Abstract
N-(2-mercaptopropionyl)glycine (tiopronin) monolayer-protected silver particles were partially displaced by single-stranded oligonucleotides through ligand exchanges. The oligonucleotide-displaced particles could be hybridized with complementary fluorophore-labeled oligonucleotides. Both the oligonucleotide-displaced and hybridized particles could be aggregated by electrostatic interactions with salt in buffer solution, and the aggregates displayed enhanced luminescence from fluorophores. This result suggests the possible application of surface-enhanced fluorescence from metallic nanoparticle aggregation for DNA detection.
Keywords: Organic monolayer-protected silver nanoparticle, Tiopronin, Ligand exchange, Oligonucleotide, Absorbance, Luminescence, Surface-enhanced fluorescence, Hybridization, Aggregation
Metallic nanoparticles have gotten a great deal of attention for their potential application to biological and chemical sensing [1-7]. They are expected to be useful for DNA analysis by coupling with complementary sequences on two particles [8-11]. Hence, the preparation of stable DNA-labeled metallic nanoparticle, which can be hybridized with complementary DNA, has become an important work of research.
The majority of recent research has focused on metallic colloids [8-11]. We are interested in organic monolayer-protected metallic nanoparticles because of their chemical stability and multiple functionalization [12]. Such nanoparticles can be made from gold, silver, platinum, copper, or alloys [12-16]. The average core size (diameter = 1-10 nm) could be controlled by a mole ratio of thiol ligand/metallic salt in the preparation [8].
In contrast to gold, which only displays quenching for excited chromophores, silver exhibits an enhanced luminescence when fluorophores are localized at a distance of 6-10 nm from the surface of silver islands [18,19]. This process can be adapted to improve the sensitivity of biological detection [20,21]. In this study, N-(2-mercaptopropionyl)glycine (abbreviated as tiopronin)-coated silver particles, which display a high chemical stability and solubility in water [22,23], were prepared and then displaced by thiolate single-stranded oligonucleotides through a ligand exchange reaction. The oligonucleotide-displaced particles were further coupled with fluorophore-labeled complementary oligonucleotides by hybridization (Scheme 1). Plasmon wavelengths of metallic particles were sensitive to the ligands on the metallic core for both displacement and hybridization. Besides the absorbance spectra, the luminescence spectra could be used to monitor the coupling of the oligonucleotide when they were fluorophore-labeled. The particles were found to aggregate in KCl buffer solution because of the electrostatic interactions between the cation K+ and negative-charged oligonucleotides attached on the metallic cores. The aggregation of particles resulted in absorbance and luminescence spectral changes, which might be utilized in high-sensitivity DNA sensing research.
Scheme 1.

Preparation, displacement by thiolate oligonucleotides, and hybridization with fluorescein-labeled complementary oligonucleotides of tiopronin-monolayer protected silver nanoparticle.
Experimental
All reagents (Aldrich) and spectroscopic grade solvents (Fisher, Aldrich) were used as received. Oligonucleotides (Oligonucleotide 1, HS-3′-TCCACACACCACTGGCCATCTTC-5′, and Oligonucleotide 2, AGGTGTGTGG-fluorescein-3′-TGACCGGTAGAAG-5′) were synthesized by the Biopolymer Laboratory of University of Maryland at Baltimore. The RC dialysis membrane (MWCO1 50,000) was available from Spectrum Laboratories. Nanopure water (>18.0 MΩ) purified using Millipore Milli-Q gradient system, was used in experiments.
Tiopronin-coated silver nanoparticles (Scheme 1, particle 1) were prepared using a modified Brust reaction with a mole ratio of tiopronin/silver nitrate = 1/1 in methanol [12,24]. In a typical reaction, AgNO3 (0.68 g, 4.0 mmol) and tiopronin (0.65 g, 4.0 mmol) were codissolved in 50 mL methanol. NaBH4 (1.5 g, 40 mmol) in 20 mL methanol was added with rapid stirring at 0 °C. The black suspension was stirred for an additional 1 h, isolated on a Millipore porous Wlter (FHLC04700, 0.45 μm), and then washed with an excess amount of methanol.
The oligonucleotide-displaced particles (Scheme 1, particle 2) were obtained by stirring a 10 mM KCl aqueous solution of particle 1 and oligonucleotide 1 for 72 h at room temperature. The mole ratio of oligonucleotide to tiopronin was 1:5 [13-16]. Uncapped oligonucleotides and any released organic components were removed by dialysis against water. The oligonucleotide 1 capped on particle 2 was hybridized by oligonucleotide 2 in a 10 mM KCl solution for 24 h and then purified by dialysis against water.
Absorption spectra were monitored with a Hewlett Packard 8453 spectrophotometer. Luminescence spectra were recorded with a Cary Eclipse fluorescence spectrophotometer. Transmission electron microscope (TEM) images was taken with a side-entry Philips electron microscope operated at 120 keV on standard carbon-coated (200-300 Å) Formvar films on copper grids (200 mesh).
Results and discussion
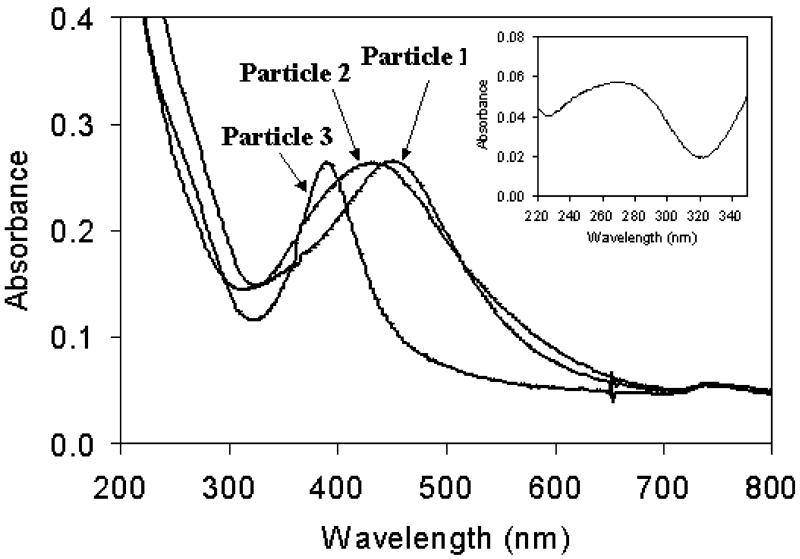
Tiopronin-protected silver particles (Scheme 1, particle 1) displayed a poly-dispersion of particles with an average diameter of about 5 nm. This corresponded to the composition of Ag1082(Tio)453 prior to ligand exchange (Fig. 1A) [17]. Because of an excess amount of sodium borohydride in the preparation, the carboxylic groups on ligands were deprotonated. Particle 1 displayed a plasmon absorbance wavelength at 453 nm in water (Fig. 2).
Fig. 1.
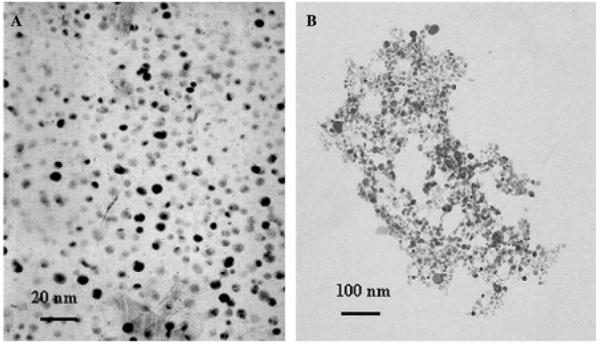
Transmission electron microscope (TEM) images of (A) particle 1 and (B) aggregates of particle 2 in 20 mM KCl buffer solution.
Fig. 2.
Absorbance spectra of particles 1, 2, and 3 in water with maxima of absorption wavelengths at 453, 426, and 396 nm, respectively. Inset represents the spectrum from attached oligonucleotides obtained by subtraction of spectrum of particle 1 from spectrum of particle 2.
In our previous research, oligonucleotide 1 has been shown to assemble well on metallic surfaces and oligonucleotides 1 and 2 to hybridize successfully each other [17]. So oligonucleotides 1 and 2 were selected for this research. The ligands on particle 1 were partially displaced by thiolate oligonucleotides 1 through ligand exchanges to yield the oligonucleotide-displaced particles (Scheme 1, particle 2), and the plasmon absorbance of particle 2 was blue-shifted to 426 nm (Fig. 2). When we subtracted the absorbance of particle 2 from particle 1, the residual spectrum displayed a maximum wavelength at 275 nm (inset of Fig. 2), close to that of the uncapped oligonucleotide 1 (absorbance wavelength = 260 nm). An absorbance increase beyond 320 nm was probably due to light scattering from the particles. The number of oligonucleotides on each particle was estimated to be 2.2 from the extinction coeYcients of the particle (6.5 × 105 cm-1 M-1) and uncapped oligonucleotide (2.6 × 105 cm-1 M-1) at their absorbance maxima in water. Although the ligand exchanges were believed to occur by a 1:1 molar ratio for organic thiols, this was unclear in this case because the oligonucleotides were much larger than the organic ligands. The displacement number of oligonucleotide was much less in this exchange than in that of regular organic thiol [13], which could be ascribed to slow rate of reaction as well as great steric hindrance in the displacement of oligonucleotides. Particle 2 displayed a TEM image with an average diameter of 5 nm, indicating that the displacement did not change the core size of the particle.
Although the metallic core of particle 2 was coated by a negative-charged ligand monolayer, the capped oligonucleotide 1 were still able to hybridize with the complementary oligonucleotide 2 in 10 mM KCl buffer solution to produce the hybridized particle (Scheme 1, particle 3). Particle 3 displayed a blue-shifted plasmon absorbance at 396 nm (Fig. 2), which was due to the effect of salt in the hybridization. The hybridization was also verified by luminescence spectrum from the fluorophore capped on particle 3. Upon excitation at 450 nm, particle 3 showed a maximum of luminescence at 527 nm, 6 nm red-shift from the uncapped oligonucleotide 2 (Fig. 3). Particle 3 had an analogous TEM image to particle 2, indicating that hybridization could not alter the properties of metallic core.
Fig. 3.
Fluorescence spectra of uncapped oligonucleotide and particle 3 in water upon excitation at 450 nm.
Some particles (2 or 3) were observed to precipitate from solution in the hybridization when the KCl concentration was above 20 mM in buffer solution. However, particle 1 was not precipitated even in 0.5 M KCl buffer solution and displayed a similar absorbance spectrum in 20 mM KCl buffer and in water; while both particles 2 and 3 displayed broadening of plasmon bands and simultaneous shoulder risings near 600 nm in 20 mM KCl buffer. This indicated that particles 2 and 3 were aggregated in 20 mM KCl buffer (Fig. 4), and the aggregation happened via electrostatic interactions of K+ cations with oligonucleotides (Scheme 2). The flexible oligonucleotide strands were expected to coil up by crosslinking with cations, resulting in compact aggregation of particles. Although the negative-charged surfaces of particles were also regarded to contribute to such aggregation, the lack of any precipitate of particle 1 in a buffer solution with high salt concentration indicated that the electrostatic interaction between the oligonucleotide and K+ cation should be the only principal factor. Particles 2 and 3 were found to take 24 h to reach precipitation equilibrium in 20 mM KCl buffer. The TEM image also showed that the particle 2 was aggregated in 20 mM KCl buffer solution (Fig. 1B). Particle 3 displayed an analogous TEM image after aggregation as particle 2.
Fig. 4.
Absorbance spectra of particles 1, 2, and 3 in 0.5 M KCl buffer solution after 1 h for aggregation. Their absorbance maxima are at 450, 425, and 396 nm, respectively.
Scheme 2.

Aggregation models of particles 2 and 3 in KCl buffer solution.
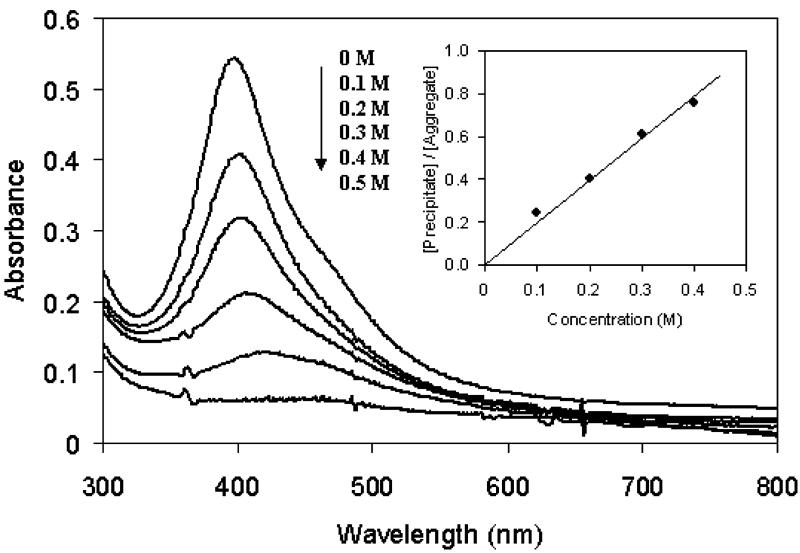
The aggregations of particles 2 and 3 were dependent on the KCl concentration in buffer solution (particle 3 in Fig. 5). The quantity of aggregate grew with an increase of KCl concentration. When the KCl concentration reached 0.5 M, almost all particles were precipitated from the solution. Although the precipitation is a complicated mechanism, it was described as a simple equilibrium shown as
| (1) |
and the equilibrium could be expressed as
| (2) |
where [Precipitate] and [Aggregate] represented the respective concentration of precipitated and dissolved aggregate in buffer solution, and K is an equilibrium constant. A plot of [Precipitate]/[Aggregate] against KCl concentration showed a good linear relation (inset of Fig. 5), and K was estimated to be 2.0 M-1. The plasmon absorbance at 0.5 M KCl was too low to be observed clearly in Fig. 5, but was visible at 456 nm when it was extended. Hence, the plasmon absorbance exhibited a red-shifted wavelength with the KCl concentration from 396 nm in water to 456 nm at 0.5 M. The properties of particle 2 were similar to those of particle 3, and the equilibrium constant was estimated to be 1.8, close to that of particle 3. Most precipitated aggregates (>80%) could be dissociated in water, implying that the electrostatic interaction of salt was the cause of particle aggregation.
Fig. 5.
Dependence of absorbance spectra of particle 3 on the KCl concentration after 24 h incubation. The inset represents dependence of concentration ratio of [Precipitate]/[Aggregate] on the KCl concentration in solution.
Particle 3 displayed a luminescence maximum at 527 nm when excited at 450 nm (Fig. 3), and the luminescence was steadily enhanced with an increase of KCl concentration in buffer solution (Fig. 6) until 20 mM. The intensity was enhanced 2.3-fold in 20 mM KCl buffer solution. The silver surface was able to enhance the brightness, and the enhancement was expected to be dependent on the presence of an enhanced electric field on the surface of particles [18]. Relative to the single particles, the electric fields between the coupling particles were expected to become stronger by overlapping, and the fluorophore in overlapping fields displayed a considerably stronger luminescence enhancement. The quantity of particle 3 aggregates increased with an increase of KCl concentration in buffer solution, corresponding to an increase of surface-enhanced fluorescence until 20 mM KCl.
Fig. 6.
Dependence of luminescence spectra of particle 3 on the KCl concentration (less than 20 mM) in water upon excitation at 450 nm. The inset represents the dependence of luminescence intensity at 523 nm on the KCl concentration.
A further increase of KCl concentration led to a decrease of emission from particles (inset of Fig. 6), which was ascribed to the precipitation of particle 3 in a high KCl concentration buffer solution. By Eq. (2), the concentration ratio of [Precipitate]/[Aggregate] was estimated to be 0.04 at 20 mM KCl concentration. When the KCl concentration reached 100 mM, the [Precipitate]/[Aggregate] was estimated to be 0.18, indicating that 15% of aggregates were precipitated from solution. The precipitated aggregates led to a direct decrease of fluorophore concentration in solution, and there was an obvious decrease in luminescence intensity at high KCl concentrations.
In conclusion, the thiolate single-stranded oligonucleotides were displaced on tiopronin—protected silver nanoparticles by a ligand-exchange reaction. The capped oligonucleotides could be hybridized by complementary oligonucleotides. Both the oligonucleotide-displaced and hybridized particles were aggregated by electrostatic interaction with KCl in water. When the complementary oligonucleotides were fluorescein-labeled, the luminescence was enhanced at low KCl concentrations and then reduced at high KCl concentrations. This paper provided some fundamental facts about displacement of oligonucleotide on the organic monolayer-protected silver particle, hybridization of complementary oligonucleotide on the oligonucleotide-displaced particle, and luminescence enhancement with aggregation of the oligonucleotide-displaced or hybridized particle in KCl buffer solution. This result suggests a possible approach to DNA detection based on the aggregation of metallic nanoparticles bound by the fluorophore-labeled oligonucleotides.
Acknowledgments
This research was supported by a grant from NIH, NCRR, RR-08119, and HG02655 of NIHGRI.
Footnotes
- MWCO
- molecular weight cutoff
- TEM
- transmission electron microscopy
References
- [1].Hayat MA, editor. Colloidal Gold: Principles, Methods, and Applications. Academic Press; San Diego: 1991. [Google Scholar]
- [2].Dai HL, Ho W, editors. Laser Spectroscopy and Photochemistry on Metal Surfaces Parts I and II. World Scientific; Singapore: 1995. [Google Scholar]
- [3].Allara D. In: Characterization of Organic Thin Films. Ulman A, editor. Butterworth-Heinemann; Boston: 1995. Chapter 4. [Google Scholar]
- [4].Ulman A. Ultrathin Organic Films. Academic Press; San Diego: 1991. [Google Scholar]
- [5].Ulman A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- [6].Kamat PV. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B. 2002;106:7729–7744. [Google Scholar]
- [7].He L, Musick MD, Nicewarner SR, Salinas FG, Benkovic SJ, Natan MJ, Keating CD. Colloidal Au-enhanced surface plasmon resonance for ultrasensitive detection of DNA hybridization. J. Am. Chem. Soc. 2000;122:9071–9077. [Google Scholar]
- [8].Nicewarner Pena SR, Raina S, Goodrich GP, Fedoroff NV, Keating CD. Hybridization and enzymatic extension of Au nanoparticle-bound oligonucleotides. J. Am. Chem. Soc. 2002;124:7314–7323. doi: 10.1021/ja0177915. [DOI] [PubMed] [Google Scholar]
- [9].Reynolds RA, III, Mirkin CA, Letsinger RL. Homogeneous, nanoparticle-based quantitative colorimetric detection of oligonucleotides. J. Am. Chem. Soc. 2000;122:3795–3796. [Google Scholar]
- [10].Taton TA, Mucic RC, Mirkin CA, Letsinger RL. The DNA-mediated formation of supramolecular mono- and multilayered nanoparticle structures. J. Am. Chem. Soc. 2000;122:6305–6306. [Google Scholar]
- [11].Templeton AC, Wuelfing WP, Murray RW. Monolayer-protected cluster molecules. Acc. Chem. Res. 2000;33:27–36. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- [12].Ingram RS, Hostetler MJ, Murray RW. Poly-hetero-ω-functionalized alkanethiolate-stabilized gold cluster compounds. J. Am. Chem. Soc. 1997;119:9175–9178. [Google Scholar]
- [13].Templeton AC, Hostetler MJ, Kraft CT, Murray RW. Reactivity of monolayer-protected gold cluster molecules: steric effects. J. Am. Chem. Soc. 1998;120:1906–1911. [Google Scholar]
- [14].Hostetler MJ, Templeton AC, Murray RW. Dynamics of place-exchange reactions on monolayer-protected gold cluster molecules. Langmuir. 1999;15:3782–3789. [Google Scholar]
- [15].Hostetler MJ, Green SJ, Stokes JJ, Murray RW. Monolayers in three dimensions: synthesis and electrochemistry of ω-functionalized alkanethiolate-stabilized gold cluster compounds. J. Am. Chem. Soc. 1996;118:4212–4213. [Google Scholar]
- [16].Hostetler MJ, Wingate JE, Zhong C-J, Harris JE, Vachet RW, Clark MR, Londono JD, Green SJ, Stokes JJ, Wignall GD, Glish GL, Porter MD, Evans ND, Murray RW. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir. 1998;14:17–30. [Google Scholar]
- [17].Malicka J, Gryczynski I, Lakowicz JR. Enhanced emission of highly labeled DNA oligomers near silver metallic surfaces. Anal. Chem. 2003;75:4408–4414. doi: 10.1021/ac020739m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Geddes CD, Cao H, Gryczynski I, Gryczynski Z, Fang JY, Lakowicz JR. metal-enhanced fluorescence (mef) due to silver colloids on a planar surface: potential applications of indocyanine green to in vivo imaging. J. Phys. Chem. A. 2003;107:3443–3449. doi: 10.1021/jp022040q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lakowicz JR, Shen YB, D’Auria S, Malicka J, Fang JY, Gryczynski Z, Gryczynski I. Radiative decay engineering. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal. Biochem. 2002;301:261–277. doi: 10.1006/abio.2001.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lakowicz JR. Radiative decay engineering: biophysical and biomedical applications. Anal. Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang T, Murray RW. Visible luminescence of water-soluble monolayer-protected gold clusters. J. Phys. Chem. B. 2001;105:12498–12502. [Google Scholar]
- [22].Huang T, Murray RW. Quenching of [Ru(bpy)3]2+ fluorescence by binding to Au nanoparticles. Langmuir. 2002;18:7077–7081. [Google Scholar]
- [23].Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman RJ. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid system. Chem. Soc., Chem. Commun. 1994:801–802. [Google Scholar]
- [24].Zhang J, Whitesell JK, Fox MA. Photophysical behavior of variously sized colloidal gold clusters capped with monolayers of an alkylstilbenethiolate. J. Phys. Chem. B. 2003;107:6051–6055. [Google Scholar]