Abstract
Vertebrate animals exhibit a spectacular diversity of social behaviors, yet a variety of basic social behavior processes are essential to all species. These include social signaling; discrimination of conspecifics and sexual partners; appetitive and consummatory sexual behaviors; aggression and dominance behaviors; and parental behaviors (the latter with rare exceptions). These behaviors are of fundamental importance and are regulated by an evolutionarily conserved, core social behavior network (SBN) of the limbic forebrain and midbrain. The SBN encodes social information in a highly dynamic, distributed manner, such that behavior is most strongly linked to the pattern of neural activity across the SBN, not the activity of single loci. Thus, shifts in the relative weighting of activity across SBN nodes can conceivably produce almost limitless variation in behavior, including diversity across species (as weighting is modified through evolution), across behavioral contexts (as weights change temporally) and across behavioral phenotypes (as weighting is specified through heritable and developmental processes). Individual neural loci may also express diverse relationships to behavior, depending upon temporal variations in their functional connectivity to other brain regions (“neural context”). We here review the basic properties of the SBN and show how behavioral variation relates to functional connectivity of the network, and discuss ways in which neuroendocrine factors adjust network activity to produce behavioral diversity. In addition to the actions of steroid hormones on SBN state, we examine the temporally plastic and evolutionarily labile properties of the nonapeptides (the vasopressin- and oxytocin-like neuropeptides), and show how variations in nonapeptide signaling within the SBN serve to promote behavioral diversity across social contexts, seasons, phenotypes and species. Although this diversity is daunting in its complexity, the search for common “organizing principles” has become increasingly fruitful. We focus on multiple aspects of behavior, including sexual behavior, aggression and affiliation, and in each of these areas, we show how broadly relevant insights have been obtained through the examination of behavioral diversity in a wide range of vertebrate taxa.
Keywords: evolution, sociality, hypothalamus, amygdala, preoptic area, lateral septum, bed nucleus of the stria terminalis, songbird, oxytocin, vasotocin, vasopressin, sexual behavior
1. Weighting dynamic limbic networks: The key to behavioral diversity?
As animals navigate through their environments, they are bombarded by stimuli that may differentially activate neural circuits that influence both avoidance and appetitive approach behaviors. Dependent upon the context and the nature of the stimulus, responses are expressed in the form of specific, complex behavioral outputs such as eating, freezing, communicating, attacking, fleeing, copulating, etc. This translation of contextual stimuli into behavioral response is largely dependent upon distributed processing across circuits of the basal forebrain and midbrain. Although these circuits are often referred to as “the limbic system,” this “system” does not function in the linear, hierarchical manner observed for most other neural systems. Rather, subsets of limbic circuits comprise various core networks that integrate the behavioral, physiological and motivational processes that relate to specific neural “tasks.” Thus, we can identify a core limbic network for social behavior, another core network for stress and anxiety, and yet others for feeding and drinking, and incentive and reward [1,3,4,8,37,47,68,92,109]. These networks are not segregated, and the neural nodes in one network (e.g., for social behavior) also contribute to processing in other networks (e.g., for stress and ingestion). Different models have been used to conceptualize the functions of these networks (e.g., [92,110]), but regardless of the model or the level of anatomical organization, it is clear that behavioral “decisions” are generated via the weighting of activity across the nodes of heavily integrated, limbic networks. More simply phrased – it is the pattern of neural activity across the networks that determines behavior. Thus, discrete brain loci can express different relationships to behavior depending upon their functional connectivity to other neural loci (an idea known as “neural context”) [87].
In the sections that follow, we will survey a variety of neuroendocrine mechanisms that underlie diversity in social behavior, including neuroendocrine processes that produce variation in behavior across seasons and contexts, and between species and phenotypes. Our goal here is not to provide an exhaustive review of the neuroendocrine mechanisms of behavioral diversity (which this paper falls far short of), and we regret the exclusion of many excellent model systems that are represented in the literature. Rather, our primary goal is to examine behavioral diversity within the context of dynamic limbic networks, and to suggest new ways of thinking about the neuroendocrine mechanisms of behavior. Throughout, we emphasize the need to move beyond a focus on discrete loci, and towards a focus on network properties that likely bear a much closer relationship to naturalistic social behavior, and to the limitless diversity that is found in that behavior.
2. The vertebrate social behavior network and neural context
2.1 Delineation of the social behavior network in mammals
Almost forty years ago, modern tract-tracing techniques became available that revolutionized the study of the brain. These advances were soon followed by the development of modern immunohistochemical techniques, and researchers quickly began to use those methods to explore the neural pathways that mediate numerous kinds of social behaviors. Arguably the first pathway to be tackled was the mating behavior circuitry in hamsters, which was initiated with the descriptions of vomeronasal projections into the brain [97,114]. As the relevant neural architecture came into greater and greater relief, however, it became apparent that the pathways being delineated were not simply mating behavior pathways, but showed extensive overlap with brain regions that regulate a variety of other social behaviors, including aggression, social communication and parental care [92]. These brain areas are now collectively referred to as the “social behavior network” (SBN) [47,86,92].
The core components of the SBN include numerous areas of the basal forebrain – the medial extended amygdala (medial amygdala, MeA, and medial bed nucleus nucleus of the stria terminalis, BSTm), preoptic area (POA), anterior hypothalamus (AH), ventromedial hypothalamus (VMH), and lateral septum (LS), as well as areas of the midbrain, most notably the central gray (CG, also known as the periaqueductal gray) and the ventral tegmental area (VTA). These areas are all reciprocally connected and absolutely essential for the expression of basic social behaviors. They are also highly enriched with steroid hormone receptors, which are essential for the sexual differentiation and activation of social behaviors [47,92]. It should be emphasized that although these structures form the core of social behavior circuitry in the brain, other areas such as the prefrontal cortex and nucleus accumbens also play important roles (e.g., [5])
Although some SBN components are more important for certain aspects of behavior than others, each is involved in the regulation of multiple aspects of behavior, and these are not always categorically similar. For instance, the POA is critically important for maternal behavior and male copulatory behavior, but is not strongly implicated in female sexual behavior or male aggression. Such findings are somewhat counterintuitive and make it difficult to characterize the functions of specific areas. However, studies of immediate early gene (IEG) expression have added a great deal of clarity. IEG studies show that while the activity of single neural loci is not predictive of specific behavioral outputs, the pattern of IEG activity across the SBN is unique for each behavioral context. Thus, it is the pattern of neural activity across the SBN that is most strongly linked to behavior [47,86,92].
2.2. The social behavior network in non-mammalian vertebrates
Although the SBN has been most extensively explored in mammals, it is conserved across all jawed vertebrate groups and is likely ancestral for vertebrates as a whole [47]. In the plainfin midshipman fish (Porichthys notatus), neurophysiologically-guided tract tracings have revealed a network of brain regions that is involved the regulation of social vocalization, comprised of sites in the POA, anterior and ventromedial hypothalamus, and midbrain CG. These areas are reciprocally connected to each other and to homologues of the tetrapod septum and subpallial amygdala [50,53]. As in tetrapods, all of these areas concentrate sex steroid hormones [40-42], and some have been implicated in other behaviors such as aggression and spawning [28,29,93]. A diverse collection of anatomical and functional studies in amphibians, birds and reptiles likewise supports the hypothesis that the fundamental structure and functions of the SBN have been strongly conserved in all vertebrate lineages. Conveniently, for most species of tetrapods [47], the names of the various SBN components are the same as those in mammals.
The most extensive data on the non-mammalian SBN come from birds, and these data are particularly valuable from a comparative standpoint, since many studies have been conducted using paradigms that are virtually identical to those used in mammals. These include studies of IEG expression in relation to sexual behavior [6,7,14,88,89,111,112], aggressive interactions [54,57,85], parental behavior [10,99], social communication [66,67,84,86], social conditioning [12], and passive processing of same-sex stimuli [56,62]. Mammal-like patterns of IEG expression are observed in all of these contexts, inclusive of prominent sex differences [6,47]. The subnuclear organization of SBN nodes in birds is likewise strongly similar to mammals, including the histochemical and functional properties of LS subdivisions [55], mediolateral organization of the VMH [56,86], columnar organization of the CG (M. A. Kingsbury and J. L. Goodson, unpub. obs.), and cytoarchitectonic organization of the VTA [43].
2.3. Behavioral control columns: an alternative framework
Newman’s SBN “patterning” model [92] has gained the attention of many researchers whose primary focus is on the neuroendocrine mechanisms of behavior – that is, behavioral endocrinologists and behavioral neuroendocrinologists. In contrast, a somewhat different framework has gained substantial traction among neuroscientists who have the primary goal of elucidating the anatomical and molecular structure of limbic behavioral networks. The “column” model of SBN function, advanced by Swanson and colleagues [15,32,33,94,110], emphasizes the fact that there are subpopulations of neurons in the medial extended amygdala and medial hypothalamus that can be distinguished based upon their connectivity and gene expression profiles. Two columns have been identified that are designated as “reproductive” and “defense” columns, and these are presented as being strongly defined by preferential connections within each column. As described in mice, the core of the reproductive column is comprised of the posterodorsal MeA, ventrolateral porttion of the ventromedial nucleus of the hypothalamus (VMN), and the ventral premammillary nucleus. The core of the defense column is comprised of the posteroventral MeA, AH, dorsomedial VMN and dorsal premammillary nucleus (e.g., [15]).
Despite the difference in packaging, the patterning and column models are extensively compatible at the anatomical level. For instance, although the column model tends to de-emphasize the reciprocal connections between columns, and the SBN patterning model tends to de-emphasize the many intermingled neuronal subpopulations that comprise various subcircuits, these are differences in points of focus and do not reflect disagreement over what is connected to what. In the end, both models are grossly over-simplified (and intentionally so) in an effort to emphasize network features that individual authors view as being particularly important.
Those compatibilities notwithstanding, the models do differ substantially in how they conceptualize the regulation of behavior. Whereas the patterning model remains sound if we extrapolate it to a larger number of nodes (e.g., representing anatomically distinct neuronal subpopulations and subcircuits), the extensive integration of the relevant cell groups is simply not consistent with the idea that different behaviors are regulated by segregated neural columns, or along labeled lines. Indeed, integration clearly occurs at an anatomical level, given the presence of a major projection from the ventrolateral VMH (“reproductive”) to the anterior hypothalamic nucleus (“defensive”) and many other smaller connections between the components of the two columns [13,110].
Importantly, functional evidence for reproduction and defense columns has been based extensively on the differential Fos responses of male mice to predator odor and female urine, although these stimuli differ along numerous other dimensions beyond reproduction and defense; e.g., in relation to valence, in terms of being conspecific versus heterospecific stimuli, and in their constituent odorant qualities. However, more naturalistic behavioral interactions typically activate components of both columns [19,104]. For instance, following resident-intruder encounters in male mice, both intruders and residents exhibit significant activation in both reproductive and defensive columns, although the relative amounts of activation in these groups differ substantially [91]. In a particularly compelling study by Sheehan et al. [102], components of the both columns were activated in female rats by interactions with pups, with differences being observed in relation to maternal response. Thus, these findings suggest that the columns are not truly segregated, but rather act in an integrated manner to determine behavioral response. Despite these concerns about interpretations, it must be emphasized that the proponents of the column model have generated some of the finest data available on the structure of limbic-hypothalamic systems, and criticisms of certain functional interpretations should not detract from that fact.
2.4. Functional diversity of discrete brain loci: neural context, functional connectivity, and neuromodulatory patterning
“Neural context” refers to the idea that the functional role of a discrete brain locus depends upon how its activity is correlated with other discrete loci, and this correlated or coupled activity is simply termed “functional connectivity” [87]. Although these concepts were developed by cognitive neuroscientists to describe the dynamic properties of cortical networks, the application to the SBN and other limbic networks is abundantly clear [16,69,100,101,118]. Indeed, the notion of neural context is implicit in Newman’s original conceptualization of the SBN [92], and subsequent authors have explicitly incorporated the idea of functional connectivity into Newman’s model [17,100,118].
Given that neural context and functional connectivity are essential concepts in SBN function, it is important to consider how neuroendocrine factors influence these variables, and how these variables impact the behavioral properties of neuroendocrine systems. To this end, we propose two simple hypotheses, each of which already has some support: 1) Neuroendocrine variables such as neuropeptides and steroid hormones produce diversity in functional connectivity and behavior based upon their patterns of action within the SBN. We will refer to this idea as “neuromodulatory patterning.” Implicit in this idea is that a given neuroendocrine variable can exert diverse (and even opposing) relationships to behavior that are derived from unique patterns of activity within the SBN (e.g., based on spatial differences in release and/or receptor distributions). This hypothesis receives strong support from recent studies of vasotocin (VT). VT can both facilitate and inhibit aggression, likely dependent upon which VT cell groups are activated [59,75]. Individual VT cell groups exhibit distinct projection patterns, and thus each will yield distinct patterns of neuromodulation. These findings are described more fully below. 2) As neuroendocrine variables adjust the relative weighting and functional connectivity of SBN nodes, social stimuli may change their relationship to the animal’s behavioral output. This hypothesis is very similar to the hypothesis above, but whereas the focus above is placed on the relationship of neuroendocrine mechanisms to behavior, the focus here is placed on the relationship of social context to social behavior output. This hypothesis receives strong support from studies of seasonal and species differences in social behavior (see remaining sections below). Importantly, while these specific hypotheses about SBN function may be somewhat novel in their formulation, they are nonetheless rooted in ideas that have a deep history in behavioral neuroendocrinology (e.g., [18]).
3. Functional connectivity in the social behavioral network is contextually dynamic and reflects social phenotype
If functional connectivity across the SBN and related structures is the primary variable that determines behavior (and hence phenotypic variation in behavior), then developmental and social factors that sculpt behavioral phenotypes should likewise sculpt functional connectivity [101]. This includes not only intrasexual differences in phenotype, but intersexual differences as well.
In a highly novel approach to this hypothesis, Sakata et al. [100] examined covariance in the metabolic capacity of brain areas (as determined by cytochrome oxidase histochemistry) in leopard geckos (Eublepharis macularius), a species that exhibits temperature-dependent sex determination. Geckos were incubated at temperatures that produce all males, all females, or a mixture that is either female-biased or male-biased. Importantly, temperature exerts numerous effects that are dissociated from gonadal sex, including effects on sociosexual behavior, reproductive physiology and sensitivity to hormones [98]. For instance, regardless of gonadal sex, animals that develop at male-biased incubation temperatures are more aggressive than those that develop at female-biased incubation temperatures [16,101]. In the experiment by Sakata et al. (2000), geckos from the male-biased incubation temperatures showed significant covariance (functional connectivity) between the AH and LS, and AH and POA, consistent with extensive data that demonstrate the importance of these areas for the regulation of aggression (particularly the AH and LS). In contrast, geckos from the female-biased temperatures showed significant covariance between the VMH and LS, areas that are strongly involved in the regulation of feminine sexual behavior. The results from this experiment therefore provide good support for the idea that phenotypic and sexual variation is reflected in different patterns of functional connectivity.
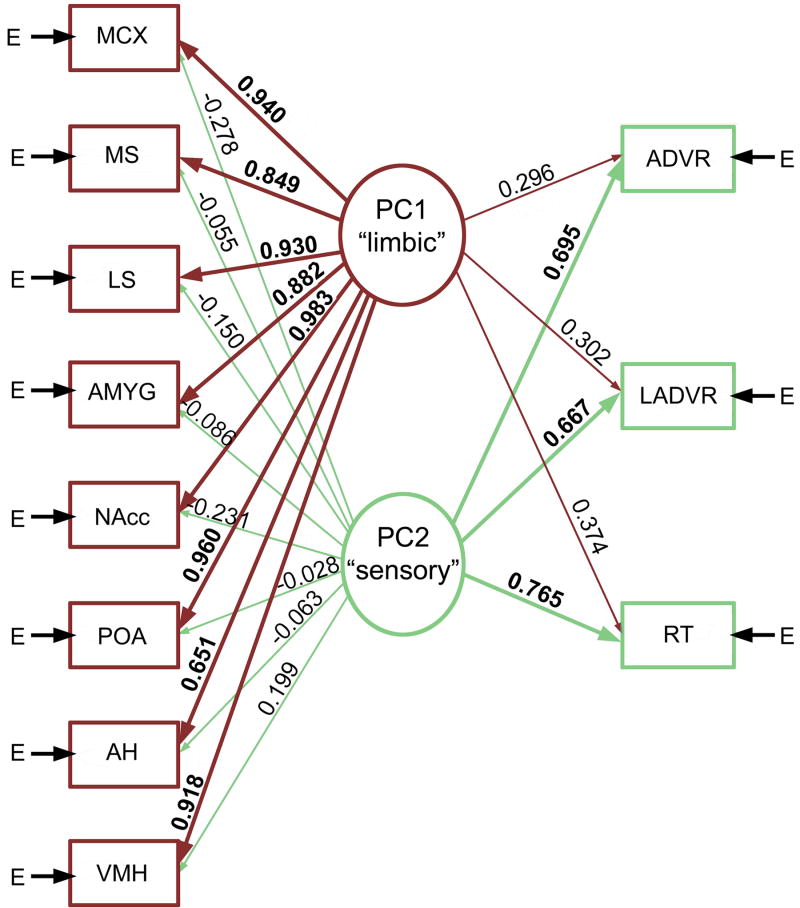
In a similar but analytically more complex experiment in male green anoles (Anolis carolinensis), Yang and Wilczynski [118] examined the metabolic capacity of numerous brain regions of males following repeated exposure to an aggressive visual stimulus (a video of a male displaying, which elicits aggressive response) or a non-social video. Unlike the approach taken above, which was based upon pairwise correlations between two brain regions at a time, metabolic capacity was analyzed using principal components analyses and structural equation modeling. As shown in Figure 1, these analyses revealed that aggressive experience organized multiple functionally connected neural networks, including a sensory network and a limbic-hypothalamic network that included all forebrain components of the SBN (VMH, LS, AH, amygdala and nucleus accumbens; midbrain loci were not examined). These findings are noteworthy in that they demonstrate that aggressive interactions do not simply influence brain regions independently, but organize functional connectivity throughout the SBN.
Fig. 1.
Aggressive experience in male anoles (repeated exposure to an aggressive video) organizes multiple functionally connected neural networks, including a sensory network and a limbic-hypothalamic network that included all forebrain components of the SBN. Shown here is a path diagram based upon the principle component (PC) structure of males exposed to videos. Arrow E represents variances contributed by factors other than the linear combination of the latent variables derived from the PCs. The thickness of the lines and the size of the numerals (PC loadings) indicate the strength of the relationships. Modified from [118]. Abbreviations: ADVR, anterior dorsal ventricular ridge; AH, anterior hypothalamus; AMYG, amygdala; LADVR, lateral ADVR; LS, lateral septum; MCX, medial cortex; MS, medial septum; NAcc, nucleus accumbens; POA, preoptic area; RT, nucleus rotundus; VMH, ventromedial hypothalamus.
In the experiments just described, metabolic capacity was examined after many days of stimulation, or after long-term developmental exposure to an environmental stimulus. However, functional connectivity can also be modified on much shorter timescales, and likely underlies rapid shifts in both perception and behavioral response. This has been demonstrated most explicitly in analyses of IEG (egr-1) expression in frogs, which show that functional connectivity among hypothalamic nuclei is rapidly remodeled based upon the behavioral relevance of acoustic stimuli [69].
4. Hormonal and seasonal factors adjust properties of the social behavior network
All nodes of the SBN express sex steroid receptors, and extensive changes in histochemistry and anatomy are observed throughout the SBN in relation to hormone levels and/or season [76-78]. These changes undoubtedly have significant impacts on functional connectivity and neuromodulatory patterning, and create unique neural contexts in which stimuli are perceived and acted upon. For species that breed or defend territories seasonally, environmental stimuli will also vary seasonally in their significance and behavioral relevance. In a particularly nice study addressing this latter idea, Maney et al. [86] recently showed that in white-throated sparrows (Zonotrichia albicollis), significant egr-1 responses to male song (versus silence and tones) are observed throughout the SBN in females that are in breeding condition, while females in nonbreeding condition only exhibit this kind of selective neural response in the lateral VMH. However, estradiol treatment in nonbreeding females fully restored the selective responses to song.
Seasonal variation in songbirds is also observed in the relationships between neural activity and behavior. Male starlings (Sturnis vulgaris) sing year-round, but whereas the function of song in the breeding season is focused on mate attraction and nest defense, singing during the nonbreeding season appears to play a social contact function within flocks [34]. Heimovics and Riters [66,67] exposed breeding-condition and nonbreeding condition male starlings to a female stimulus and found that although males in both conditions sang about the same amount, correlations between neural activity and singing were very different across conditions. In breeding-condition males, song number correlated positively with Fos labeling in the medial preoptic nucleus, VTA, VMH, AH, and BSTm. These relationships were not present in the nonbreeding context, but nonbreeding song correlated positively with Fos labeling in three zones of the LS. Thus, the relationships between behavior and neural activity can be remodeled seasonally, tracking shifts in the significance of the behavior. Although not specifically addressed in these studies, it seems quite likely that the seasonal changes in brain-behavior relationships are based in hormone-mediated shifts in functional connectivity and neural context.
Sex steroids do not simply activate components of the SBN during periods of breeding, and in fact, it is becoming increasing clear that sex steroids of neural origin play important roles in behavioral regulation, even during periods of complete gonadal collapse, and that neurosteroids may exert seasonally variable patterns of effects [106]. The best evidence from this comes from male song sparrows (Melospiza melodia), which are territorial year-round [116]. Whereas castration reduces territorial behavior during the breeding season, it has no effect in non nonbreeding males [115]. Nonetheless, aromatase blockade reduces agonistic behavior in the nonbreeding season [107,108], indicating that a non-gonadal source of androgens supports nonbreeding territoriality. Steroidogenic enzymes show region-specific patterns of variation across seasons in male song sparrows [105], providing good support for the idea that neurosteroids exert seasonally variable influences on behavior that are based in patterns of modulation.
Although these studies strongly suggest that sex steroids create seasonally variable and context-specific patterns of functional connectivity, and thereby variable neural contexts, they do not directly demonstrate effects on functional connectivity. This represents a major gap in our knowledge of SBN and hormonal function. Indeed, although we hypothesize that hormonally mediated shifts in SBN functional connectivity represent one of the most important ways in which hormones influence vertebrate social behaviors, we are aware of no experiments that provide direct support for this idea.
5. The vertebrate nonapeptides: Neuromodulatory patterning and behavioral diversity
5.1. Nonapeptide systems are built for diversity
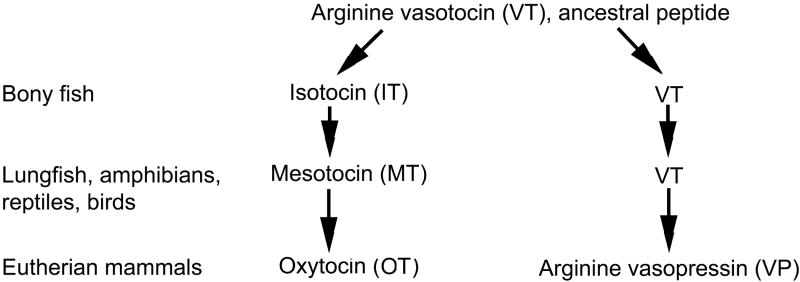
Of the many neuropeptide and transmitter systems in the brain, the nine amino acid “nonapeptides,” such as arginine vasotocin (VT), its mammalian homologue arginine vasopressin (VP), and the related peptide oxytocin (OT) arguably show the greatest variation and plasticity, including seasonal and hormone-mediated changes in virtually every respect (e.g., mRNA, peptide distributions, projections, receptor densities, etc.). Nonapeptide systems and nonapeptide behavioral functions show an equally wide range of variation across species, sexes, phenotypes, and contexts [11,20,26,51]. The evolutionary history of this family is shown in Figure 2, and has recently been reviewed in depth [45].
Fig. 2.
Evolutionary history of the vertebrate nine-amino acid “nonapeptides.” Arginine vasotocin (VT) is the ancestral peptide form. Separate clades giving rise to mammalian vasopressin (VP) and oxytocin (OT) originated with a duplication of the VT gene in early jawed vertebrates. Each change in form (e.g., from isotocin, IT, to mesotocin, MT) represents a single amino acid substitution, but OT differs from VT at only one position. Only major features of the family are shown, and some species show unique peptide forms. For reviews, see [2,70,71].
Given that nonapeptides are made in multiple cell groups that each have distinct patterns of activity and neural release (see section 5.3), much of the apparent variation in VT/VP function across species or phenotypes may 1) reflect the differential involvement of specific VT/VP cell groups, which creates differences in neuromodulatory patterning, and 2) reflect the fact that the neuromodulator action is being superimposed upon distinct neural contexts within the SBN. Indeed, as presented below, studies of the nonapeptides are beginning to yield some of the most direct evidence for phenotype- and species-specific neuromodulatory patterning within the SBN.
5.2. Receptor distribution patterns and social diversity in voles
In the early 1990’s, studies of monogamous and non-monogamous mouse species (Peromyscus spp.) [72], and monogamous and non-monogamous vole species (Microtus spp.) [73], demonstrated an extraordinary level of species-specificity in OT receptor (OTR) and VP V1a receptor (V1aR) distributions. Subsequent experiments in monogamous species have demonstrated that activation of VP receptors is necessary and sufficient for selective partner preference (a measure of monogamous-like behavior) in males, and that a comparable role is played by OT in females [81,119]. However, in comparing the receptor distributions in mice to those in voles, it is clear that most species differences do not show good correspondence to mating system, with the exception of V1aR density in the ventral pallidum, which is reliably higher in monogamous species. Numerous experiments now demonstrate that selective partner preference can be manipulated by site-specific manipulations in the ventral pallidum [82,96], and males of a non-monogamous vole species can be induced to exhibit partner preference by V1aR over-expression in this area [80].
These findings strongly encourage a single-locus view of monogamy, but other lines of evidence in the monogamous prairie vole (Microtus ochrogaster) suggest that species-specific patterns of bonding are related to a distributed pattern of V1aR. For instance, the LS is also an important site for V1aR-mediated effects on pair bonding [83], and partner preference behavior correlates with V1aR density in at least 12 neural loci [65]. Those loci include multiple components of the SBN (e.g., CG and MeA) and numerous other limbic areas. This distributed pattern of correlations is perhaps not surprising, though, given that V1aR densities covary across many brain regions, based upon shared developmental origins [95].
Although these findings do not directly demonstrate a role for neuromodulatory patterning in the evolution of mating systems, they do suggest the intriguing possibility that bonding behavior is most tightly coupled to species-specific patterns of covariation in V1aR densities across brain areas, rather than being driven solely by the density of V1aR in a single locus. That is, when we focus on single loci across the vole and mouse studies, only the ventral pallidum shows a reliable relationship to behavior, but relative V1aR densities across many brain areas may be an even more powerful variable. This line of thought is implicit in much of the mating system literature, but the necessary analyses (e.g., as in [118]; see section 3) have not yet been applied.
5.3. Good stuff, bad stuff and functionally distinct VT/VP cell groups
VT/VP release in the SBN originates in multiple cell groups, each of which responds to environmental stimuli in its own unique fashion [51,79]. These cell groups also exhibit different patterns of projections throughout the brain. Hence, activation of VT cell group X should impact behavior differently than activation of VT cell group Y, based on differences in neuromodulatory patterning (as defined in section 2.4). Although this hypothesis might appear logically sound, it should be noted that neuromodulatory patterning cannot be fully elucidated using common experimental approaches, such as site-specific and intracerebroventricular (i.c.v.) drug manipulations, since neither of those methods allows us to mimic or block a natural pattern of peptide release throughout the brain. In the remaining sections, we will show how we have demonstrated the importance of neuromodulatory patterning using i.c.v. peptide manipulations combined with analyses of neuronal activation (using combined immunocytochemistry for VT and Fos), and will discuss promising new findings obtained using VT antisense, which does allow the manipulation of natural VT release patterns.
How many of the numerous VT/VP cell groups do we need to be concerned with? The answer to this is not entirely clear, but at least four have been shown to be important contributors to SBN function: 1) PVN and homologues. Much of the VT/VP innervation of the basal forebrain and brainstem appears to originate from VT/VP neurons in the PVN (in amniotes) or homologous neurons [90] in the parvocellular preoptic area of anamniotes (cf. [52,53]). As expounded upon below, these neurons regulate both physiological and behavioral responses to stress [68]. 2) BSTm. Tetrapods exhibit a derived VT/VP cell group in the BSTm [45,90], which is implicated in a variety of social behavior functions [61,62,113]. 3) Anterior hypothalamus. Small populations of VP neurons are also found in the anterior hypothalamus, including the nucleus circularis, and are considered together here because they are not clearly differentiated in several functional studies. These neurons facilitate agonistic behavior, at least in hamsters [39] (also see [44]), and topographically similar (homologous?) neurons are found in other tetrapods as well [90]. 4) SON and homologues. Finally, magnocellular VT/VP neurons (e.g., in the SON or magnocellular preoptic area in anamniotes) may also contribute to the regulation of social behavior [27,64,79], but their behavioral roles are poorly understood. These neurons are best known for their physiological functions, such as regulation of hydromineral balance, but may generate dynamic patterns of neuromodulation via dendritic peptide release [36,79].
Although we have examined all of these populations in recent studies of songbirds and mice (see below), positive results have been obtained only for the BSTm and PVN, and thus we will focus mostly on those two cell groups. Interestingly, the VT/VP neurons of the BSTm and PVN are activated by positive and negative stimuli, respectively, and thus appear to be virtual opposites. We will first describe these differences, and then proceed to show how they might contribute to context- and phenotype-specific behavioral regulation.
PVN
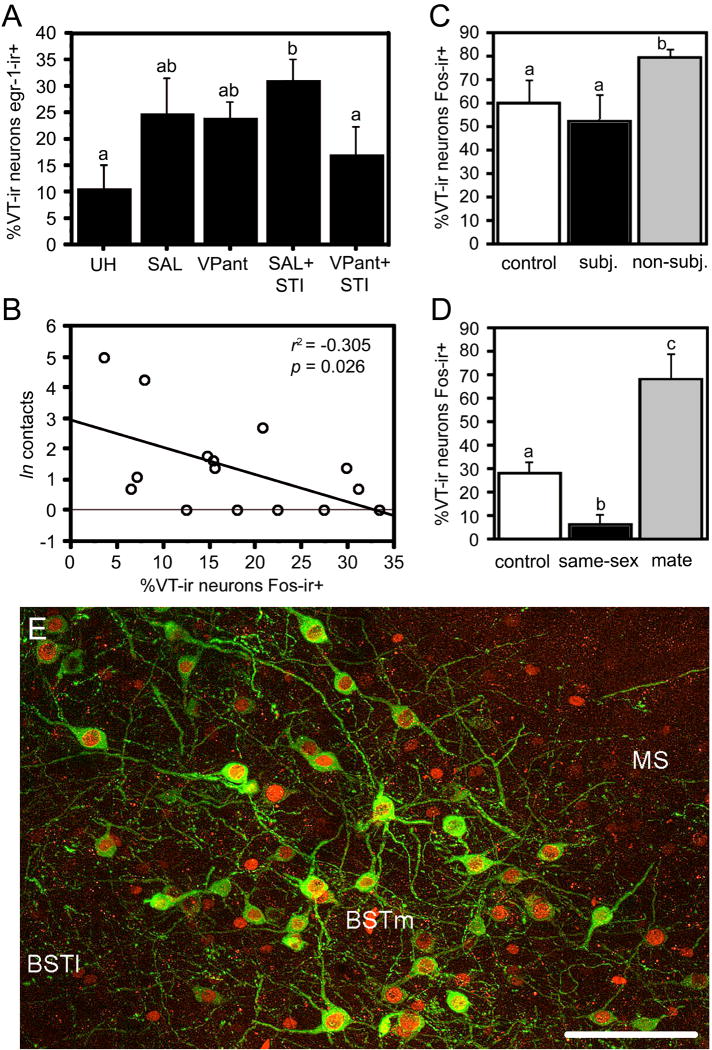
Across all vertebrate taxa, the neurons in the PVN (or parvocellular preoptic neurons in anamniotes) release VT/VP into the anterior pituitary in response to stress, and project to other stress-responsive brain regions, including the lateral BST and central amygdala [51,68]. These neurons respond relatively weakly to physiological stressors, but very robustly to emotional stressors such as forced swimming and agonistic encounters [35,54,117]. Figure 3A shows an example of this from male song sparrows housed in field-based flight cages. VT-immunoreactive (-ir) cells of the PVN tended to show increased colocalization of egr-1 (“Zenk”) following handling and restraint (for i.c.v. infusion of saline or a VP antagonist) and showed very robust increases in colocalization when this was followed by a simulated territorial intrusion (STI) [54]. Despite this increase, recent findings in song sparrows show that VT-Fos colocalization correlates negatively with aggression (Fig. 3B; J.L. Goodson, A.K. Evans and K.K. Soma, unpubl. obs.), suggesting that increased activity of the PVN VT neurons is most pronounced in less aggressive (typically subordinate?) animals. Notably, no change in VT-Fos colocalization was observed in the BSTm following STI (~21% VT-ir neurons colocalized Fos in silent controls vs. ~17% following STI), or in any of the small populations of the anterior hypothalamus. Comparable results are obtained in resident-intruder tests with male C57/BL6 mice: Subordinate mice exhibit significantly higher levels of VP-Fos colocalization in the PVN than do dominant mice, and fighting does not elevate colocalization in the BSTm above the level of non-aggressive chemoinvestigation (J.M. Ho, J.M. Murray, G.E. Demas, and J.L. Goodson; unpubl. obs.).
Fig. 3.
Functional profiles of VT cell groups in the PVN and BSTm of songbirds, showing that these cell groups respond to negative and positive stimuli, respectively. (A) Colocalization of VT and egr-1 (Zenk) in male song sparrows housed on semi-natural territories (field-based flight cages) following control conditions (unhandled; UH); capture and restraint for i.c.v. infusion of saline (SAL) or a VP antagonist (VPant); or infusions followed by simulated territorial intrusions (STI; presentation of a caged decoy and playback of song). Total n = 21. Modified from [54]. Although VT-Zenk colocalization increases following STI, findings in male song sparrows (B) show that VT-Fos colocalization in the PVN correlates negatively with an index of aggressive response (“contacts;” n = 16; J.L. Goodson, A.K. Evans and K.K. Soma, unpubl. obs.). Whereas VT-Fos colocalization in the BSTm was unaffected by STI (not shown), it does increase in zebra finches following aggressive competition for mates (C), but not in subjects that are intensely subjugated (subj.) and not allowed to court (total n = 15; sexes pooled). This pattern of results for the BSTm reflects a sensitivity to stimulus valence. For instance, whereas exposure to a same-sex conspecific produces a significant increase in VT-Fos colocalization in the BSTm of zebra finches, which are gregarious (see section 5.4), same-sex stimuli produce a decrease in VT-Fos colocalization in the BSTm of territorial violet-eared waxbills (D). Violet-eared waxbills nonetheless exhibit robust increases in colocalization following exposure to a positive social stimulus (the subject’s pair bond partner, or “mate;” also in panel D; total n = 16; sexes pooled). Modified from [62]. Bar graphs present means ± SEM. Different letters above the error bars denote significant group differences. (E) Representative labeling for VT (Alexa Fluor 488; green) and Fos (Alexa Fluor 594; red) in the BSTm of a male zebra finch following exposure to a female. Abbreviations: BSTl, lateral bed nucleus of the stria terminalis; MS, medial septum. Scale bar = 50 μm. Modified from [61].
BSTm
In strong contrast to the profile of the PVN VT/VP neurons, recent findings in songbirds (a phylogenetically controlled comparison of five estrildid finch species that differ selectively in sociality; see section 5.5) demonstrate that VT neurons of the BSTm increase their Fos protein expression only in response to stimuli that are of a positive social valence – that is, stimuli that are associated with affiliation. Negative social stimuli either have no effect, or actually produce decreases in Fos within the BSTm VT-ir neurons [62]. For instance, zebra finches that are engaged in aggressive competition over a potential mate exhibit increases in VT-Fos colocalization, but only if the subject is not intensely subjugated and is allowed to court (Fig. 3C). This valence sensitivity also sets up significant species differences in neuronal response, such that exposure to a same-sex conspecific produces increases in VT-Fos colocalization in highly gregarious finch species, while producing decreases in colocalization in relatively asocial, territorial species. The different valence effects are particularly pronounced in the territorial violet-eared waxbill (Estrildidae: Uraeginthus granatina), which shows a significant decrease in VT-Fos colocalization in response to a same-sex conspecific (a negative stimulus), but a dramatic increase to the subject’s pair bond partner (a positive stimulus; Fig. 3D) [62]. Finally, this exquisite sensitivity to valence is coupled with an equally remarkable selectivity for social stimuli. That is, although BSTm VT neurons respond strongly to positive social stimuli, they show a convincing lack of response to a positive non-social reinforcer [61]. As with the PVN, comparable findings are obtained in C57/BL6 mice. Thus, VP-Fos colocalization in the BSTm increases modestly (but significantly) following nonaggressive chemoinvestigation, and a very large increase is observed following copulation (J.M. Ho, J.M. Murray, G.E. Demas, and J.L. Goodson; unpubl. obs.).
Despite the very different functional profiles of the PVN and BSTm cell groups, they are closely juxtaposed and show apparently commingled terminal fields (Figs. 4 and 5). Hence, VT/VP release from these populations likely reaches many of the same brain areas via a combination of direct projections and diffusion to distal sites [36,79]. For instance, while direct VT/VP projections to the LS appear to arise almost exclusively from the BSTm [21,26], physiological data demonstrate that the PVN cell group also exerts significant effects on the LS [30,31]. This mixture of signals about “good” and “bad” stimuli is highly counterintuitive if we take a single-locus view of peptide action. However, no such conundrum exists if we consider the actions of the PVN and BSTm cell groups within the frameworks of neuromodulatory patterning and neural context, since each cell group will generate a unique pattern of release, and will do so under different social conditions.
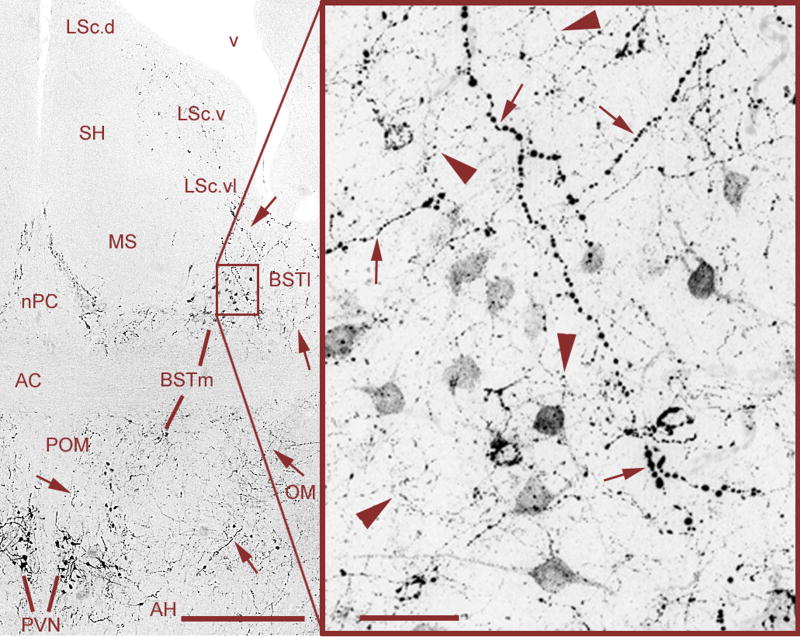
Fig. 4.
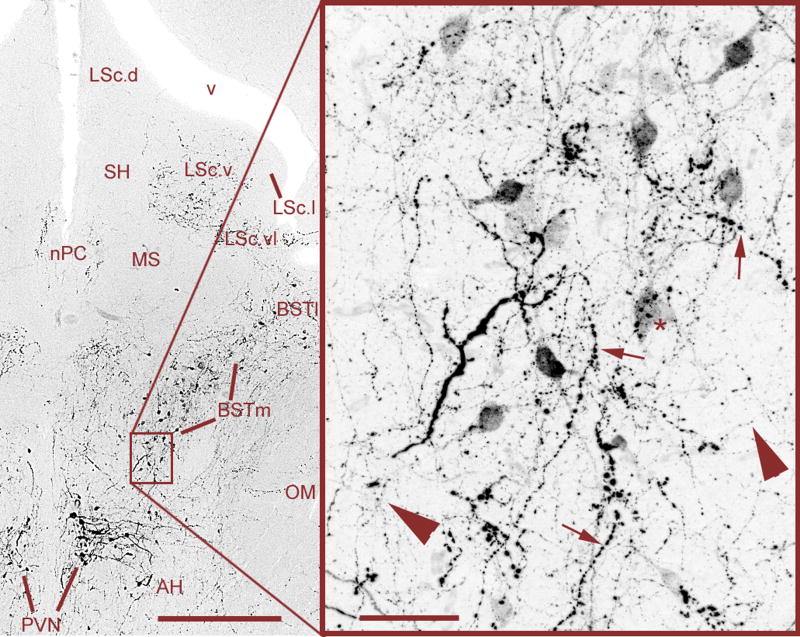
Mixed VT signals in the BSTm and LS of a male zebra finch, originating from VT neurons in the PVN and BSTm that are primarily responsive to negative and positive stimuli, respectively (see section 5.3). Large-caliber, heavily beaded axons (small arrows) are observed coursing from the PVN through the BSTm and directly into the ventrolateral zone of the caudal LS (LSc.vl). Relatively heavier projections are observed to the lateral BST (BSTl), but terminate immediately adjacent to the BSTm and LSc.vl. Within the BSTm (box), fine-caliber, beaded axons of local origin (large arrowheads) mix with the heavier axons of apparent PVN origin. Photos were taken at the rostral (supracommissural) level of the BSTm, where the spatial separation between the BSTm and PVN is greatest. As shown in Fig. 5, axonal commingling also occurs caudally in the vicinity of the PVN. Scale bars = 200 μm (left) and 20 μm (right). Other abbreviations: AC, anterior commissure; AH, anterior hypothalamus; LSc.d, dorsal zone of the LSc; LSc.v, ventral zone of the LSc; MS, medial septum; nPC, nucleus of the pallial commissure; OM, occipital-mesencephalic tract; POM, medial preoptic nucleus; SH, septohippocampal septum.
Fig. 5.
Mixed VT signals in the caudal BSTm and immediate vicinity of the PVN. Large-caliber, heavily beaded axons from the PVN (small arrows) and fine-caliber, beaded axons from the BSTm (large arrowheads) commingle in the ventral BSTm and putative terminals of a large-caliber axon are observed on a BSTm VT neuron (asterisk). Scattered BSTm-like neurons are often observed in the fibers immediately lateral to the PVN, and the mixture of large-caliber and fine-caliber fibers (as shown in the box) is observed throughout the entire area. Scale bars = 200 μm (left) and 20 μm (right). Abbreviations as in Fig. 4.
5.4. Distinct cell groups in action: Neuromodulatory patterning of aggression varies across contexts and phenotypes
Early studies of the VP circuitry of the BSTm and LS showed that this circuitry is sexually dimorphic (male-biased) and strongly regulated by sex steroids in rats [22-25]. Subsequent studies in Syrian hamsters (Mesocricetus auratus) demonstrated that septal VP promotes flank marking, an agonistic communication behavior [38,74]. This combination of findings suggested the fairly straightforward hypothesis that septal VP promotes aggressive behavior, particularly in males. Consistent with this idea, intraseptal VT infusions in male field sparrows (Spizella pusilla) selectively increase the use of an agonistic song type during the “dawn song” period [46], and VT infusions (either intraseptal or i.c.v.) likewise increase aggressive behavior during mate competition in zebra finches [49,60]. However, despite the effects on agonistic song, intraseptal VT infusions decrease territorial (resident-intruder) aggression in field sparrows [46], and also decrease resident-intruder aggression in the violet-eared waxbill [48], a species that is virtually identical to the zebra finch in much of its behavior and ecology, other than being territorial.
These complex findings have generally been regarded as evidence for species differences in the relationship between VT and aggression (e.g., [51]). However, the species differences are confounded with context (i.e., resident-intruder versus mate competition). Furthermore, the role of endogenous VT, as assessed with the use of a V1a antagonist, had only been assessed for mate competition aggression in zebra finches [49,60], and thus the importance of endogenous VT for territorial aggression remained to be demonstrated.
In order to address these issues, we recently developed a mate competition paradigm for male violet-eared waxbills, and then compared the effects of a novel V1a antagonist (which readily crosses the blood-brain barrier; delivered systemically) on aggression in both resident-intruder and mate competition tests. The antagonist significantly inhibited aggression in the mate competition tests, but appeared to have absolutely no effect in the resident-intruder test (Fig. 6A-B) [59].
Fig. 6.
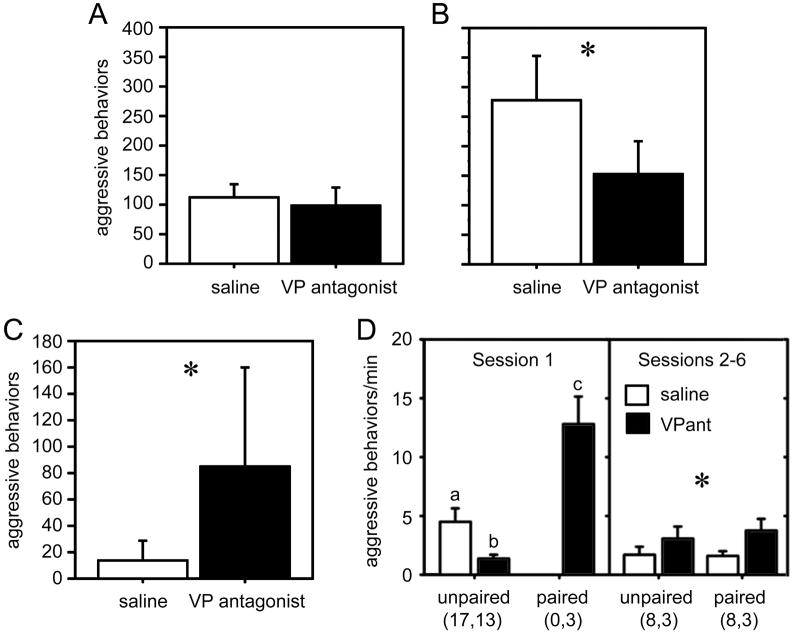
Neuromodulation of aggression varies across contexts and phenotypes. (A-B) Total numbers of aggressive behaviors (means ± SEM) exhibited by male violet-eared waxbills in the context of (A) territorial defense (resident-intruder tests) and (B) mate competition. Subjects were tested in a within-subjects design following injections of saline control or JNJ-17308616, a novel V1a antagonist that crossed the blood-brain barrier. Tests were 7 min. Total n = 9 for all *p = 0.015. (C) Aggressive behaviors exhibited in resident-intruder tests (as in panel A) by male violet-eared waxbills that were typically subordinate. Total n = 6; *p = 0.043. Modified from [59]. (D) Total aggression levels per minute off of the nest (means ± SEM) exhibited by male zebra finches in colony cages containing 4 males and 5 females. Focal 10-min observations were conducted in the morning and afternoon for three days (corresponding to sessions 1-6). Data are shown separately for session 1, soon after focal males were introduced to the females, and during sessions 2-6 when all birds had been housed together for a period of hours to days. Data are displayed separately for unpaired and pair-bonded individuals, and those receiving VP V1 antagonist or saline infusions (i.c.v.; twice daily). In session 1, paired males exhibited more aggression than unpaired males (p = 0.0002), and VP antagonist treatment resulted in a decrease in aggression relative to treatment with saline (p = 0.006). Different letters above the error bars denote significant group differences (p < 0.05). In sessions 2 to 6, the antagonist resulted in an increase in aggression levels relative to saline treatment (*p = 0.04). Group n’s are shown below the figure. Data for all males are shown for session 1; analyses for sessions 2-6 are restricted to males for which unpaired and paired data are available. Modified from [75].
These findings for mate competition replicate those in zebra finches, and are also consistent with the fact that VT-Fos colocalization increases in the BSTm during mate competition and courtship [61,62]. However, the lack of effects on territorial aggression were surprising, given that 1) VT-Fos colocalization in the PVN correlates negatively with aggressive behavior, and 2) exogenous VT inhibits overt aggression in territorial sparrows and waxbills [46,48]. Both of these observations suggest that the V1a antagonist should facilitate territorial aggression, which it did not. However, the subjects had been screened for robust aggression, and we therefore hypothesized that the lack of antagonist effects in the resident-intruder test reflected the fact that we were testing the “wrong” birds. That is, the findings for VT-Fos colocalization in the PVN suggest that less aggressive males exhibit higher levels VT neuronal activity during territorial encounters, and that more aggressive males actually reduce the activity of PVN neurons from baseline. We therefore conducted another experiment using less aggressive male waxbills that were typically subordinate in resident-intruder tests. These males exhibited virtually no aggression when injected with saline, but showed a striking increase in aggression when treated with the V1a antagonist (Fig. 6C) [59]. This phenotype difference in endogenous VT function is strongly corroborated by microdialysis data from male rats, showing that aggressive, low-anxiety males decrease intraseptal VP release during resident-intruder tests, whereas less aggressive, high-anxiety males increase release [9].
Importantly, the pattern of results just described for violet-eared waxbills is precisely what we would predict based upon the stimulus-response characteristics of the BSTm and PVN neurons. Hence, these findings provide compelling support for the hypothesis that context- and phenotype-specific effects of VT are a product of distinct neuromodulatory patterns deriving from neurons of the BSTm and PVN. However, we must also consider that VT release is being superimposed upon neural networks that are in different conformations of functional connectivity (i.e., in relation to the social context of mate competition versus the social context of resident-intruder testing). Thus, the diverse effects of VT on aggression are likely based on a combination of both neuromodulatory patterning and neural context. Indeed, the field sparrow data described above provide good evidence for neural context effects: When VT infusions are administered in the context of a resident-intruder test, aggression is inhibited, but in the context of dawn singing, the same infusions selectively facilitate the use of a strictly agonistic song type [46].
A recent experiment in male zebra finches provides a good parallel to the violet-eared waxbill experiments, and further demonstrates that the relationship of endogenous VT to aggression changes across social contexts. Male zebra finches were introduced to colony nesting cages in groups of four, and five females were provided as potential partners. High levels of aggression were exhibited at the time of introduction, mostly focused on competition for mates. As in other mate competition tests (see above), aggression was significantly lower in unpaired males that received central VP antagonist infusions. However, this effect was completely reversed over subsequent days in the colony environment, such that the VP antagonist instead increased aggression as most males paired and began to nest (Fig. 6D) [75]. Aggression in paired zebra finches is largely focused on nest defense [120], a behavior that is similar in many ways to territorial aggression. Therefore, endogenous VT inhibits aggression in both resident-intruder tests (e.g., in territorial sparrows and waxbills) [46,48] and colony nesting experiments (in zebra finches) [75], suggesting that the neural mechanisms of nest defense and territoriality are similar.
5.5. Neural context and neuromodulatory patterning: Mechanisms of social motivation differences in territorial and flocking songbirds
In most analyses of IEG activity in the SBN, animals are sacrificed following active interactions with conspecifics, and it is therefore difficult to determine whether neural activity patterns reflect perceptual and motivational processes, or overt behavioral performance. Overt behavior is thus a strong concern when comparing animals that express distinct behavioral phenotypes, since animals that perform different behaviors will show differences in neural activity that are associated with overt “behaving” in addition to differences that relate to perceptual and motivational processes. Assuming that species differences in characters such as sociality are based primarily in the mechanisms of perception and motivation, the elucidation of relevant neural processes (e.g., through analyses of IEG induction) therefore require the elimination of species differences in overt responses to social stimuli.
Thus, in order to compare the neural processing of social stimuli in gregarious and territorial finch species, we constructed a paradigm that limits the overt behavioral performance of subjects while exposing them to same-sex conspecifics. Subjects are acclimated to a quiet room outside of the normal housing environment, which suppresses territorial behavior, and then subjects are exposed to a same-sex conspecific through a wire barrier. Although subjects exhibit arousal, the majority of behaviors that typify physical interactions are eliminated or greatly reduced [56]. When tested in this manner, territorial subjects exhibit significantly higher levels of IEG response (Fos and egr-1) than do three gregarious species within the ventrolateral LS, medial extended amygdala, anterior hypothalamus, and lateral VMH. Interestingly, this pattern is virtually identical to a pattern of activity that distinguishes female rats that express “pup aversion” from fully maternal rats [102,103], and therefore appears to be distinctive for animals that are exposed to socially aversive stimuli.
What coordinates these species-specific neural responses to conspecific stimuli? Neuromodulatory patterning by neuropeptides seems to be a good place to look for answers, particularly in relation to nonapeptide systems, which exhibit profound evolutionary lability [45]. We therefore conducted studies to examine the Fos activation of VT neurons [61,62], and also to examine the distributions of nonapeptide receptors and other relevant receptor types [58]. These experiments are described below and were conducted in five estrildid finch species, including two relatively asocial, territorial species that live as male-female pairs year-round (violet-eared waxbill and melba finch, Pytilia melba); two highly gregarious, colonially breeding species that exhibit modal group sizes of approximately 100 (zebra finch and spice finch, Lonchura punctulata); and a modestly gregarious species, the Angolan blue waxbill (Uraeginthus angolensis), which exhibits a modal group size of approximately 20. These species are all monogamous (exhibiting long-term or life-long pairbonds), biparental, and share most other aspects of behavior and ecology [58,63]. Importantly, the two territorial species likely evolved territoriality independently from a modestly gregarious ancestor, and the colonial species also evolved independently from more modestly gregarious ancestors [58]. Finally, the modestly gregarious species is a sympatric congener of the territorial violet-eared waxbill. Combined, these species provide an excellent opportunity to examine convergent and divergent evolution in sociality.
Using the same paradigm described above, we found that the VT-ir neurons of the BSTm exhibited significantly different Fos responses to a same-sex stimulus across the five species, and in a pattern that closely matched sociality. Thus, over a period of 90 min, VT-Fos colocalization decreased in the territorial species, increased in the highly gregarious species, and increased only slightly (and non-significantly) in the intermediate species [62]. No such differences were observed in the PVN (J.L. Goodson and Y. Wang, unpubl. obs.). As described above (see section 5.3), these differences reflect the valence of the stimulus. For instance, although VT-Fos colocalization decreases significantly in violet-eared waxbills following exposure to a same-sex conspecific, it increases following exposure to the subject’s pair bond partner [62]. By themselves, such species differences in the response of VT neurons should be sufficient to create differences in neuromodulatory patterning, but these differences are magnified in the estrildid finches in multiple ways: Baseline VT-Fos colocalization is significantly higher in the three flocking species as compared to the two territorial species, and the two highly gregarious species exhibit almost ten times more VT-ir neurons than do the less social species [62].
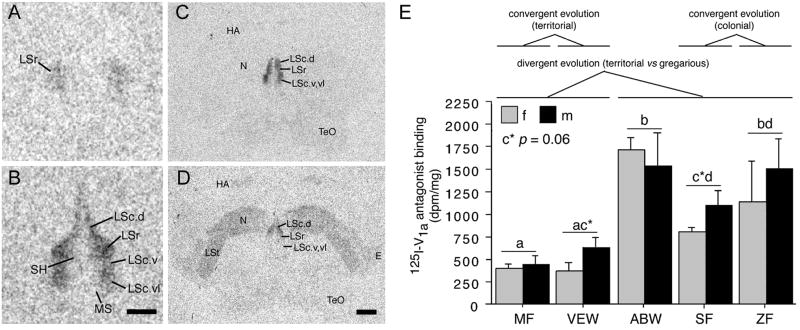
In order to determine whether species differences in neuropeptide receptor systems may also contribute to species differences in neural activity, Goodson et al. [58] examined binding sites for VT (using an iodinated V1a antagonist), corticotropin releasing factor (using iodinated antisauvagine-30), and vasoactive intestinal polypeptide (VIP). All three of these peptides are known to influence social behavior in context- and phenotype-specific ways. Clear cases of convergent and divergent evolution were observed for all three ligands, most prominently in the septal complex, where sociality-related differences in binding were found for each of the ligands. In order to be deemed sociality-related, we required that binding densities for the two territorial species differ significantly from the two colonial species, and that the modestly gregarious species be intermediate or fall in line with the two colonial species. The most convincing comparisons were those that met these criteria, with significant differences also being observed between the territorial and gregarious Uraeginthus species. Figure 7 shows one such pattern for the density of V1a-like receptor density in the dorsal LS, and similar species differences are observed in several other septal subdivisions. Outside of the septal complex, sociality related differences were also observed for antisauvagine-30 binding in the VMH and VIP binding in the BSTm, both biased towards the gregarious species, and for antisauvagine-30 binding in the infundibular hypothalamus, which was biased towards the territorial species.
Fig. 7.
Species differences in linear 125I-V1a antagonist binding reflect convergent and divergent evolution in sociality. (A-B) Representative binding in the septum of the territorial violet-eared waxbill (VEW; A), and moderately gregarious Angolan blue waxbill (ABW; B). (C-D) Representative sections for a male Angolan blue waxbill (C) and male spice finch (colonial; D), showing species differences in binding for the nidopallium (N) and other areas of the forebrain. E. Linear 125I-V1a antagonist binding in the dorsal (pallial) portion of the lateral septum (LS), shown as decompositions per minute/mg (dpm/mg; means ± SEM). Different letters above the error bars denote significant species differences (p < 0.05). The scale bar in B corresponds to 500 μm in panels A-B; the scale bar in D corresponds to 1 mm in panels C-D. Abbreviations: E, entopallium; HA, apical part of the hyperpallium; LSc, caudal division of the lateral septum (dorsal, ventrolateral, and ventral zones denoted as LSc.d, LSc.vl, and LSc.v, respectively); LSr, rostral division of the lateral septum; LSt, lateral striatum; MS, medial septum; N, nidopallium; SH, septohippocampal septum; TeO, optic tectum. Modified from [58].
Overall, these findings strongly suggest that species differences in social processing are extensively based in species differences in neuromodulatory patterning. However, given the constitutive nature of some variables (e.g., in receptor distributions, VT-ir cell numbers in the BSTm, and baseline VT-Fos colocalization), it is also likely that baseline differences in peptide signaling produce constitutive, species-specific neural contexts.
5.6. Context-specific neuromodulatory patterns directly influence avian sociality
The findings just described strongly suggest that sociality is facilitated by VT derived from the BSTm. We recently addressed this hypothesis through a series of experiments in the zebra finch. First, in order to establish that endogenous VT modulates sociality, male and female zebra finches were given an injection of saline or a novel V1a antagonist that crosses the blood-brain barrier (as in section 5.5). Subjects were placed in a long central cage that adjoined a smaller cage at each end. One of these smaller cages contained two same-sex individuals, and the other contained ten. As expected, the V1a antagonist reduced the amount of time spent in close proximity to the large group. However, this effect was only observed when the stimulus animals were familiar to the subjects. When the social stimuli were novel, the pattern was reversed (J. L. Goodson, D. Kabelik, and S. E. Schrock, unpub. obs.).
This reversal in antagonist effects could reflect variations in neural context and/or neuromodulatory patterning. If we were to find that patterns of VT release in the brain are comparable during exposure to familiar and novel conspecifics, then the reversal is a product of neural context. Alternatively, the reversal could be based in the context-dependent activity of VT cell groups that each bear a different relationship to behavior (as addressed in sections 5.3 and 5.4). This latter hypothesis suggests a neuromodulatory tug of war, with behavior being determined by the relative weighting of activity across different VT cell groups, and hence by the resultant pattern of VT release. If this is so, then VT derived from any given cell group should bear a consistent relationship to sociality, (although it could be overridden by VT from other cell groups) regardless of whether the social stimuli are novel or familiar. In contrast, if the reversal in antagonist effects are based in neural context, then VT derived from any given cell group should bear a relationship to sociality that depends upon whether the social stimuli are novel or familiar.
To test these alternative hypotheses, we are currently attempting to down VT production by unilateral antisense oligodeoxynucleotide infusions targeting the BSTm of male zebra finches (D. Kabelik, J.L. Goodson, and R.R. Thompson, unpub. obs.). This approach should allow us to manipulate neuromodulatory patterning that derives from the BSTm while leaving the influence of other VT cell groups intact. Thus, if VT knockdown reduces sociality in tests with both novel and familiar stimuli, then the contextual variation in VT effects that we have observed with the antagonist must derive from competing cell groups.
6. Conclusions
Behavioral outputs of the SBN and associated limbic networks appear to be based in distributed patterns of activity across network nodes, such that the functional connectivity of SBN nodes varies dynamically in relation to context and phenotype. We have here presented evidence that these context- and phenotype-specific activity patterns arises through differences in neuromodulatory patterning, and that variation in patterning can occur as a product of 1) differential activation of cell groups (e.g., differential activation of the VT cell groups in the PVN and BSTm, which exhibit distinct projections and response profiles), and 2) differences in the distribution of receptors. Within this framework, it is the distributed pattern of modulation that determines the behavioral impact of the neuromodulator, not the effects at single loci. Differences in neuromodulatory patterning are strongly implicated in the production of individual differences in aggression phenotype, as well as species differences in sociality and mating system. In addition, limited evidence suggests that a given neuroendocrine variable can exert diverse behavioral effects depending upon the neural context that the variable is superimposed upon. For instance, VT release in the LS both promotes and inhibits agonistic behavior in field sparrows, depending upon the context. Unfortunately, the experimental dissection of distributed processes and functional connectivity is not well suited to the traditional tools of behavioral neuroscience, which are based in site-specific or wholly nonspecific approaches to neuroendocrine function. However, manipulation of distributed systems is increasing possible through the use of such tools as RNA interference and antisense, which allow specific cell groups to be knocked down, and through transgenics, which allows distributed patterns of receptors to be manipulated in toto.
Acknowledgments
Support provided by National Institutes of Health grant MH62656.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul Pept. 2008;149:3–10. doi: 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acher R. Chemistry of the neurohypophysial hormones: an example of molecular evolution. Endocrinology, American Physiological Society; Washington, D.C.: 1972. pp. 119–130. [Google Scholar]
- 3.Adamantidis A, de Lecea L. Sleep and metabolism: shared circuits, new connections. Trends Endocrinol Metab. 2008;19:362–370. doi: 10.1016/j.tem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balfour ME, Brown JL, Yu L, Coolen LM. Potential contributions of efferents from medial prefrontal cortex to neural activation following sexual behavior in the male rat. Neuroscience. 2006;137:1259–1276. doi: 10.1016/j.neuroscience.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Ball GF, Balthazart J. Ethological concepts revisited: Immediate early gene induction in response to sexual stimuli in birds. Brain Behav Evol. 2001;57:252–270. doi: 10.1159/000047244. [DOI] [PubMed] [Google Scholar]
- 7.Ball GF, Tlemcani O, Balthazart J. Induction of the Zenk protein after sexual interactions in male Japanese quail. Neuroreport. 1997;8:2965–2770. doi: 10.1097/00001756-199709080-00032. [DOI] [PubMed] [Google Scholar]
- 8.Balleine BW. Neural bases of food-seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 9.Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007;26:3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- 10.Buntin L, Berghman LR, Buntin JD. Patterns of Fos-like immunoreactivity in the brains of parent ring doves (Streptopelia risoria) given tactile and nontactile exposure to their young. Behav Neurosci. 2006;120:651–664. doi: 10.1037/0735-7044.120.3.651. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell HK, Lee H-J, Macbeth AH, Young WS., III Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Can A, Domjan M, Delville Y. Sexual experience modulates neuronal activity in male Japanese quail. Horm Behav. 2007;52:590–599. doi: 10.1016/j.yhbeh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a phaseolus-vulgaris-leukoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 14.Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- 15.Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Crews D. The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol. 2003;43:1–10. doi: 10.1002/dev.10115. [DOI] [PubMed] [Google Scholar]
- 17.Crews D, Lou W, Fleming A, Ogawa S. From gene networks underlying sex determination and gonadal differentiation to the development of neural networks regulating sociosexual behavior. Brain Res. 2006;1126:109–121. doi: 10.1016/j.brainres.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crews D, Moore MC. Evolution of mechanisms controlling mating behavior. Science. 1986;231:121–125. doi: 10.1126/science.3941893. [DOI] [PubMed] [Google Scholar]
- 19.Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- 20.De Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- 21.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 22.De Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- 23.De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain - presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 24.De Vries DJ, Buijs RM, van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- 25.De Vries GJ, Duetz W, Buijs RM, Van Heerikhuize J, Vreeburg JTM. Effects of androgens and estrogens on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 1986;399:296–302. doi: 10.1016/0006-8993(86)91519-2. [DOI] [PubMed] [Google Scholar]
- 26.De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- 28.Demski LS. Feeding and aggressive behavior evoked by hypothalamic stimulation in a cichlid fish. Comp Biochem Physiol A. 1973;44:685–692. doi: 10.1016/0300-9629(73)90134-5. [DOI] [PubMed] [Google Scholar]
- 29.Demski LS, Knigge KM. The telencephalon and hypothalamus of the bluegill Lepomis macrochirus: Evoked feeding, aggressive and reproductive behavior With representative frontal sections. J Comp Neurol. 1971;143:1–16. doi: 10.1002/cne.901430102. [DOI] [PubMed] [Google Scholar]
- 30.Disturnal JE, Veale WL, Pittman QJ. Modulation by arginine vasopressin of glutamate excitation in the ventral septal area of the rat brain. Can J Physiol Pharmacol. 1987;65:30–35. doi: 10.1139/y87-006. [DOI] [PubMed] [Google Scholar]
- 31.Disturnal JE, Veale WL, Pittman QL. The ventral septal area: electrophysiological evidence for putative arginine vasopressin projections onto thermoresponsive neurons. Neuroscience. 1986;19:795–802. doi: 10.1016/0306-4522(86)90299-x. [DOI] [PubMed] [Google Scholar]
- 32.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: Implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- 34.Eens M. Understanding the complex song of the European starling: An integrated ethological approach Advances in the Study of Behavior. Vol. 26. Elsevier Academic Press Inc; San Diego: 1997. pp. 355–434. [Google Scholar]
- 35.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol. 2000;85:125S–130S. doi: 10.1111/j.1469-445x.2000.tb00015.x. Spec No. [DOI] [PubMed] [Google Scholar]
- 37.Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferris CF, Delville Y, Irvin RW, Potegal M. Septo-hypothalamic organization of a stereotyped behavior controlled by vasopressin in golden hamsters. Physiol Behav. 1994;55:755–759. doi: 10.1016/0031-9384(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 39.Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fine ML, Chen FA, Keefer DA. Autoradiographic localization of dihydrotestosterone and testosterone concentrating neurons in the brain of the oyster toadfish. Brain Res. 1996;709:65–80. doi: 10.1016/0006-8993(95)01275-3. [DOI] [PubMed] [Google Scholar]
- 41.Fine ML, Keefer DA, Russel-Mergenthal H. Autoradiographic localization of estrogen-concentrating cells in the brain and pituitary of the oyster toadfish. Brain Res. 1990;536:207–219. doi: 10.1016/0006-8993(90)90027-9. [DOI] [PubMed] [Google Scholar]
- 42.Forlano PM, Maruska KP, Sower SA, King JA, Tricas TC. Differential distribution of gonadotropin-releasing hormone- immunoreactive neurons in the stingray brain: functional and evolutionary considerations. Gen Comp Endocrinol. 2000;118:226–248. doi: 10.1006/gcen.2000.7467. [DOI] [PubMed] [Google Scholar]
- 43.Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol. 2006;96:2295–2306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- 44.Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- 45.Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- 47.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- 49.Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 50.Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- 51.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 52.Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost, Porichthys notatus. J Comp Neurol. 2000;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 53.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 54.Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B Biol Sci. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 2005;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodson JL, Kabelik D, Schrock SE. Dynamic neuromodulation of aggression by vasotocin: influence of social context and social phenotype in territorial songbirds. Biol Lett. 2009 doi: 10.1098/rsbl.2009.0316. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci U S A. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodwin D. Estrildid finches of the world. Cornell University Press; Ithaca, NY: 1982. [Google Scholar]
- 64.Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc R Soc Lond B Biol Sci. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammock EA, Lim MM, Nair HP, Young LJ. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes Brain Behav. 2005;4:289–301. doi: 10.1111/j.1601-183X.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 66.Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) 2006 doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- 68.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci U S A. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoyle CHV. Neuropeptide families and their receptors: evolutionary perspectives. Brain Res. 1999;848:1–25. doi: 10.1016/s0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- 71.Hoyle CHV. Neuropeptide families: evolutionary perspectives. Regul Pept. 1998;73:1–33. doi: 10.1016/s0167-0115(97)01073-2. [DOI] [PubMed] [Google Scholar]
- 72.Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- 73.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–700. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- 75.Kabelik D, Klatt JD, Goodson JL. Modulation of male zebra finch aggression by a vasopressin V1a antagonist reverses across behavioral contexts in a colony environment. 2009 submitted. [Google Scholar]
- 76.Kabelik D, Weiss SL, Moore MC. Arginine vasotocin (AVT) immunoreactivity relates to testosterone but not territorial aggression in the tree lizard, Urosaurus ornatus. Brain Behav Evol. 2008;19:283–294. doi: 10.1159/000174248. [DOI] [PubMed] [Google Scholar]
- 77.Kabelik D, Weiss SL, Moore MC. Steroid hormone mediation of limbic brain plasticity and aggression in free-living tree lizards, Urosaurus ornatus. Horm Behav. 2006;49:587–597. doi: 10.1016/j.yhbeh.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Kabelik D, Weiss SL, Moore MC. Steroid hormones alter neuroanatomy and aggression independently in the tree lizard. Physiol Behav. 2008;93:492–501. doi: 10.1016/j.physbeh.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 81.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 82.Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 84.Lynch KS, Diekamp B, Ball GF. Catecholaminergic cell groups and vocal communication in male songbirds. Physiol Behav. 2008;93:870–876. doi: 10.1016/j.physbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- 86.Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- 87.McIntosh AR. Contexts and catalysts - A resolution of the localization and integration of function in the brain. Neuroinformatics. 2004;2:175–181. doi: 10.1385/NI:2:2:175. [DOI] [PubMed] [Google Scholar]
- 88.Meddle SL, Foidart A, Wingfield JC, Ramenofsky M, Balthazart J. Effects of sexual interactions with a male on fos-like immunoreactivity in the female quail brain. J Neuroendocrinol. 1999;11:771–784. doi: 10.1046/j.1365-2826.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- 89.Meddle SL, King VM, Follett BK, Wingfield JC, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- 90.Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- 91.Motta SC, Goto M, Gouveia FV, Baldo MVC, Canteras NS, Swanson LW. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci U S A. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 93.Peter RE. The preoptic nucleus in fishes: A comparative discussion of function-activity relationships. Am Zool. 1977;17:775–785. [Google Scholar]
- 94.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 95.Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J Comp Neurol. 2003;466:564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- 96.Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Powers JB, Winans SS. Vomeronasal organ - critical role in mediating sexual behavior of male hamster. Science. 1975;187:961–963. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- 98.Rhen T, Crews D. Embryonic temperature and gonadal sex organize male-typical sexual and aggressive behavior in a lizard with temperature-dependent sex determination. Endocrinology. 1999;140:4501–4508. doi: 10.1210/endo.140.10.7083. [DOI] [PubMed] [Google Scholar]
- 99.Ruscio MG, Adkins-Regan E. Immediate early gene expression associated with induction of brooding behavior in Japanese quail. Horm Behav. 2004;46:19–29. doi: 10.1016/j.yhbeh.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Sakata JT, Coomber P, Gonzalez-Lima F, Crews D. Functional connectivity among limbic brain areas: differential effects of incubation temperature and gonadal sex in the leopard gecko, Eublepharis macularius. Brain Behav Evol. 2000;55:139–151. doi: 10.1159/000006648. [DOI] [PubMed] [Google Scholar]
- 101.Sakata JT, Crews D. Developmental sculpting of social phenotype and plasticity. Neurosci Biobehav Rev. 2004;28:95–112. doi: 10.1016/j.neubiorev.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 102.Sheehan TP, Cirrito J, Numan MJ, Numan M. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav Neurosci. 2000;114:337–352. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- 103.Sheehan TP, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- 104.Shelley DN, Meisel RL. The effects of mating stimulation on c-Fos immunoreactivity in the female hamster medial amygdala are region and context dependent. Horm Behav. 2005;47:212–222. doi: 10.1016/j.yhbeh.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- 106.Soma KK, Scotti M-AL, Newman AEM, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 107.Soma KK, Sullivan K, Wingfield J. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen Comp Endocrinol. 1999;115:442–453. doi: 10.1006/gcen.1999.7334. [DOI] [PubMed] [Google Scholar]
- 108.Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. Proc R Soc Lond B Biol Sci. 2000;267:1089–1096. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: Neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- 110.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 111.Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur J Neurosci. 2006;23:1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- 112.Tlemcani O, Ball GF, D’Hondt E, Vandesande F, Sharp PJ, Balthazart J. Fos induction in the Japanese quail brain after expression of appetitive and consummatory aspects of male sexual behavior. Brain Res Bull. 2000;52:249–262. doi: 10.1016/s0361-9230(00)00233-1. [DOI] [PubMed] [Google Scholar]
- 113.Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- 114.Winans SS, Scalia F. Amygdaloid nucleus - new afferent input from vomeronasal organ. Science. 1970;170:330–&. doi: 10.1126/science.170.3955.330. [DOI] [PubMed] [Google Scholar]
- 115.Wingfield JC. Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm Behav. 1994;28:1–15. doi: 10.1006/hbeh.1994.1001. [DOI] [PubMed] [Google Scholar]
- 116.Wingfield JC, Hahn TP. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim Behav. 1994;47:77–89. [Google Scholar]
- 117.Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: A novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci. 1996;16:7725–7732. doi: 10.1523/JNEUROSCI.16-23-07725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang EJ, Wilczynski W. Social experience organizes parallel networks in sensory and limbic forebrain. Dev Neurobiol. 2007;67:285–303. doi: 10.1002/dneu.20347. [DOI] [PubMed] [Google Scholar]
- 119.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 120.Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]