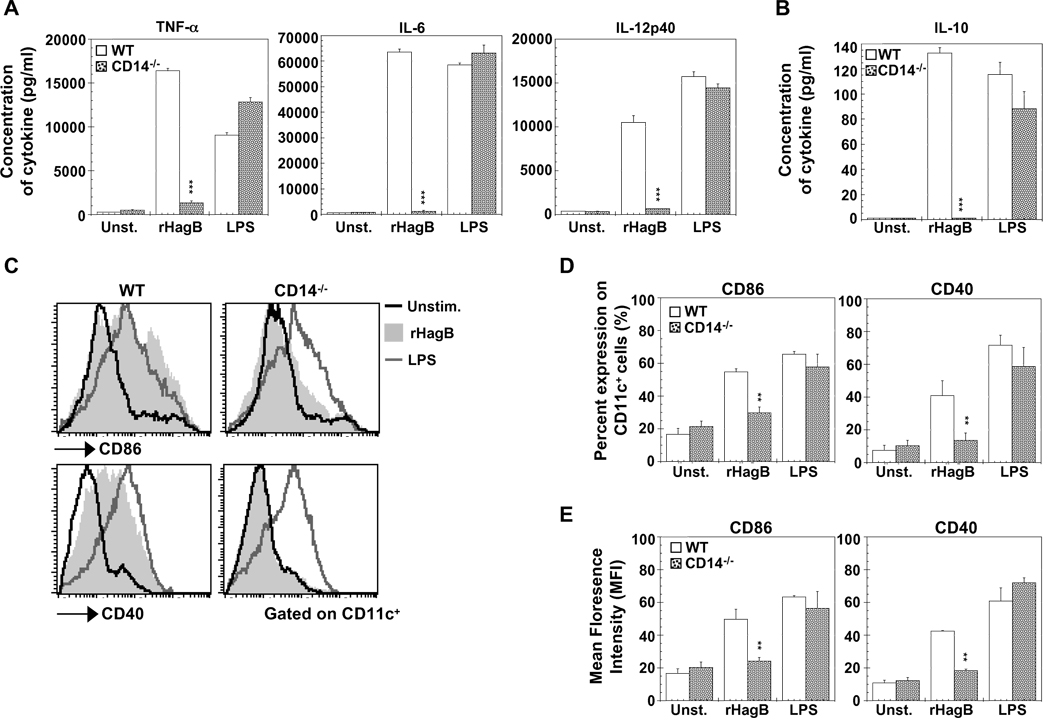
3.9. CD14 is necessary for the immunostimulatory activity of HagB
CD14 is a glycosyl-phosphatidylinositol GPI-anchored cell membrane receptor that upon LPS stimulation is thought to deliver the LPS-LPS binding protein complex to TLR4 (Akashi et al., 2003; Wright et al., 1990). Therefore, we next wanted to assess the role of CD14 in the ability of rHagB to stimulate DC. Stimulation of DC from CD14−/− mice with rHagB failed to induce the production of the pro-inflammatory cytokines TNF-α, IL-6 and IL-12p40 (Fig. 9A), or the production of the anti-inflammatory cytokine IL-10 (Fig. 9B). Moreover, stimulation of CD14−/− DC with rHagB did not result in an upregulation in the expression of costimulatory molecules CD86 or CD40 (Fig. 9C, D and E) or in the phosphorylation of p38, ERK1/2, CREB, Akt, GSK3 or the activation of NF-κB or IRF-3 (data not shown). In addition, the production of IFN-β was completely abolished (data not shown). These results demonstrate that CD14 is absolutely required for rHagB activation of DC. This result was in contrast to that seen with LPS stimulated DC where a comparable level of activation was seen with DC derived from WT and CD14−/− mice, in terms of cytokine production (Fig. 9A and B), costimulatory molecules (Fig. 9C, D and E) or activation of the indicated signaling molecules (data not shown). In our experiments, the media used was supplemented with serum, which is rich with soluble CD14 (Bazil et al., 1989). Upon stimulation of DC with serum free media, the response of LPS stimulated DC derived from CD14−/− was reduced (data not shown). This agrees with other studies that have proposed that CD14 participates in loading of LPS to the TLR4-MD2 complex (Akashi et al., 2003); however, signal transduction was not absolutely dependent on membrane bound (mCD14), since LPS signaling can still occur in mCD14 independent manner (Gangloff et al., 2005; Perera et al., 1997). These results suggested that, unlike LPS, which can be delivered to TLR4 independently from mCD14, rHagB absolutely requires mCD14 to signal through TLR4.
Fig. 9.
CD14 is required for rHagB to activate DC. DC (2×105 ) from WT (open bars) and CD14−/− (grey dotted bars) mice were stimulated with 40 µg/ml rHagB (shaded histograms), 100 ng/ml of E. coli K12 LPS (grey lines) or left untreated (black lines). (A and B) Culture supernatants were harvested 24 h post-stimulation and assayed for levels of TNF-α, IL-6, IL-12p40 and IL-10 by ELISA. Results are expressed as the mean ± standard error of triplicate cultures from one of three representative experiments. *** Significant differences at P < 0.001 compared to WT cultures stimulated with rHagB. (C, D and E) Cells were harvested 16 h post-stimulation and stained with fluorescent-labeled antibodies against CD11c, CD86 and CD40. Histogram plots were gated on CD11c+ cells (C) and bar graphs represent the percentage expression of CD86 and CD40 on CD11c+ cells (D) or the mean florescence intensity on CD11c+ cells (E). Results are expressed as the mean ± standard error of three independent experiments. ** Significant difference at P < 0.01 compared to WT cultures stimulated with rHagB.
4. Discussion
In the present study, we investigated the immunostimulatory activity of HagB from P. gingivalis on murine bone marrow-derived DC. Our results demonstrate that rHagB is a TLR4 agonist and that mCD14, MyD88 and TRIF are critical participants of the TLR4 signaling complex. Furthermore, rHagB stimulation of DC resulted in the activation of Akt and the p38 and ERK1/2 MAP kinases. Although the signaling pathways implicated in the cytokine response of murine macrophages to rHagB had been previously described (Zhang et al., 2005a), our current results with DC differ from those obtained with macrophages, thus underlying the uniqueness of each cell type (Argueta-Donohue et al., 2008; Jang et al., 2008; Pompei et al., 2007; Siegemund et al., 2007; Werling et al., 2004). The dissimilarities observed were; (i) the relative amount of IL-12p40 and IL-10 produced by each cell type, (ii) the differential requirements of p38, ERK or NF-κB in the production of pro- and anti-inflammatory cytokines, and (iii) the induction of phosphorylated JNK in macrophages, but not in DC. The differences between DC and macrophages in response to rHagB can perhaps be explained by the variability in the engaged signaling mechanism(s). Werling et al. showed that an increase in IL-10 production and variations in MAP kinase activation could be due to differential level of TLR expression on DC and macrophages (Werling et al., 2004). In addition, differences in the nuclear translocation of NF-κB could explain the variations among the two cell types (Argueta-Donohue et al., 2008).
TLR2 and TLR4 are expressed in periodontal tissues of patients with periodontitis, hence, implicating their potential participation in the inflammatory response to periodontal pathogens (Mori et al., 2003). Whereas previous studies have shown the involvement of TLR2 and CD14 in the inflammatory response to P. gingivalis, its LPS and fimbriae components (Asai et al., 2001, 2005; Burns et al., 2006; Hajishengallis et al., 2006; Wang and Ohura, 2002), the role of TLR4 remains unclear since studies have seen similar inflammatory responses, bone resorption and delay in bacterial clearance in TLR4−/− and wild type mice after P. gingivalis infection (Burns et al., 2006). However, it is critical to understand that the response to the whole pathogen can differ from that mediated by its antigenic components. In this regard, each antigen, depending on its own specificity and its interaction with the host cell, will initiate a unique response. Evidently, our results highlight this phenomenon that is not exclusive of P. gingivalis, as attested by investigations with Mycobacterium and Francisella species (Asai et al., 2001, 2005; Ashtekar et al., 2008; Bulut et al., 2005; Burns et al., 2006; Cole et al., 2007; Duenas et al., 2006; Hajishengallis et al., 2006; Hong et al., 2007; Katz et al., 2006; Li et al., 2006; Ogawa et al., 2002; Pulendran et al., 2001). Since P. gingivalis seems to induce an immune response mainly through TLR2, the immunodominant antigen of this pathogen could be its LPS or fimbriae, but not HagB.
Previous studies have shown that microbial molecules not structurally related to LPS have the capacity to interact with TLR4 and induce the activation of immune cells (Aosai et al., 2006; Ashtekar et al., 2008; Bulut et al., 2005; Kurt-Jones et al., 2000). We demonstrate in the present study that HagB, like LPS, is a TLR4 agonist; however, the response induced by each of these antigens is not the same. While rHagB stimulation was completely abrogated in CD14−/− DC, LPS signaling was intact since LPS can utilize both mCD14 and sCD14. The complete dependency of rHagB on mCD14 and its inability to utilize sCD14 suggests that mCD14 might participate or even associate with the TLR4 complex to activate DC. This dependency on mCD14 has been shown with P. gingivalis fimbriae, but not with P. gingivalis LPS (Hajishengallis et al., 2006; Wang and Ohura, 2002), suggesting that the difference in the requirements for membrane versus soluble CD14 by an antigen could be due to structural differences. In addition, rHagB, but not LPS, failed to induce the phosphorylation of JNK and the upregulation of the costimulatory molecule CD80. These events can be linked since Lim et al. has shown that LPS induced CD80 upregulation involved the activation of JNK MAP kinase, and that blocking JNK prevents CD80 upregulation (Lim et al., 2005). We also observed a differential regulation of TNF-α production with p38 and ERK1/2 inhibitors in cultures stimulated with rHagB or LPS. Our results differ somewhat from those observed by others regarding LPS responses following ERK1/2 inhibition (Ardeshna et al., 2000; Arrighi et al., 2001; Nakahara et al., 2004); however, the disparities may be due to differences in cell types, dose of inhibitor or type of inhibitor used. The fact that LPS signaling is held as the standard for TLR4 immune responses should not preclude the possibility that other TLR4 agonist molecules will induce a different immune pattern. Indeed, as shown in the present study, two structurally distinct microbial molecules signaling through TLR4 induce a different outcome.
We have demonstrated for the first time that both MyD88 and TRIF are indispensable for the optimal pro- and anti-inflammatory cytokine response by rHagB stimulated DC. The defect in MyD88−/− or TRIFLps2 DC to induce cytokines was not due to a defect in the activation of MAP kinase (Kawai et al., 1999, 2001), although NF-κB may play a partial role in the abrogated cytokine response of TRIFLps2 DC, since its activation was diminished compared to WT DC. Moreover, the phosphorylation kinetics of p38, ERK1/2 and NF-κB were similar in WT and MyD88−/− DC. This is in contrast to the findings of others showing that TLR4 signaling causes the activation of MAP kinases and NF-κB in two phases, an early TRIF dependent phase followed by a late MyD88 dependent phase (Hoebe et al., 2003a; Kawai et al., 2001; Yamamoto et al., 2003). In the case of LPS, the delayed response in MyD88−/− cells was attributed to the time required for the TRIF pathway to initiate IRF-3 activation, followed by TNF-α production, which in turn acts on the TNF receptor to induce a delayed NF-κB activation (Covert et al., 2005). However, the similarity in kinetics between rHagB stimulated WT and MyD88−/−DC suggests that rHagB signaling through MyD88 and TRIF may occur in two independent or simultaneous signaling events. Our results further suggest that while MyD88 and TRIF are of equal importance in HagB signaling through TLR4 for cytokine production, they have different roles in the activation of NF-κB or the inhibition of GSK3, which are more dependent on TRIF and MyD88, respectively. The selective requirement for one adaptor molecule over the other by a TLR4 agonist has been reported previously. Mata-haro et al. showed that the TLR4 agonist monophosphoryl lipid A (a lower toxicity derivative of LPS) preferentially utilized the TRIF dependent pathway (Mata-Haro et al., 2007). Other studies from our laboratory have shown that the induction of IL-6 and IL-12p40 production by F. tularensis DnaK via TLR4 was more dependent on MyD88 than TRIF (Ashtekar et al., 2008).
We have also shown that the upregulation in CD40 expression was completely abolished in stimulated TRIFLps2 DC and that no activation of IRF-3 or production of IFN-β was seen, which is in agreement with findings of others (Hoebe et al., 2003b; Hoshino et al., 2002; Kaisho et al., 2001; Weighardt et al., 2004). However, examination of rHagB or LPS stimulated MyD88−/− DC indicated that rHagB, unlike LPS, did not lead to IFN-β production or upregulation of CD40. Furthermore, even though both rHagB and LPS stimulated MyD88−/− DC showed a reduction in IFN-β levels compared to WT DC (~ 80%), LPS stimulated MyD88−/− DC were still capable of CD40 upregulation. These results suggest that the activation of IRF-3 alone is not sufficient for the induction of CD40 upregulation in MyD88−/− DC, but rather the amount of IFN-β available to the cell since LPS stimulated MyD88−/− DC were producing significantly more IFN-β than unstimulated controls. Hoshino et al. showed that type I interferon signaling through STAT-1 is required for the upregulation of CD40 and that in the absence of the IFN-α/β receptor, CD40 expression was abrogated (Hoshino et al., 2002). Therefore, our results indicate that IFN-β production is partially dependent on MyD88. Whether the requirement for MyD88 to produce IFN-β is a direct or an indirect effect through the activation of another pathway is still to be determined.
In conclusion, we have demonstrated for the first time that HagB is a TLR4 agonist and that the signaling events and the resulting response induced by this antigen are unique and different from that exerted by LPS. Our results extend the findings of others showing the importance of studying different cell types and the uniqueness of their response to a particular antigen. Lastly, we have expanded the current knowledge regarding the P. gingivalis HagB antigen, providing information that will aid in designing better therapeutic and preventive methods against periodontitis and the systemic consequences of this disease process.
Acknowledgements
We thank Wayne Duck for his help with HagB protein purification protocol modification. This work was supported by grants DE14215 from the National Institute of Dental and Craniofacial Research (J.K.) and D.E.G was supported in part by Training Grant T32 AI-007051 from National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, Kosugi A, Miyake K. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosai F, Rodriguez Pena MS, Mun HS, Fang H, Mitsunaga T, Norose K, Kang HK, Bae YS, Yano A. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4. Cell Stress Chaperones. 2006;11:13–22. doi: 10.1379/CSC-138R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- Argueta-Donohue J, Carrillo N, Valdes-Reyes L, Zentella A, Aguirre-Garcia M, Becker I, Gutierrez-Kobeh L. Leishmania mexicana: participation of NF-kappaB in the differential production of IL-12 in dendritic cells and monocytes induced by lipophosphoglycan (LPG) Exp. Parasitol. 2008;120:1–9. doi: 10.1016/j.exppara.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- Asai Y, Hashimoto M, Fletcher HM, Miyake K, Akira S, Ogawa T. Lipopolysaccharide preparation extracted from Porphyromonas gingivalis lipoprotein-deficient mutant shows a marked decrease in toll-like receptor 2-mediated signaling. Infect. Immun. 2005;73:2157–2163. doi: 10.1128/IAI.73.4.2157-2163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai Y, Ohyama Y, Gen K, Ogawa T. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect. Immun. 2001;69:7387–7395. doi: 10.1128/IAI.69.12.7387-7395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtekar AR, Zhang P, Katz J, Deivanayagam CC, Rallabhandi P, Vogel SN, Michalek SM. TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis. J. Leukoc. Biol. 2008;84:1434–1446. doi: 10.1189/jlb.0308215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banus S, Stenger RM, Gremmer ER, Dormans JA, Mooi FR, Kimman TG, Vandebriel RJ. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 2008;9:21. doi: 10.1186/1471-2172-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R. Control of adaptive immune responses by Tolllike receptors. Curr. Opin. Immunol. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- Bazil V, Baudys M, Hilgert I, Stefanova I, Low MG, Zbrozek J, Horejsi V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol. Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J. Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J. Biol. Chem. 2005;280:20961–20967. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche AC, Michalek SM, Vogel SN. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect. Immun. 2007;75:4127–4137. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- Craig RG. Inflammation, cardiovascular disease and destructive periodontal diseases The evolving role of the dental profession. N. Y. State Dent. J. 2004;70:22–26. [PubMed] [Google Scholar]
- Craig RG, Kotanko P, Kamer AR, Levin NW. Periodontal diseases--a modifiable source of systemic inflammation for the end-stage renal disease patient on haemodialysis therapy? Nephrol. Dial. Transplant. 2007;22:312–315. doi: 10.1093/ndt/gfl604. [DOI] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. Embo J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Duenas AI, Aceves M, Orduna A, Diaz R, Sanchez Crespo M, Garcia-Rodriguez C. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E coli LPS. Int. Immunol. 2006;18:785–795. doi: 10.1093/intimm/dxl015. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect. Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek DM, Progulske-Fox A, Brown TA. Systemic and mucosal immune responses in mice orally immunized with avirulent Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin. Infect. Immun. 1994;62:1652–1657. doi: 10.1128/iai.62.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek DM, Progulske-Fox A, Whitlock J, Brown TA. Isolation and characterization of a cloned Porphyromonas gingivalis hemagglutinin from an avirulent strain of Salmonella typhimurium. Infect. Immun. 1993;61:940–946. doi: 10.1128/iai.61.3.940-946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Gangloff SC, Zahringer U, Blondin C, Guenounou M, Silver J, Goyert SM. Influence of CD14 on ligand interactions between lipopolysaccharide and its receptor complex. J. Immunol. 2005;175:3940–3945. doi: 10.4049/jimmunol.175.6.3940. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama S, Ratti P, Schifferle RE, Lyle EA, Triantafilou M, Triantafilou K, Yoshimura F. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus.Cell. Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- Hajjar AM, O'Mahony DS, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB. Cutting edge: functional interactions between tolllike receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- Hirose K, Isogai E, Mizugai H, Ueda I. Adhesion of Porphyromonas gingivalis fimbriae to human gingival cell line Ca9-22. Oral Microbiol. Immunol. 1996;11:402–406. doi: 10.1111/j.1399-302x.1996.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003a;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trifindependent pathways. Nat. Immunol. 2003b;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- Hong KJ, Wickstrum JR, Yeh HW, Parmely MJ. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect. Immun. 2007;75:5338–5345. doi: 10.1128/IAI.00561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 2002;14:1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G. Isolation of Dendritic cells. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevac EM, Strober W, editors. Current Protocols in Immunology. John Wiley and Sons, Inc; 1998. pp. 3.7.1–3.7.15. [Google Scholar]
- Jang S, Uzelac A, Salgame P. Distinct chemokine and cytokine gene expression pattern of murine dendritic cells and macrophages in response to Mycobacterium tuberculosis infection. J. Leukoc. Biol. 2008;84:1264–1270. doi: 10.1189/jlb.1107742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int. Immunol. 2002;14:695–700. doi: 10.1093/intimm/dxf039. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 2001;166:5688–5694. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- Kato S, Ding J, Du K. Differential activation of CREB by Akt1 and Akt2. Biochem. Biophys. Res. Commun. 2007;354:1061–1066. doi: 10.1016/j.bbrc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- Katz J, Black KP, Michalek SM. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect. Immun. 1999;67:4352–4359. doi: 10.1128/iai.67.9.4352-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect. Immun. 2000;68:1441–1449. doi: 10.1128/iai.68.3.1441-1449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect. Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- Kuramitsu HK. Proteases of Porphyromonas gingivalis: what don't they do? Oral Microbiol. Immunol. 1998;13:263–270. doi: 10.1111/j.1399-302x.1998.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine G, Progulske-Fox A. Duplication and differential expression of hemagglutinin genes in Porphyromonas gingivalis. Oral. Microbiol. Immunol. 1996;11:65–78. doi: 10.1111/j.1399-302x.1996.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Li H, Nookala S, Bina XR, Bina JE, Re F. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J. Leukoc. Biol. 2006;80:766–773. doi: 10.1189/jlb.0406294. [DOI] [PubMed] [Google Scholar]
- Lim W, Gee K, Mishra S, Kumar A. Regulation of B7.1 costimulatory molecule is mediated by the IFN regulatory factor-7 through the activation of JNK in lipopolysaccharide-stimulated human monocytic cells. J. Immunol. 2005;175:5690–5700. doi: 10.4049/jimmunol.175.9.5690. [DOI] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Mori Y, Yoshimura A, Ukai T, Lien E, Espevik T, Hara Y. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol. Immunol. 2003;18:54–58. doi: 10.1034/j.1399-302x.2003.180109.x. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Uchi H, Urabe K, Chen Q, Furue M, Moroi Y. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int. Immunol. 2004;16:1701–1709. doi: 10.1093/intimm/dxh171. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger's Principles of Biochemistry. 4th edition. New York, NY: WH Freeman; 2005. [Google Scholar]
- O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–290. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 1996;67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Asai Y, Hashimoto M, Takeuchi O, Kurita T, Yoshikai Y, Miyake K, Akira S. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int. Immunol. 2002;14:1325–1332. doi: 10.1093/intimm/dxf097. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksy A, Banbula A, Bugno M, Travis J, Potempa J. Proteolysis of interleukin-6 receptor (IL-6R) by Porphyromonas gingivalis cysteine proteinases (gingipains) inhibits interleukin-6-mediated cell activation. Microb. Pathog. 2002;32:173–181. doi: 10.1006/mpat.2002.0491. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Perera PY, Vogel SN, Detore GR, Haziot A, Goyert SM. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J. Immunol. 1997;158:4422–4429. [PubMed] [Google Scholar]
- Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A, Hickman SP, Salgame P. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J. Immunol. 2007;178:5192–5199. doi: 10.4049/jimmunol.178.8.5192. [DOI] [PubMed] [Google Scholar]
- Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology. 2000;24:153–192. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- Progulske-Fox A, Tumwasorn S, Holt SC. The expression and function of a Bacteroides gingivalis hemagglutinin gene in Escherichia coli. Oral Microbiol. Immunol. 1989;4:121–131. doi: 10.1111/j.1399-302x.1989.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Progulske-Fox A, Tumwasorn S, Lepine G, Whitlock J, Savett D, Ferretti JJ, Banas JA. The cloning, expression and sequence analysis of a second Porphyromonas gingivalis gene that codes for a protein involved in hemagglutination. Oral Microbiol. Immunol. 1995;10:311–318. doi: 10.1111/j.1399-302x.1995.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells as sensors of infection. Immunity. 2001;14:495–498. doi: 10.1016/s1074-7613(01)00136-4. [DOI] [PubMed] [Google Scholar]
- Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat. Immunol. 2001;2:925–931. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- Rodionova E, Conzelmann M, Maraskovsky E, Hess M, Kirsch M, Giese T, Ho AD, Zoller M, Dreger P, Luft T. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood. 2007;109:1584–1592. doi: 10.1182/blood-2006-06-028951. [DOI] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Siegemund S, Schutze N, Freudenberg MA, Lutz MB, Straubinger RK, Alber G. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella enteritidis. Immunobiology. 2007;212:739–750. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J. Clin. Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tong L, Pav S, White DM, Rogers S, Crane KM, Cywin CL, Brown ML, Pargellis CA. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat. Struct. Biol. 1997;4:311–316. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- Wang PL, Ohura K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit. Rev. Oral Biol. Med. 2002;13:132–142. doi: 10.1177/154411130201300204. [DOI] [PubMed] [Google Scholar]
- Weighardt H, Jusek G, Mages J, Lang R, Hoebe K, Beutler B, Holzmann B. Identification of a TLR4- and TRIF-dependent activation program of dendritic cells. Eur. J. Immunol. 2004;34:558–564. doi: 10.1002/eji.200324714. [DOI] [PubMed] [Google Scholar]
- Werling D, Hope JC, Howard CJ, Jungi TW. Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists. Immunology. 2004;111:41–52. doi: 10.1111/j.1365-2567.2003.01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell. Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yang QB, Martin M, Michalek SM, Katz J. Mechanisms of monophosphoryl lipid A augmentation of host responses to recombinant HagB from Porphyromonas gingivalis. Infect. Immun. 2002;70:3557–3565. doi: 10.1128/IAI.70.7.3557-3565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dong H, Kashket S, Duncan MJ. IL-8 degradation by Porphyromonas gingivalis proteases. Microb. Pathog. 1999;26:275–280. doi: 10.1006/mpat.1998.0277. [DOI] [PubMed] [Google Scholar]
- Zhang P, Martin M, Michalek SM, Katz J. Role of mitogen-activated protein kinases and NF-kappaB in the regulation of proinflammatory and anti-inflammatory cytokines by Porphyromonas gingivalis hemagglutinin B. Infect. Immun. 2005a;73:3990–3998. doi: 10.1128/IAI.73.7.3990-3998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Martin M, Yang QB, Michalek SM, Katz J. Role of B7 costimulatory molecules in immune responses and T-helper cell differentiation in response to recombinant HagB from Porphyromonas gingivalis. Infect. Immun. 2004;72:637–644. doi: 10.1128/IAI.72.2.637-644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yang QB, Balkovetz DF, Lewis JP, Clements JD, Michalek SM, Katz J. Effectiveness of the B subunit of cholera toxin in potentiating immune responses to the recombinant hemagglutinin/adhesin domain of the gingipain Kgp from Porphyromonas gingivalis. Vaccine. 2005b;23:4734–4744. doi: 10.1016/j.vaccine.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang P, Yang QB, Marciani DJ, Martin M, Clements JD, Michalek SM, Katz J. Effectiveness of the quillaja saponin semi-synthetic analog GPI-0100 in potentiating mucosal and systemic responses to recombinant HagB from Porphyromonas gingivalis. Vaccine. 2003;21:4459–4471. doi: 10.1016/s0264-410x(03)00438-9. [DOI] [PubMed] [Google Scholar]