Summary
Background
Previous studies yielded conflicting results regarding the presence of an association between celiac disease (CD) and psychiatric disorders including depression. This association has not been studied in the United States.
Aim
To determine the prevalence of psychiatric and autoimmune disorders in patients with CD in the US compared to control groups.
Methods
In a case control study, the prevalence of psychiatric and autoimmune disorders was compared in 600 CD patients, 200 irritable bowel syndrome (IBS) patients and 200 healthy controls.
Results
The prevalence of depression in CD was 17.2% and was similar to that in IBS (18.5%, P = 0.74) and controls (16.0%, P = 0.79). Among CD patients, type I DM was identified as a significant risk factor for depression (P < 0.01) with 37% of patients with both CD and type I DM having clinical depression.
Conclusion
The prevalence of depression in CD is similar to that in other chronic gastrointestinal diseases and healthy controls. However, there is a markedly elevated risk of depression in patients with both type I DM and CD. Differing rates of type 1 DM among celiac populations may account for disparity in published rates of depression in patients with CD.
Keywords: Celiac disease, Psychiatric disorders, Autoimmune disorders
Introduction
The prevalence of celiac disease (CD) in the general population in the United States (US) is now believed to be approximately 1% 1. The clinical characteristics of this large CD population are still being defined and our perspectives on CD are altering with the changing demographics of those being diagnosed. Along with the many gastrointestinal, nutritional and metabolic consequences of CD, there has been significant concern about increased rates of psychological and psychiatric disorders occurring in association with CD. Although this topic has been studied previously, all prior studies looking at the prevalence of psychiatric disorders in CD, most aimed at depression, anxiety, bipolar disorder, schizophrenia and panic disorder, have been performed in Europe. It is well accepted that cultural and social differences may result in significant alterations in the manifestation of psychological disorders and that reported rates of depression and other psychiatric illnesses differ greatly from region to region. This may partially explain the wide variation in the reported prevalence of depression in CD, between 6% and 57%. Further, some studies have found a significant positive association between CD and depression 2–8 while others did not 9–12. For these reasons, it is important to assess the burden of psychiatric disease in a US celiac population.
Aside from differences between populations, the existing data on the burden of psychological diseases in CD is limited for multiple reasons. First, most studies include small numbers of participants with celiac disease, ranging from 35 to 176. The exception to this is the report by Ludvigsson et al. which included 13,667 CD patients and over 66,000 controls in a study of the risk of subsequent depression after diagnosis of celiac disease 7. Among the small number of studies with more than 100 CD patients, few have used a control population 7, 8, 10, 11 and most have used self-rating questionnaires to diagnose depression 2–6, 8, 9 which may not adequately represent the prevalence of clinical depression.
It is also clear that CD is associated with a variety of co-morbid disorders and, in particular, an increased risk for autoimmune diseases. Ventura et al, for example, showed that 14.8% of CD patients had autoimmune disorders 13. Many studies have shown an association of CD with type I DM 14–17 and with autoimmune thyroid disease 17. There is a suggestion that adherence to a gluten free diet may reduce the risk of development of subsequent autoimmune disorders but studies have shown conflicting results and the effects, if any, of gluten avoidance on the development and course of co-morbid autoimmune diseases remains unclear 18, 19. Nonetheless, it is clear that certain autoimmune disorders, type 1 DM in particular, are associated with an increased risk of depression. For this reason, it may be important to control for the presence of co-morbid auto-immune disorders when evaluating the risk of psychiatric disorders in individuals with celiac disease.
This study examines the prevalence of psychiatric disorders in a large group of subjects with CD in the US and compares the findings to both healthy and disease control groups. The prevalence of type 1 DM, autoimmune thyroid disease and other autoimmune disorders in CD was also determined and this information was used to evaluate the effect that these co-morbid diseases have on the prevalence of psychiatric disorders in CD.
Methods
The study used a case-control design. We have a large database of CD patients which is created using the International Classification of Diseases (ICD) code (579.0). Cases were defined as patients with biopsy proven celiac disease cared for at The Celiac Center at Beth Israel Deaconess Medical Center (BIDMC), Boston, Massachusetts, USA. Individuals without a duodenal biopsy consistent with CD were excluded. Six hundred CD patients were selected randomly from this database. Similarly 200 age and gender matched IBS patients and 200 age and gender matched controls were selected from IBS database and healthy control database respectively. We did not contact any of these patients. Data was gathered solely from electronic medical records. In our medical record, medical problem list and medication list are listed and maintained separately. Thus any patient with a discordant result; e.g. no recorded psychiatric diagnosis but prescribed a psychiatric medication or vice versa, was targeted for review. Charts for which discrepancies could not be resolved were excluded from further analysis.
The diagnosis of IBS was the result of the global assessment of an attending gastroenterologist. We required that all patients be seen on at least two occasions by the same attending physician to ensure that tests prompted by the first visit were reviewed and the diagnosis of IBS confirmed. Patients seen primarily by a resident or trainee were excluded. The healthy controls database consisted of patients coming to the hospital for annual physical examinations. The diagnosis of psychiatric disorders including depression was made from primary care provider notes and gastroenterologist notes. Only a minority of patients with psychiatric disorders were actively followed by a psychiatrist. Due to state laws we are forbidden to access psychiatric records without express patient permission, which was not possible in this type of study. The diagnoses were established by review of `past medical history', `concurrent-medical problems' and/or medication lists as recorded by the treating gastroenterologist or primary care physician. Data were collected on age, gender, diagnosis of depression, anxiety, other psychiatric disorders, autoimmune thyroid disease, DM type 1 and other autoimmune disorders.
The sample size for the cases and the controls was calculated to provide 80% power to assess a 10% difference in prevalence between CD and control populations for either aggregated autoimmune diseases or psychiatric disorders. Statistical analyses were performed using MedCalc for Windows, version 9.3.2.0 (MedCalc Software, Mariakerke, Belgium). Chi square test was used to analyze categorical data. Yates correction was applied when appropriate. The study was approved by the BIDMC Institutional Review Board. None of the study subjects were contacted.
Results
Characteristics of the study subjects are shown in Table 1. Of the 600 CD patients, 75% were women (mean age 49 years) and 25% were men (mean age 54 years). The age and gender distribution of IBS and healthy controls were similar (no statistically significant differences) in accordance with the age- and gender-matched study design: 200 IBS patients, 75% women (mean age 45.4 years) and 25% men (mean age 47.9 years); 200 healthy, primary care controls, 75% women (mean age 46.8 years) and 25% men (mean age 52.1 years).
Table 1.
Characteristics of the study subjects
| Celiac disease | IBS | Controls | P value (CD & IBS) | P value (CD & Controls) | |
|---|---|---|---|---|---|
| Total number | 600 | 200 | 200 | ||
|
| |||||
| Gender | |||||
| Men | 150 | 50 | 50 | 0.92 | 0.92 |
| Women | 450 | 150 | 150 | 0.92 | 0.92 |
|
| |||||
| Mean age in years | |||||
| Men | 54 | 47.9 | 52.1 | 0.09 | 0.49 |
| Women | 49 | 45.4 | 46.8 | 0.08 | 0.14 |
The prevalence of psychiatric disorders in the three study groups is shown in Table 2. The prevalence of depression was similar in CD (17.2%), IBS (18.5%) and primary care controls (16%). There was a trend towards a higher prevalence of anxiety in CD (8.7%) than in controls (4.5%) which did not reach statistical significance (P = 0.08). Anxiety was significantly more prevalent in the IBS group (12%) than in primary care controls (P = 0.011) but similar to CD patients (P=0.21). The total prevalence of patients with any psychiatric diagnosis was similar in the 3 groups. Table 3 shows the number of participants with various psychiatric disorders in the 3 study groups. 80% of patients with concurrent psychiatric disorders were on medications for these indications. Low dose tricyclic antidepressants in patients with documented IBS were not considered psychiatric medications.
Table 2.
Prevalence of psychiatric disorders
| CD (%) | IBS (%) | Controls (%) | P value (CD vs Controls) | P value (IBS vs Controls) | P value (CD vs IBS) | |
|---|---|---|---|---|---|---|
| Depression | 17.2 | 18.5 | 16.0 | 0.79 | 0.60 | 0.75 |
| Anxiety | 8.7 | 12.0 | 4.5 | 0.08 | 0.01 | 0.21 |
| Bipolar disorder | 1.2 | 2.5 | 0.5 | 0.31 | 0.22 | 0.68 |
| Eating disorder | 2.0 | 1.0 | 0 | 0.26 | 1.0 | 0.53 |
| Panic attacks | 0.2 | 1.5 | 1.0 | 0.32 | 1.0 | 0.08 |
| Aggregated total | 25.2 | 27.5 | 26.0 | 0.89 | 0.82 | 0.58 |
Table 3.
Number of subjects with other psychiatric disorders in CD, IBS and healthy controls (apart from those shown in table 2)
| Celiac disease (n=600) | IBS (n=200) | Healthy controls (n=200) | |
|---|---|---|---|
| Alcohol abuse | 6 | 0 | 0 |
| Attention deficit disorder | 4 | 2 | 0 |
| Obsessive compulsive disorder | 4 | 0 | 2 |
| Post traumatic stress disorder | 4 | 5 | 1 |
| Substance abuse | 3 | 0 | 0 |
| Schizophrenia | 1 | 0 | 4 |
| Borderline personalty disorder | 0 | 2 | 0 |
| Hypochondriosis | 0 | 1 | 0 |
| Schizoaffective Disorder | 0 | 1 | 0 |
| Scizotypal disorder | 0 | 0 | 1 |
| Seasonal affective disorder | 0 | 0 | 1 |
| Somatization disorder | 0 | 1 | 0 |
Out of the total 600 CD patients, 390 were seen at diagnosis while the remaining 210 were referred by health care providers outside of the Celiac Center after diagnosis. The rate of depression in the patients seen at diagnosis was 17.2% (67 of 390) while that for patients not seen at diagnosis was 17.1% (36 of 210). In patients with depression who were seen at diagnosis, 58 of 67 had depression before the diagnosis of celiac disease and four of 67 patients were diagnosed with depression after CD was detected. All four patients who did not have depression at diagnosis were diagnosed with depression within 1 year of the diagnosis of CD. In remaining five of 67 patients, records did not clearly identify the timing of the diagnosis of depression. We have insufficient information in the referral population regarding timing of depression. However, given that the overall rate of depression is identical in patients referred to the Celiac Center and diagnosed by the Celiac Center the populations are likely to be similar.
The prevalence of autoimmune disorders in the three study groups is shown in Table 4. In CD patients the prevalence of type I DM (5.8%) was significantly higher than in IBS (1.5%, P=0.02) or primary care controls (2.0%, P=0.05). Similarly the prevalence of autoimmune thyroid disease was significantly higher in CD (13.0%) than in IBS (7.0%, P=0.03) or primary care controls (6.5%, P=0.02). Of the 600 CD patients 2 had autoimmune hepatitis, 3 had primary biliary cirrhosis and 1 had primary sclerosing cholangitis. None of the IBS patients or primary care controls had autoimmune liver diseases. CD patients also had a greater prevalence of inflammatory bowel diseases (IBD) (6 patients had Crohn's disease and 2 had ulcerative colitis) than primary care controls (1 patient with Crohn's disease). Sarcoidosis was found in 8 CD patients but in none of the IBS patients or controls. The trend for a higher prevalence of autoimmune liver diseases, IBD and sarcoidosis in CD compared to the control groups did not reach statistical significance. The total number of patients with autoimmune disorders was significantly higher in CD (24.3%) than in IBS (12.5%, P<0.01) and in primary care controls (10.0%, P<0.01). Table 5 shows the number of subjects with other autoimmune disorders in the 3 study groups.
Table 4.
Prevalence of various autoimmune disorders
| CD (%) | IBS (%) | Controls (%) | P value (CD vs Controls) | P value (IBS vs Controls) | P value (CD vs IBS) | |
|---|---|---|---|---|---|---|
| Autoimmune thyroid disease | 13.0 | 7.0 | 6.5 | 0.02 | 1.0 | 0.03 |
| DM type 1 | 5.8 | 1.5 | 2.0 | 0.05 | 1.0 | 0.02 |
| Autoimmune liver diseases | 1.0 | 0 | 0 | 0.34 | 1.0 | 0.34 |
| IBD | 1.3 | 0 | 1.0 | 0.56 | 1.0 | 0.22 |
| Sarcoidosis | 1.3 | 0 | 0 | 0.22 | 1.0 | 0.22 |
| Aggregated total | 24.3 | 12.5 | 10.0 | < 0.0001 | 0.53 | 0.0006 |
Table 5.
Number of subjects with other autoimmune disorders in CD, IBS and healthy controls (apart from those shown in table 4)
| Celiac disease (n=600) | IBS (n=200) | Healthy controls (n=200) | |
|---|---|---|---|
| Raynaud's disease | 7 | 0 | 0 |
| Psoriasis | 6 | 3 | 1 |
| Rheumatoid arthritis | 4 | 3 | 0 |
| Vitiligo | 3 | 0 | 0 |
| Eczema | 2 | 0 | 0 |
| Lupus | 2 | 2 | 1 |
| Addison's disease | 1 | 0 | 0 |
| Behcet's syndrome | 1 | 0 | 0 |
| IgA nephropathy | 1 | 0 | 0 |
| Microscopic colitis | 1 | 0 | 0 |
| Palindromic polyarthritis | 1 | 0 | 0 |
| Polymyalgia rheumatica | 1 | 0 | 0 |
| Polymyositis | 1 | 0 | 0 |
| Primary hyperparathyroidism | 1 | 0 | 0 |
| Systemic sclerosis | 1 | 0 | 0 |
| Temporal arteritis | 1 | 0 | 0 |
| Immune thrombocytopenic purpura | 0 | 1 | 0 |
| Pernicious anemia | 0 | 0 | 1 |
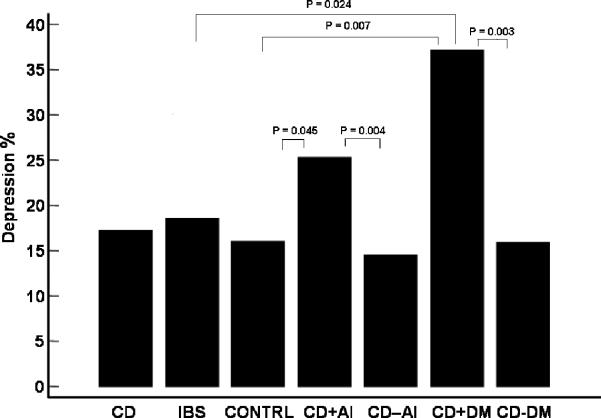
An important aspect of this study was the evaluation of the interaction between the prevalence of psychiatric disorders in CD patients and the presence of co-morbid autoimmune disorders. For CD patients the prevalence of depression was significantly higher in patients who had autoimmune disorder (25.3%) than in patients who did not have any autoimmune disorder (14.5%, P < 0.01). More specifically, CD patients with type I DM had a higher depression rate (37.1%) than those without type I DM (15.9%) (P < 0.01) (Figure 1). After controlling for type 1 DM, the prevalence of depression in CD patients was no longer significantly different in patients with and without autoimmune disorders (P = 0.09). Also for CD patients, the overall prevalence of any psychiatric disorder was higher among patients with autoimmune disorders (34.2%) than in patients without autoimmune disorders (22.2%) (P = 0.005). IBS patients with type 1 DM were also found to have a higher prevalence of anxiety than IBS patients without type I DM (P = 0.04), however, this analysis is limited by the small number (n=3) of patients in the IBS groups with DM1.
Figure 1.
Prevalence of depression in the 3 study groups and CD subgroups.
CD + AI: CD patients with AI disorders
CD − AI: CD patients without AI disorders
CD + DM: CD patients with type I DM
CD − DM: CD patients without type I DM
Discussion
In this study, we found the prevalence of depression in CD in a large US population to be 17%, similar to the rate found in both our IBS and healthy control populations, and within the 6% – 24% range found in prior studies 8, 10, 11, 20. However, previous studies that found a positive association between CD and psychiatric disorders did not adjust for the presence of type I DM or other autoimmune disorders 2, 5, 6, 8, 21. This is especially pertinent given the increased prevalence of co-morbid autoimmune disorders in the CD population 14–17 and the fact that multiple studies have confirmed the high prevalence of depression in individuals with type 1 DM and other auto-immune disorders 22, 23. The duel association of type 1 DM with both CD and depression makes this a classic confounder, hindering the assessment of the prevalence of psychological disorders in CD. This factor may account for much of the disparity in prevalence rates of depression reported in CD patients.
In light of the above observations, when looking at the prevalence of psychiatric disorders in CD we adjusted for confounders including age, gender and the presence of co-morbid autoimmune disorders including type I DM. As expected, the total number of patients with autoimmune disorders was significantly higher in CD than in IBS patients or controls. Seventy eight percent of the total autoimmune disease burden in CD was accounted for by either type I DM or autoimmune thyroid disease. After removing type I DM and hypothyroid patients, we still found a significantly higher proportion of CD patients with other autoimmune disorders when compared to healthy controls (P = 0.04) but not when compared to IBS. In addition to type I DM and autoimmune thyroid disease, other major autoimmune disorders in CD were IBD, autoimmune liver diseases and sarcoidosis each of which has previously been reported 24–26.
Our findings support the hypothesis that enrichment for Type 1 DM in patients with celiac disease accounts for much of the increased prevalence of depression in this patient population. In fact, 37% of patients with both CD and Type 1 DM in our study suffered from depression compared to 16% with celiac disease alone. Similarly, we found that CD patients with any co-existing autoimmune disorder had a significantly higher prevalence of depression (34%) than CD patients who did not have another autoimmune disorder (22%). Nearly all of the increase in prevalence of depression in the group of celiac patients with co-morbid autoimmune disorders was attributable to the effect of Type 1 DM.
Although we are able to report only the association of depression and type 1 diabetes in individuals with celiac disease, we are not able to make any conclusions regarding the cause of depression in this population. The relatively high prevalence of depression in CD patients without type I DM suggests that there are other factors apart from diabetes which contribute to depression. While one may speculate that depression may be triggered or exacerbated by factors such as metabolic abnormalities or stress related to disease coping, we are unable to support these hypotheses at this time. Additionally, there is no definitive evidence on the effect of gluten free diet on depression, however, the fact that there seem to be few incident cases of depression after diagnosis of CD does suggest a salutary effect. Further study of these issues is clearly warranted.
Unlike depression, anxiety was not significantly more prevalent in the CD population. Whether anxiety is associated with CD is unclear, as past studies have yielded conflicting results. Carta et al found a higher prevalence of anxiety in CD patients (27.7%) but this did not reach statistical significance 21. Fera et al and Addolorato et al found significantly higher scores for anxiety on State and Trait Anxiety Inventory 2, 11 although the clinical relevance of this finding is unclear. These data suggest that celiac disease patients usually present with state anxiety 35, which disappears after gluten withdrawal. In our study, differentiating between state anxiety and trait anxiety was not possible as diagnosis was determined through evaluation of medical records rather than patient evaluation. Anxiety was significantly more common in IBS patients than in CD patients or healthy controls suggesting that there was adequate power to detect a clinically significant difference had one existed.
Clearly the most likely explanation for the association of psychological disorders with CD and autoimmune disorders is the psychosocial burden that these conditions place on patients. It is worth noting, however, that other hypotheses have been proposed. For instance, autoimmune mechanisms may alter the neurotransmitter pathways leading to depression, as suggested by Carta et al 21. Additionally, malabsorption of certain dietary elements such as tryptophan and folic acid has been suggested to result in depression and other psychiatric disorders in CD patients 32, 33. If this were the case, gluten withdrawal should resolve the malabsorption in CD, normalizing nutritional status; yet treatment has not been conclusively shown to improve the depressive symptoms in CD patients 8, 34. For this reason, although dietary deficiencies might contribute to psychiatric disorders in CD, there are clearly other factors which play a role and remain to be defined.
Although data on the prevalence of psychiatric disorders in CD has been presented previously, the size of our study population and the use of controls are significant strengths. The large sample size gives us adequate power to detect a clinically meaningful difference between the study groups. We chose, in addition to healthy controls, to use IBS patients to act as chronic gastrointestinal disease controls. The only study on this topic to use chronic GI disease controls was reported by Ciacci et al who used chronic hepatitis patients as controls. Although a valid comparison group, it is worth noting that the use of a chronic liver disease population as a control group for the study of psychological disorders may be problematic due to the risk factors for, and manifestations of, liver disease 4. The use of IBS patients as controls may be more suitable as it corrects for the influence of chronic gastrointestinal symptoms without adding new variables which may be social or physiological in nature.
One limitation of this study is the ascertainment of psychiatric disorders from the medical record. Further state laws forbid access to psychiatric records which may lead to detection bias. However, as the methodology was uniform between groups, it would be expected that, while absolute rates may be biased, the ratio of disorders between groups should be accurate. Using a cross sectional design we are unable to study the temporal relationship between CD and psychiatric disorders. For this reason speculation into whether CD specifically plays a role in the causation of the psychiatric disorder is not possible. Ludwigsson et al7 did conduct a population based longitudinal study and has shown a positive association of CD with subsequent depression which was not affected by the presence of DM or thyroid disease. We also were not able to look at the effect of the gluten free diet on the prevalence of psychiatric disorders in CD. Had we been able to assess patients prior to diagnosis of CD the rate of depression might have been higher.
In this study, the presence of psychiatric disorders in our study was made by physicians as opposed to through questionnaires as was done in most prior studies. Although this strategy may miss some patients with mild or subclinical psychiatric disease, by focusing on patients whose symptoms are noted by treating physicians we can be more secure that our data is of clear clinical relevance. On the other hand, individuals who score high on a mood disorder survey only may be compensated so that there is no effect on clinical outcomes. The diagnoses of CD and IBS were identified using ICD-9 codes and individually verified from physician notes. Although in any retrospective study, there is the potential for missed diagnoses and misclassification of patients, the ubiquity of the institution's electronic medical record increases reliability and as the methodology for case and control data collection was identical, inaccuracies should not have biased any group over another.
In summary, we found a clear association between autoimmune disorders, especially type 1 DM and thyroid disease, and celiac disease; however, we did not find a significant increase in prevalence of depression or anxiety in an unselected CD population compared to IBS or healthy controls. We did however find that CD patients with type I DM have a significantly higher prevalence of depression than was seen in either of the control groups or in the overall CD group. This is significant, as differing rates of Type 1 DM may account for the large discrepancy in the prevalence of depression seen in previous CD studies.
Future research into the role of psychiatric disorders in celiac disease should control for co-morbid autoimmune disorders as these appear to be significant confounding variables. Further, the prevalence of depression in patients with both CD and type 1 DM is striking, affecting over 35% of this population. This is a group that should be targeted and potentially screened for depression as treatment may improve both adherence to medical treatment and overall quality of life. Future studies will be required to assess the effect of gluten withdrawal on psychiatric disorders in CD and to evaluate the utility of screening for depression in patients with CD and type 1 DM.
Acknowledgement
We appreciate the assistance of Harold Calderon in Medical Records for his help with data procurement. This work was funded in part by a NIH T32 training grant (DK07760 to DL).
Funding: Authors' declaration of personal interests: SV Kane has served as a consultant/scientific advisor for Abbott Laboratories, Centocor, Inc., Elan, Procter & Gamble Pharmaceuticals, Inc., Shire Pharmaceuticals Group, and UCB Pharma. She has received grant/research support from AstraZeneca, Elan Pharmaceuticals, Procter & Gamble Pharmaceuticals, Inc., Shire Pharmaceuticals Group, and UCB Pharma. D Brixner has served as a consultant/scientific advisor for Procter & Gamble Pharmaceuticals, Inc. NA Accortt and S Magowan are employees of Procter & Gamble Pharmaceuticals, Inc. Declaration of funding interests: Procter and Gamble Pharmaceuticals, Inc. provided funding for this study and for professional writing services.
References
- 1.AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977–80. doi: 10.1053/j.gastro.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addolorato G, Capristo E, Ghittoni G, et al. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: a longitudinal study. Scand J Gastroenterol. 2001;36:502–6. doi: 10.1080/00365520119754. [DOI] [PubMed] [Google Scholar]
- 3.Addolorato G, Stefanini GF, Capristo E, Caputo F, Gasbarrini A, Gasbarrini G. Anxiety and depression in adult untreated celiac subjects and in patients affected by inflammatory bowel disease: a personality “trait” or a reactive illness? Hepatogastroenterology. 1996;43:1513–7. [PubMed] [Google Scholar]
- 4.Ciacci C, Iavarone A, Mazzacca G, De Rosa A. Depressive symptoms in adult coeliac disease. Scand J Gastroenterol. 1998;33:247–50. doi: 10.1080/00365529850170801. [DOI] [PubMed] [Google Scholar]
- 5.Hallert C, Astrom J. Psychic disturbances in adult coeliac disease. II. Psychological findings. Scand J Gastroenterol. 1982;17:21–4. doi: 10.3109/00365528209181038. [DOI] [PubMed] [Google Scholar]
- 6.Hallert C, Derefeldt T. Psychic disturbances in adult coeliac disease. I. Clinical observations. Scand J Gastroenterol. 1982;17:17–9. doi: 10.3109/00365528209181037. [DOI] [PubMed] [Google Scholar]
- 7.Ludvigsson JF, Reutfors J, Osby U, Ekbom A, Montgomery SM. Coeliac disease and risk of mood disorders--a general population-based cohort study. J Affect Disord. 2007;99:117–26. doi: 10.1016/j.jad.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Siniscalchi M, Iovino P, Tortora R, et al. Fatigue in adult coeliac disease. Aliment Pharmacol Ther. 2005;22:489–94. doi: 10.1111/j.1365-2036.2005.02619.x. [DOI] [PubMed] [Google Scholar]
- 9.Accomando S, Fragapane ML, Montaperto D, et al. Coeliac disease and depression: two related entities? Dig Liver Dis. 2005;37:298–9. doi: 10.1016/j.dld.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Cicarelli G, Della Rocca G, Amboni M, et al. Clinical and neurological abnormalities in adult celiac disease. Neurol Sci. 2003;24:311–7. doi: 10.1007/s10072-003-0181-4. [DOI] [PubMed] [Google Scholar]
- 11.Fera T, Cascio B, Angelini G, Martini S, Guidetti CS. Affective disorders and quality of life in adult coeliac disease patients on a gluten-free diet. Eur J Gastroenterol Hepatol. 2003;15:1287–92. doi: 10.1097/00042737-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Roos S, Karner A, Hallert C. Psychological well-being of adult coeliac patients treated for 10 years. Dig Liver Dis. 2006;38:177–80. doi: 10.1016/j.dld.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 14.Acerini CL, Ahmed ML, Ross KM, Sullivan PB, Bird G, Dunger DB. Coeliac disease in children and adolescents with IDDM: clinical characteristics and response to gluten-free diet. Diabet Med. 1998;15:38–44. doi: 10.1002/(SICI)1096-9136(199801)15:1<38::AID-DIA520>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Cronin CC, Feighery A, Ferriss JB, Liddy C, Shanahan F, Feighery C. High prevalence of celiac disease among patients with insulin-dependent (type I) diabetes mellitus. Am J Gastroenterol. 1997;92:2210–2. [PubMed] [Google Scholar]
- 16.Schuppan D, Hahn EG. Celiac disease and its link to type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2001;14(Suppl 1):597–605. doi: 10.1515/jpem.2001.14.s1.597. [DOI] [PubMed] [Google Scholar]
- 17.Talal AH, Murray JA, Goeken JA, Sivitz WI. Celiac disease in an adult population with insulin-dependent diabetes mellitus: use of endomysial antibody testing. Am J Gastroenterol. 1997;92:1280–4. [PubMed] [Google Scholar]
- 18.Sategna Guidetti C, Solerio E, Scaglione N, Aimo G, Mengozzi G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut. 2001;49:502–5. doi: 10.1136/gut.49.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viljamaa M, Kaukinen K, Huhtala H, Kyronpalo S, Rasmussen M, Collin P. Coeliac disease, autoimmune diseases and gluten exposure. Scand J Gastroenterol. 2005;40:437–43. doi: 10.1080/00365520510012181. [DOI] [PubMed] [Google Scholar]
- 20.Ciacci C, D'Agate C, De Rosa A, et al. Self-rated quality of life in celiac disease. Dig Dis Sci. 2003;48:2216–20. doi: 10.1023/b:ddas.0000004530.11738.a2. [DOI] [PubMed] [Google Scholar]
- 21.Carta MG, Hardoy MC, Boi MF, Mariotti S, Carpiniello B, Usai P. Association between panic disorder, major depressive disorder and celiac disease: a possible role of thyroid autoimmunity. J Psychosom Res. 2002;53:789–93. doi: 10.1016/s0022-3999(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 23.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53:1053–60. doi: 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 24.Caprai S, Vajro P, Ventura A, Sciveres M, Maggiore G. Autoimmune Liver Disease Associated With Celiac Disease in Childhood: A Multicenter Study. Clin Gastroenterol Hepatol. 2008 doi: 10.1016/j.cgh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Hwang E, McBride R, Neugut AI, Green PH. Sarcoidosis in patients with celiac disease. Dig Dis Sci. 2008;53:977–81. doi: 10.1007/s10620-007-9974-2. [DOI] [PubMed] [Google Scholar]
- 26.Leeds JS, Horoldt BS, Sidhu R, et al. Is there an association between coeliac disease and inflammatory bowel diseases? A study of relative prevalence in comparison with population controls. Scand J Gastroenterol. 2007;42:1214–20. doi: 10.1080/00365520701365112. [DOI] [PubMed] [Google Scholar]
- 27.Drossman DA, McKee DC, Sandler RS, et al. Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology. 1988;95:701–8. doi: 10.1016/s0016-5085(88)80017-9. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead WE, Bosmajian L, Zonderman AB, Costa PT, Jr., Schuster MM. Symptoms of psychologic distress associated with irritable bowel syndrome. Comparison of community and medical clinic samples. Gastroenterology. 1988;95:709–14. doi: 10.1016/s0016-5085(88)80018-0. [DOI] [PubMed] [Google Scholar]
- 29.Mikocka-Walus AA, Turnbull DA, Moulding NT, Wilson IG, Andrews JM, Holtmann GJ. Controversies surrounding the comorbidity of depression and anxiety in inflammatory bowel disease patients: a literature review. Inflamm Bowel Dis. 2007;13:225–34. doi: 10.1002/ibd.20062. [DOI] [PubMed] [Google Scholar]
- 30.Golden J, O'Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry. 2005;27:431–8. doi: 10.1016/j.genhosppsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Gutteling JJ, de Man RA, Busschbach JJ, Darlington AS. Overview of research on health-related quality of life in patients with chronic liver disease. Neth J Med. 2007;65:227–34. [PubMed] [Google Scholar]
- 32.Hallert C, Astrom J, Sedvall G. Psychic disturbances in adult coeliac disease. III. Reduced central monoamine metabolism and signs of depression. Scand J Gastroenterol. 1982;17:25–8. doi: 10.3109/00365528209181039. [DOI] [PubMed] [Google Scholar]
- 33.Hernanz A, Polanco I. Plasma precursor amino acids of central nervous system monoamines in children with coeliac disease. Gut. 1991;32:1478–81. doi: 10.1136/gut.32.12.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauser W, Gold J, Stein J, Caspary WF, Stallmach A. Health-related quality of life in adult coeliac disease in Germany: results of a national survey. Eur J Gastroenterol Hepatol. 2006;18:747–54. doi: 10.1097/01.meg.0000221855.19201.e8. [DOI] [PubMed] [Google Scholar]
- 35.Cannings-John R, Butler C, Prout H. A case-control study of presentations in general practice before diagnosis of coeliac disease. Br J Gen Pract. 2007;57:636–42. [PMC free article] [PubMed] [Google Scholar]