Abstract
The arsenic (+3 oxidation state) methyltransferase (As3mt) gene encodes a 43 kDa protein that catalyzes methylation of inorganic arsenic. Altered expression of AS3MT in cultured human cells controls arsenic methylation phenotypes, suggesting a critical role in arsenic metabolism. Because methylated arsenicals mediate some toxic or carcinogenic effects linked to inorganic arsenic exposure, studies of the fate and effects of arsenicals in mice which cannot methylate arsenic could be instructive. This study compared retention and distribution of arsenic in As3mt knockout mice and in wild-type C57BL/6 mice in which expression of the As3mt gene is normal. Male and female mice of either genotype received an oral dose of 0.5 mg of arsenic as arsenate per kg containing [73As]-arsenate. Mice were radioassayed for up to 96 hours after dosing; tissues were collected at 2 and 24 hours after dosing. At 2 and 24 hours after dosing, livers of As3mt knockouts contained a greater proportion of inorganic and monomethylated arsenic than did livers of C57BL/6 mice. A similar predominance of inorganic and monomethylated arsenic was found in the urine of As3mt knockouts. At 24 hours after dosing, As3mt knockouts retained significantly higher percentages of arsenic dose in liver, kidneys, urinary bladder, lungs, heart, and carcass than did C57BL/6 mice. Whole body clearance of [73As] in As3mt knockouts was substantially slower than in C57BL/6 mice. At 24 hours after dosing, As3mt knockouts retained about 50% and C57BL/6 mice about 6% of the dose. After 96 hours, As3mt knockouts retained about 20% and C57BL/6 mice retained less than 2% of the dose. These data confirm a central role for As3mt in metabolism of inorganic arsenic and indicate that phenotypes for arsenic retention and distribution are markedly affected by the null genotype for arsenic methylation, indicating a close linkage between the metabolism and retention of arsenicals.
Introduction
Many animal species, including humans, convert inorganic arsenic to mono-, di-, and trimethylated products. The Challenger pathway for the conversion of inorganic arsenic to methylated products (1) is summarized in the general scheme
Here, each oxidative methylation reaction is preceded by reduction of arsenic from the pentavalent to the trivalent oxidation state. These reactions are catalyzed by a cytosolic enzyme, arsenic (+3 oxidation state) methyltransferase (As3mt), which uses S-adenosylmethionine (AdoMet) as a methyl group donor and requires a dithiol-containing reductant (e.g., thioredoxin) for catalysis (2). Because some methylated metabolites formed in As3mt-catalyzed reactions are more reactive and toxic than inorganic arsenicals, methylation of arsenic can be regarded as an activation process (3).
Genome annotation has identified orthologous As3mt genes in organisms as diverse as the sea urchin Strongylocentrotus purpuratus and Homo sapiens (2). There is a strong correlation between occurrence of an As3mt ortholog in the genome of a species and evidence of the capacity of that species to methylate inorganic arsenic (2, 4, 5). These orthologs share amino acid sequences common non-DNA methyltransferases and strongly conserved cysteinyl residues that function catalytically (2, 6). In cultured human uroepithelial cells, heterologous expression of rat As3mt confers capacity to methylate inorganic arsenic (7). Reducing expression of AS3MT in HepG2 hepatoma cells by RNA interference decreases cellular arsenic methylation and alters sensitivity to the cytotoxicity of methylated arsenicals (8). Because distribution and retention of arsenicals are affected both by the oxidation state of arsenic and the extent of methylation, changes in the capacity to methylate arsenic would be expected to change distribution and retention of arsenic.
Here we have compared metabolism, distribution, and clearance of arsenic in wild-type and As3mt knockout mice. The altered As3mt genotype affected tissue distribution, the rate of whole body clearance of arsenic, and the extent of conversion of inorganic arsenic into its methylated metabolites. Changes in phenotypes for methylation, distribution, and retention of arsenic in As3mt knockout mice confirm the central role of methylation in metabolism of inorganic arsenic.
Methods
Animal Origin and Maintenance
Production of As3mt knockout mice
A 12 kb DNA fragment that contains exons 1 through 7 of the mouse As3mt gene was identified by screening a lambda phage library made of genomic DNA from mouse embryonic stem cells (129S6 strain). A targeting construct was designed to delete after homologous recombination exons 3 through 5 that encode for amino acids important for the methyltransferase activity. A 1.2 kb Hind3/Sac1 fragment and a 6.6 kb Eag1/Nsi1 fragment was assembled as 5′ and 3′ arms of homology flanking a positively selectable marker gene, pMC1neo, and the herpes simplex TK gene was inserted into the plasmid as a negatively selectable marker gene (Supplemental Figure 1). The plasmid DNA of 14kb in length was linearized by digestion with Sal1 and introduced the targeting vector DNA into 129S6 strain ES cells by electroporation. G418-resistant ES cell colonies were expanded, screened by PCR followed by Southern blot analyses for the correct modification. Chimeras made with two of the five correctly modified ES cells transmitted the modified gene. The mutation was initially maintained in 129S6 mice. The mutant As3mt gene was transferred onto a C57BL/6 background through a series of backcrosses and the C57BL/6 heterozygous for mutant As3mt were intercrossed to generate homozygous mutant As3mt mice. As3mt Primer 1 (GACGCTGATGAGACTCACAA) and Primer 2 (CCATAGTAGTTCTGACTCAGC) or Primer 3 (CTTCCTCGTGCTTTACGGTA) were used to genotype mice. Amplification of DNA from the wild-type As3mt gene using Primers 1 and 2 yielded a 330bp band; for the modified As3mt gene amplification with Primers 1 and 3 yielded a 400bp band.
Because As3mt knockout mouse were fertile, homozygous knockouts were maintained by brother-sister matings.
Wild-type mice
Wild-type mice for this study were adult (> 8 weeks old) male and female C57BL/6 mice obtained from Charles River Laboratory (Raleigh, NC). Previous studies have shown that these mice rapidly converted arsenate to a variety of methylated metabolites (9) and that immunoreactive As3mt was present in various tissues (Z. Drobna, unpublished observations). C57BL/6 mice are hereafter referred to as wild-type mice.
Animal maintenance
All mice were routinely maintained in a 12 hour light-12 hour dark photocycle at about 20-22°C and 50% relative humidity with free access to pelleted rodent chow (TestDiet, Richmond, IN) and tap water. Before use in metabolism and disposition studies, mice were acclimated by individual housing in metabolic cages (Nalgene, Rochester, NY) for three days. Throughout the acclimation and experimental periods, mice had free access to AIN-93G powdered rodent diet (10) obtained from Dyets (Bethlehem, PA) and tap water. The Institutional Animal Care and Use Committee of the National Health and Environmental Effects Research Laboratory of the U.S. Environmental Protection Agency approved all procedures involving use of mice.
Dosing Solutions
Arsenic acid, sodium salt heptahydrate, used for dosing solutions was obtained from Sigma-Aldrich (St. Louis, MO). [73As]-arsenic acid used as a radiotracer in some dosing solutions was produced at Los Alamos National Laboratory and obtained through the National Isotope Program (Oak Ridge National Laboratory, Oak Ridge, TN). HPLC grade water (Burdick & Jackson, Muskegon, MI) was used to prepare all dosing solutions.
Animal Dosing Regimens
Whole body retention, tissue distribution, and excretion of orally administered [73As]-arsenate
Male and female As3mt knockout mice and wild-type mice received single oral doses of 0.5 mg of arsenic as arsenate per kg body. The orally administered dose of arsenate delivered to each mouse contained about 2 μCi of [73As] sodium arsenate. Immediately after dosing, each mouse was radioassayed for 2 minutes in a Canberra model 2290-S small animal counter (Canberra, Meriden, CT). The whole body burden of 73As determined immediately after dosing was designated as the initial body burden. After determination of the initial body burden, each mouse was returned to its metabolic cage with free access to tap water and powdered AIN-93G diet. Mice were radioassayed again at 2, 4, 8, 12, 24, 48, 72, and 96 hours after dosing. Urine and feces were collected from metabolic cages at 24, 48, 72, and 96 hours after dosing. At 24 hours after dosing, one group of mice was euthanized by cardiac puncture under CO2-induced anesthesia and lung, liver, kidney, urinary bladder, brain, heart, gonads (testes or ovaries), skin, and residual carcass were taken for [73As] radioassay in a Model 5530 gamma counter (Perkin Elmer, Waltham, MA, USA).
Tissue distribution and excretion of arsenicals after oral administration of arsenate
In parallel studies with stable arsenate, male and female wild-type or As3mt knockout mice received a single oral dose of 0.5 mg of arsenic as arsenate per kg body weight. These mice were maintained in metabolic cages with free access to tap water and powdered AIN-93G diet. At 2 and 24 hours after dosing, mice were euthanized by cardiac puncture under CO2 anesthesia and tissues collected for quantitation of arsenicals and immunoblotting. Urine and feces were collected from metabolic cages of the 24 hours after dosing group.
Analytical Methods
As3mt immunoblot analysis
Livers were collected from male and female wild-type or As3mt knockout mice at 24 hours after a single oral dose of 0.5 mg of arsenic as arsenate per kg body. Liver homogenates (1:10 weight/volume) were prepared in a buffer containing 25 mM Hepes (pH 7.4), 1% Nonidet P-40, 100 mM NaCl, 2% glycerol, 5 mM sodium fluoride, 1 mM EDTA, 1 mM NaVO3, 1 mM PMSF, and 1:200 v/v PIC. Homogenates were centrifuged at 20,000 × g for 15 min at 4°C to prepare supernates. Protein contents of supernates were determined using the BCA assay (Sigma-Aldrich, St Louis, MO) with bovine serum albumin as the protein standard. Aliquots containing 100 μg of supernatant protein were mixed with loading sample buffer, boiled for 5 minutes, and then applied to a 10% SDS-PAGE mini gel for electrophoresis. Proteins were transferred from gel to PVDF membrane at 100 V for 1 hour. Membranes were blocked with 5% non-fat milk prepared in TBS-T buffer (TBS buffer, BioRad, Hercules, CA, containing 0.1% Tween 20) for 1 hour at room temperature and then were incubated with polyclonal rabbit anti-mouse As3mt antibody diluted 1:500 in 5% non-fat milk prepared in TBS-T. After overnight incubation at 4°C with the primary antibody, membranes were washed 3 times for 5 minutes in TBS-T and then incubated with secondary anti-rabbit antibody conjugated with HRP diluted 1:5,000 in 5% non-fat milk prepared in TBS-T for 1 hour at room temperature. After repeated washing in TBS-T, enhanced chemiluminescence procedures (Denville Scientific Inc, Metuchen, NJ) were used to detect protein complexes on membranes. A monoclonal antibody against β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect expression of this housekeeping protein which was used as a control for protein loading.
High pressure liquid chromatography-inductively coupled plasma mass spectrometry
Inductively coupled plasma-mass spectrometry (ICP-MS) was used to detect and quantify arsenicals separated by high pressure liquid chromatography (HPLC).
In one set of analyses, emphasis was placed on determination of concentrations of inorganic arsenic and its mono-, di-, and trimethylated metabolites in liver and urine. Here, liver samples were digested in 2N phosphoric acid (Ultrex grade, Mallinckrodt Baker, Inc. (Phillipsburg, NJ, USA) as previously described (11). Digestates and urine samples were diluted in chromatographic mobile phase (35 mM ammonium bicarbonate buffer, pH 8.2 in 5% methanol). Diluted samples were injected onto a polymer-based, strong anion-exchange column (150×4.1 mm; PRP-X100, Hamilton, Reno, NV) using an 1100 series HPLC system from Agilent Technologies, Inc. (Santa Clara, CA) including solvent tray, G1311A QuatPump, G1367A WPALS autosampler, G1314A variable wavelength detector, G1322A degasser and G1330B ALSTherm. Arsenic in eluate (flow rate = 0.8 ml per minute) was monitored with an Agilent HP 7500 ICP MS (Yokogawa Analytical Systems, Hachiouji, Japan) equipped with an octopole reaction system (ORS) with a He flow of 3.0 ml per minute to prevent molecular interference by 40Ar35Cl+ (signal at m/z 75). Online ICP-MS data were processed with software developed in-house.
All reagents for this separation were analytical grade. All solutions were prepared in Milli-Q water (Millipore, Billerica, MA). Sodium arsenite, sodium arsenate, and dimethylarsinic acid from Sigma (St. Louis, MO) and methylarsonic acid and arsenobetaine from Tri Chemical Laboratories, Inc. (Yamanashi, Japan) were used as analytical standards. Iododimethylarsine was prepared as previously described (12, 13). Methyl- and dimethyl thioarsenicals used as analytical standards were prepared by the reduction of methylarsonic acid or dimethylarsinic acid with hydrogen sulfide (14). Nitric acid, hydrogen chloride, sodium sulfide, sodium thiosulfate and ammonium bicarbonate were purchased from Sigma.
In the other set of analyses, concentrations of inorganic and methylated thioarsenicals in urine were determined. Detection and quantitation of thioarsenicals present in urine were initially performed by HPLC-ICP-MS using an Agilent 1100 HPLC (Agilent, Palo Alto, CA) and an Agilent 7500ce ICP-MS (Agilent Technologies, Tokyo, Japan). For these analyses, about 20 μg of each mouse urine sample was diluted 1:49 with mobile phase (10 mM NH4NO3/NH4H2PO4, pH 6.0, adjusted with NH4OH) in deionized water (DIW). The diluted analyte (0.05 ml) was injected on a Hamilton PRP-X100 column (250mm × 4.1mm, 5 μm) with a guard column and eluted at a flow rate of 1.0 ml per minute. Under these conditions, arsenite, arsenate, methylarsonic acid, dimethylarsinic acid, dimethylthioarsinic acid, and monothioarsenate were detected in urine samples. Identities of each arsenical were confirmed by a combination of retention time matching and fortifying the sample with the corresponding authentic standard. Compounds were quantified based on external calibration using standards.
Identities of monothioarsenate and dimethylthioarsinic acid in urine were confirmed by other chromatographic methods using ICP-MS for detection. For identification of dimethylthioarsinic acid, urines were diluted in mobile phase (15 mM NH4COOCH3, pH 4.6, with 4% MeOH (w/w) in DIW) and 0.05 ml of diluted urine was injected on a Phenomenex Inertsil ODS-2 C18 column (250 × 4.6 mm, 5 μm) with a guard column that was eluted at a flow rate of 0.5 ml per minute. Dimethylthioarsinic acid in urine was identified on the basis of retention time matching and fortifying the sample with an authentic dimethylthioarsinic acid standard. For identification of monothioarsenate, urine was diluted with mobile phase (0.67% tetramethylammonium hydroxide (w/w) in deionized water) and 0.05ml injected on a Dionex IonPac AS16 column (250 × 4 mm) with a guard column in a Dionex GP50 system (Dionex Corporation, Sunnyvale, CA, USA). The column was eluted with 0.5 ml of mobile phase per minute. Thioarsenate in urine was identified on the basis of retention time matching and fortifying of samples with an authentic monothioarsenate standard.
Arsenate and arsenite standards were purchased from Spex Certiprep (Metuchen, NJ, USA). Dimethylarsinic acid and methylarsenic acid standards (both 98% pure) were purchased from Chem Service (West Chester, PA, USA). Dimethylthioarsinic acid was prepared as previously decscribed (15 16). Monothioarsenate was synthesized as previously described (17) and was purified by HPLC fraction collection. Deionized water (DIW, 18MΩ, Millipore, Bedford, MA, USA) was used for the preparation of all mobile phases. Iron sulfide (FeS), ammonium hydroxide (NH4OH), methanol (MeOH), ammonium acetate (NH4COOCH3), and acetic acid were obtained from Fisher Scientific (Pittsburgh, PA, USA). Potassium monohydrogen phosphate (K2HPO4) was from J.T. Baker (Phillipsburg, NJ, USA).
Pharmacokinetic Analysis
Data on time-dependent changes in the body burden of 73As as fractions of the initial body burden data were converted to μg of arsenic. Pharmacokinetic parameters were estimated with the numerical module of the software program SAAM II version 1.0.2 (SAAM Institute, Seattle, WA). Data were fitted to the biexponential equation
where y(t) is the amount (μg) of arsenic retained at time t, A and B represent the y-axis intercepts, and α and β are the first-order rate constants. The distribution (α) and elimination (β) half-lives were calculated by the following equations: t½α = 0.693/α; t½β = 0.693/β.
Statistical Methods
The Mann Whitney rank sum test was used for comparisons of the summed concentrations of inorganic and methylated arsenicals in liver and urines and of the fractions of the initial body burden in tissues and excreta of As3mt knockout mice and wild-type mice. To test for an effect of genotype on retention of [73As] after oral administration of [73As]-arsenate, data on the fraction of the initial body burden retained up to 96 hours after dosing were arcsine transformed and evaluated using a Kruskal-Wallis one-way analysis of variance on ranks. Differences with a P level < 0.05 were considered statistically significant.
Results
Effect of genotype on As3mt expression
Western blotting of lysates prepared from livers of As3mt knockout mice did not detect immunoreactive As3mt; As3mt was detected in the livers of wild-type mice (Figure 1). Because liver is considered a primary site for the conversion of inorganic arsenic to its methylated metabolites (18), disruption of As3mt in these mice would be expected to affect the phenotype for arsenic methylation.
Figure 1.
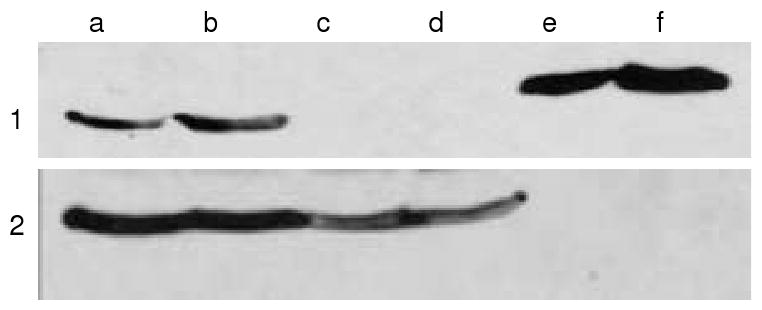
Expression of As3mt in livers of male and female As3mt knockout and C57BL/6 wild-type mice.
- Immunoblots with rabbit anti-mouse As3mt antibody using homogenates from a) male and b) female C57BL/6 wild-type mice or from c) male and d) female As3mt knockout mice or e) 10 ng and f) 50 ng of recombinant His-tagged rat recombinant As3mt (∼45 kDa).
- Immunoblots with anti β-actin antibody using homogenates from a) male and b) female C57BL/6 wild-type mice or from c) male and d) female As3mt knockout mice or e) 10 ng and f) 50 ng of recombinant His-tagged rat As3mt (∼45 kDa).
Effects of genotype and sex on arsenic speciation in liver and urine
In either As3mt knockout or wild-type mice there was no evidence of overt toxicity related to treatment. Both genotype and sex affected accumulation and loss of arsenicals from livers of As3mt knockout and wild-type mice (Figure 2). Initial comparisons focused on male-female differences within knockout or wild-type genotypes. Two hours after oral administration of arsenate, the summed concentrations of inorganic arsenic, methyl arsenic, and dimethyl arsenic differed significantly in livers of male and female As3mt knockout mice (P=0.008) and in livers of male and female wild-type mice (P=0.016). At 24 hours after oral administration of arsenate, the summed concentrations of inorganic arsenic, methyl arsenic, and dimethyl arsenic differed significantly in livers of male and female wild-type mice (P=0.008) but not in livers of male and female As3mt knockout mice. Comparisons between the two genotypes found that the summed concentrations of inorganic arsenic, methyl arsenic, and dimethyl arsenic in livers differed significantly (P=0.008) in both male and female mice at two hours after oral administration of arsenate. At 24 hours after treatment, there was no difference in the summed concentrations of inorganic arsenic, methyl arsenic, and dimethyl arsenic in livers of male As3mt knockout or wild-type mice; in female mice, genotype was associated with a significant difference (P=0.008) in summed concentrations of inorganic arsenic, methyl arsenic, and dimethyl arsenic in liver. Genotype also affected relative proportions of mono- and dimethylated arsenicals in liver. In As3mt knockout mice, these two methylated arsenicals accounted for 28 to 32% of arsenic in liver at 2 and 24 hours after dosing. In wild-type mice, mono- and dimethylated arsenic accounted for 80 to 84 percent of arsenic in liver at 2 hours after dosing and for 62 to 68% of arsenic in livers at 24 hours after dosing. Notably, monomethylated arsenic accounted for a larger fraction of the total concentration of arsenic in livers of As3mt knockout mice than in livers of wild-type mice.
Figure 2.
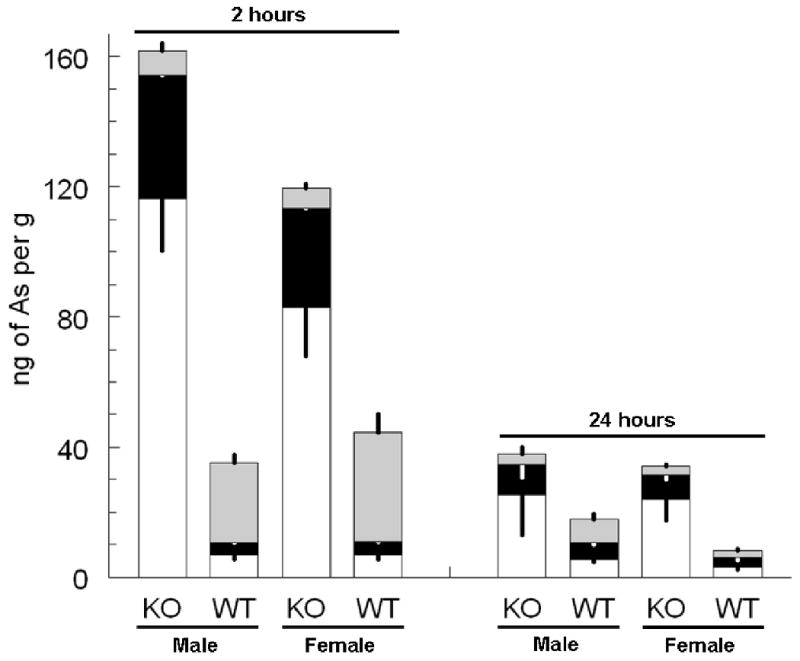
Concentrations of arsenicals in livers of a) male and b) female As3mt knockout (KO) and C57BL/6 wild-type (WT) mice at 2 and 24 hours after oral administration of 0.5 mg of arsenic as arsenate per kg. Mean and standard deviation shown for the summed concentrations of inorganic arsenic (□), methyl arsenic (■), and dimethyl arsenic ( ) in liver. Standard deviations projected downward for inorganic and methyl arsenic and upward for dimethyl arsenic (n = 5).
) in liver. Standard deviations projected downward for inorganic and methyl arsenic and upward for dimethyl arsenic (n = 5).
Profiles of inorganic arsenic and its methylated metabolites were compared in urine collected from As3mt knockout and wild-type mice during the 24-hour intervals immediately before and after oral administration of arsenate (Figure 3). For both genotypes, inorganic arsenic was the predominant arsenical detected in urine before treatment and genotype did not have a significant effect on the concentration of arsenic in urine. After dosing with arsenate, the summed concentrations of inorganic arsenic, methyl arsenic, dimethylarsenic, and trimethylarsenic in urine increased significantly (P=0.008), irrespective of genotype or sex, as compared to pretreatment values. Genotype affected relative contributions of inorganic arsenic and its methylated metabolites to arsenic excreted in urine. In male and female As3mt knockout mice, the sum of concentrations of methyl arsenic, dimethylarsenic, and trimethylarsenic in urine accounted for 22 to 28% of the total. In male and female wild-type mice, the summed concentrations of these methylated species accounted for about 92% of the total. After arsenate treatment, the predominant thioarsenical species in urine of wild-type mice was dimethylthioarsinic acid (about 50 to 300 ng of per ml). In contrast, monothioarsenate (about 5 to 65 ng per ml) was the predominant thioarsenical in urine of arsenate-treated As3mt knockout mice.
Figure 3.
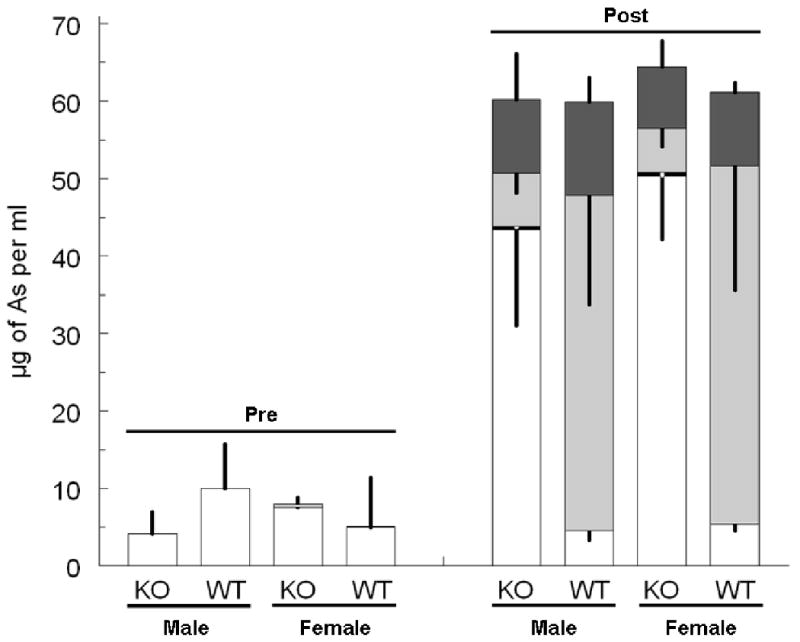
Concentrations of arsenicals in urines of a) male and b) female As3mt knockout (KO) and C57BL/6 wild-type (WT) mice collected during 24 hours before (Pre) or 24 hours after (Post) after oral administration of 0.5 mg of arsenic as arsenate per kg. Mean and standard deviation shown for the summed concentrations of inorganic arsenic (□), methyl arsenic (■), dimethyl arsenic ( ) and trimethyl arsenic (
) and trimethyl arsenic ( ) in urine. Standard deviations project upward for inorganic arsenic for pretreatment samples; for posttreatment samples, standard deviations project downward for inorganic, methyl, and dimethyl arsenic and upward for trimethyl arsenic (n = 5 to 10).
) in urine. Standard deviations project upward for inorganic arsenic for pretreatment samples; for posttreatment samples, standard deviations project downward for inorganic, methyl, and dimethyl arsenic and upward for trimethyl arsenic (n = 5 to 10).
Effect of genotype on tissue distribution and whole body retention of arsenic
Retention of arsenic in tissues and the extent of clearance in urine and feces of wild-type and As3mt knockout mice were determined at 24 hours after dosing (Figure 4). For these comparisons, 73As contents of tissues and excreta were calculated as percentages of the initial body burden of 73As. For both genotypes, there were significant male-female differences (P≤0.008) in retention of arsenic in urinary bladder and gonads; therefore, all comparisons were made on the basis of genotype and sex. In liver, kidney, urinary bladder, lung, heart, and brain, As3mt knockout mice retained higher percentages of the initial body burden of arsenic than did wild-type mice. These statistically significant differences (P≤0.003) were found in male and female mice. In skin, the difference between genotypes was only significant for females (P<0.03). Percentages of the initial body burden retained in gonads did not differ between genotypes. For carcass (which consisted of all residual tissues), genotype significantly affected (P≤0.003) the percentage of the initial body burden retained at 24 hours after dosing. Genotype also affected the pattern of arsenic excretion in urine and feces. The percentage of the initial body burden of arsenic excreted in urine over the first 24 hours after oral administration of arsenate was lower in both male and female As3mt knockout mice than in wild-type mice. These differences were statistically significant (P≤0.006). In contrast, fecal excretion of arsenic was unaffected by genotype.
Figure 4.
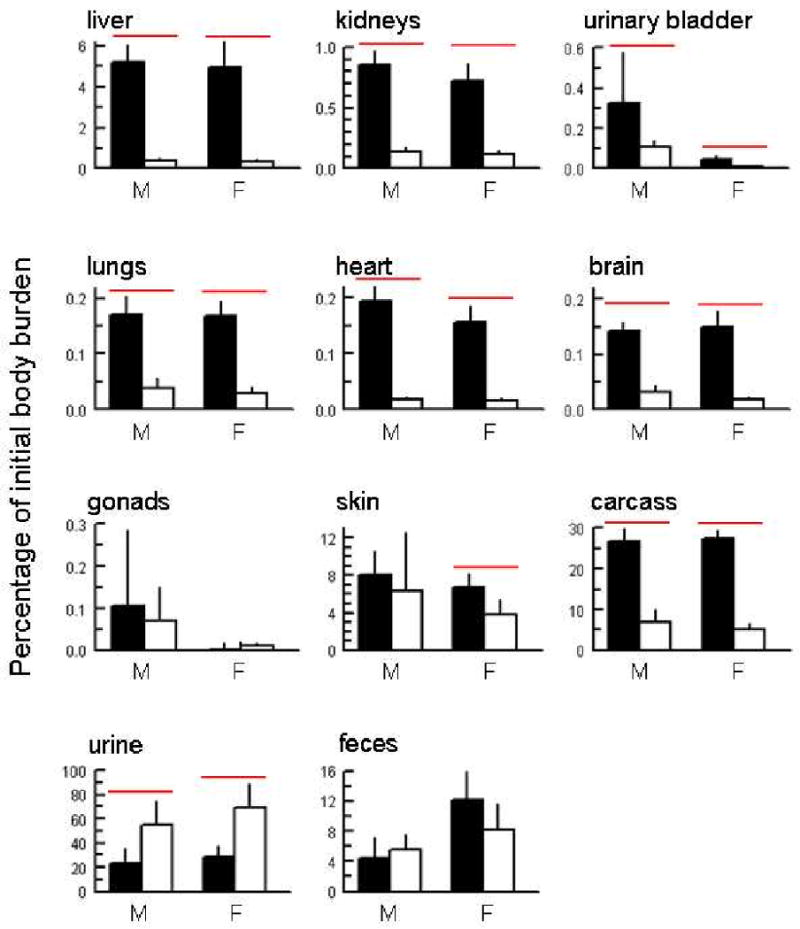
Percentage of initial body burden of arsenic in tissues or cumulative excreta from male (M) or female (F) As3mt knockout (■) and C57BL/6 wild-type (□) mice at 24 hours after oral administration of 0.5 mg of arsenic as [73As]-labeled per kg. a – liver, b – kidneys, c – urinary bladder, d – lungs, e – heart, f - brain, g – gonads, h – skin, i – carcass, j – urine, k – feces. Mean and standard deviation shown (n=5). A statistically significant difference (P < 0.03) between mice of the same sex and different genotype is indicated by a horizontal bar ( ).
).
Genotype had a striking effect upon whole body retention of arsenic in mice for up to 96 hours after arsenate treatment (Figure 5). At 24 hours after dosing, As3mt knockout mice retained on average about 50 percent and wild-type mice retained on average about 6 percent of the initial body burden of arsenic. This genotypic difference in retention was persistent. At 96 hours, As3mt knockout mice retained on average about 22 percent and wild-type mice retained on average about 2 percent of the initial body burden of arsenic. Over the 96 hour experimental period, As3mt genotype was associated with statistically significant difference (P≤0.001) in the median percentage of the initial body burden of radioarsenic that was retained.
Figure 5.
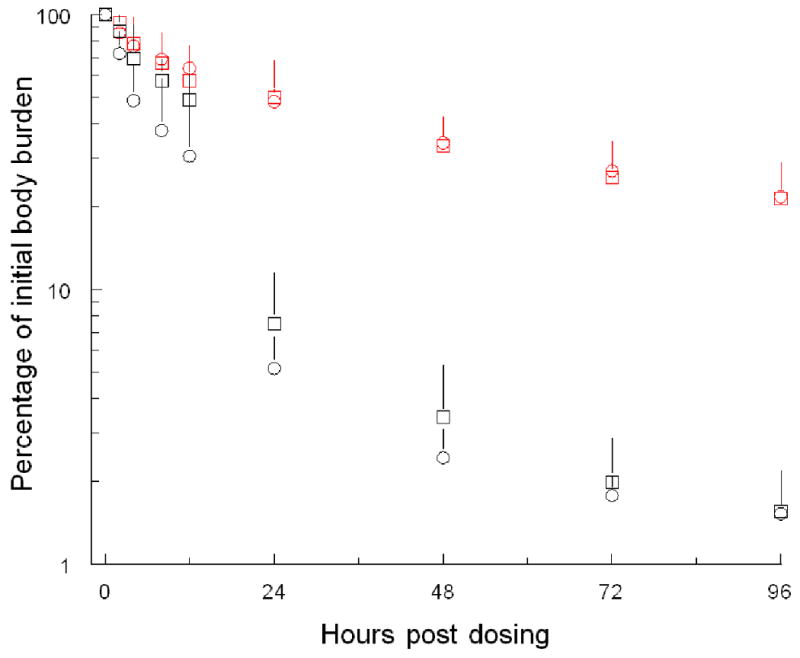
Whole body retention of [73As] by male ( ) or female (
) or female ( ) As3mt knockout and male (○) or female (□) C57BL/6 wild-type mice up to 96 hour after oral administration of 0.5 mg of arsenic as [73As]-labeled arsenate per kg. Whole body retention calculated as percentage of initial body burden of 73As determined within two minutes of dosing. Mean and standard deviation shown (n=5).
) As3mt knockout and male (○) or female (□) C57BL/6 wild-type mice up to 96 hour after oral administration of 0.5 mg of arsenic as [73As]-labeled arsenate per kg. Whole body retention calculated as percentage of initial body burden of 73As determined within two minutes of dosing. Mean and standard deviation shown (n=5).
Pharmacokinetic analysis of whole body data showed that genotype markedly affected retention of arsenic during the distribution and elimination phases (Table 1). In both As3mt knockout and wild-type mice, the estimated half-life of whole body clearance of arsenic during the distribution phase was short (4 to 7 hours). In wild-type mice, clearance of over 97% of the whole body burden of arsenic occurred during the distribution phase. In As3mt knockout mice, about 42 to 44% of the clearance of the whole body burden of arsenic occurred during the distribution phase. In wild-type mice, the half-life for whole body clearance of arsenic during the elimination phase differed markedly in males (250 hours) and females (91 hours). In comparison, the half-life estimates for whole body clearance of arsenic during the elimination phase in male and female As3mt knockout mice were similar (68.6-66.8 hours).
Table 1.
Pharmacokinetic parameters for whole body clearance of arsenic in C57BL/6 wild-type mice and As3mt knockout mice over 96 hours after oral administration of 0.5 mg of arsenic as [73As]-arsenate per kilogram
| Strain | Sex | A intercept | B intercept | Distribution half-life (hr) | Elimination half-life (hr) |
|---|---|---|---|---|---|
| C57BL/6 wild-type | Male | 13.4 | 0.3 | 7.3 | 250.0 |
| Female | 10.0 | 0.3 | 4.4 | 90.5 | |
| As3mt knockout | Male | 8.0 | 10.2 | 6.1 | 68.6 |
| Female | 6.3 | 8.9 | 6.7 | 68.8 |
Data were fitted using the biexponential equation y(t) = Ae-αt + Be-βt
Discussion
Disruption of the As3mt gene eliminated expression of immunoreactive As3mt. This genotypic change was associated with altered patterns for metabolism, distribution, and clearance of arsenic after oral administration of arsenate. These linkages between genotype and phenotype demonstrate that formation of methylated metabolites of inorganic arsenic catalyzed by As3mt is a critical determinant of patterns of arsenic distribution and retention. The higher retention of arsenic by knockout mice is consistent with the hypothesis that formation of methylated arsenicals as metabolites of inorganic arsenic has the salutary effect by increasing the rate of clearance of arsenic (19). For example, in B6C3F1 mice, rapid whole body clearance of arsenic has been reported after dosing with either methylarsonic acid or dimethylarsinic acid (20). Pharmacokinetic analyses of clearance data from mice treated with these arsenicals found that 70% or more of the body burden of arsenic was eliminated during an α phase with a half-life of less than 3 hours. Although the half life for α phase elimination of arsenic in As3mt knockout was similar to that in wild-type mice, overall whole body retention of arsenic was markedly greater in knockout mice. This difference reflected the predominance of α phase elimination in wild-type mice. Thus, the longer half-life for β phase clearance in wild-type mice than in knockout mice had little effect on overall retention because only a small fraction of the arsenic dose was cleared during this phase.
The slow rate of whole body clearance of arsenic in As3mt knockout mouse is also consistent with earlier findings in animals in which the capacity for formation of methylated arsenicals is minimal. For example, in the marmoset monkey or the chimpanzee, species with a very low or no capacity for the methylation of inorganic arsenic, whole body retention of arsenic following arsenate treatment is relatively high. At 24 hours after intravenous administration of arsenate, the chimpanzee retained about 40 to 50 percent and the marmoset monkey about 70 percent of the dose (21, 22). Administration of protein- or lipotrope-deficient diets to rabbits reduced availability of AdoMet needed for methylation of inorganic arsenic and increased retention of arsenic in tissues (23). Similarly, treatment of rabbits, mice, or rats with periodate-oxidized adenosine which inhibits activities of many AdoMet-dependent methyltransferases immediately before exposure to arsenate or arsenite diminished formation of methylated metabolites and increased tissue concentrations of inorganic arsenic (24-26).
Under the conditions used in the current study, the As3mt knockout genotype did not result in the complete absence of methylated metabolites in tissues and urine following arsenate dosing. The presence of methylated arsenicals in urine and liver of treated As3mt knockout mice suggests that there are alternative pathways for arsenic methylation in these animals. Conceivably, there could be another methyltransferase encoded in the mouse genome that catalyzes requisite reactions for production of methylated metabolites of inorganic arsenic. The presence of methylated metabolites in urines and livers from arsenate-treated As3mt knockout mice may also reflect metabolism by the microbiota of the gastrointestinal tract. Genomes of microorganisms commonly resident in the gastrointestinal tract encode proteins that catalyze reduction, methylation, and transport of arsenicals (27, 28). The anaerobic microbiota of mouse cecum can use arsenate and dimethylarsinic acid as substrates for a variety of methylation and thiolation reactions (29-31). Hence, metabolism of arsenate in the gastrointestinal tract of As3mt knockout mice before systemic absorption could contribute to the pattern of methylated metabolites found in tissues and urine. Manipulation of the microflora of the gastrointestinal tract could affect the pattern and extent of metabolism of arsenicals before systemic absorption. The patterns of thioarsenicals found in the urine of wild-type or As3mt knockout mice were concordant with the arsenic methylation phenotype. In either case, the predominant thioarsenical in urine matched the predominant oxoarsenical species present in urine. Thioarsenicals found in the urine of mice of either genotype may be formed using H2S generated by bacteria present in the gastrointestinal tract or could be produced from H2S generated in tissues.
For both genotypes, distribution and retention of arsenicals in tissues after treatment with arsenate were sexually dimorphic. This is reflected by male-female differences in summed concentration of arsenicals in liver and retention of arsenic in urinary bladder and gonads. There is little information on sexual dimorphism of metabolism of arsenic in experimental animals, although there is evidence of differences in sensitivity to the carcinogenic effects of arsenic (32, 33). Male-female differences in the urinary clearance of dimethylated arsenic have been found in populations chronically exposed to inorganic arsenic in drinking water (34-37). Pregnancy may also modulate the pattern of arsenic in humans (38, 39). The As3mt knockout mouse may be a useful model for identifying other genetic modifiers that contribute to male-female differences in arsenic metabolism and retention.
The null genotype for As3mt gene in the knockout mice increased retention of arsenic in tissues and was associated with higher retention of arsenic in lung, liver, and urinary bladder that are targets for arsenic-induced carcinogenesis in humans (40). For example, at the time points examined in this study, the null genotype increased concentration of inorganic arsenic present in the liver. If tissue injury is proportional to the integrated exposure to inorganic arsenic, then one would postulate that As3mt knockout mice would be more susceptible to adverse effects. Notably, in the present study, the concentration of arsenic attained in liver of As3mt knockout mice at 2 hours after dosing was below the concentration that has been associated with biochemical evidence of liver or kidney injury in arsenite-exposed mice (41). Additional characterization is needed of the dose-response relations for accumulation of arsenicals in tissues of As3mt knockout mice and indicators of biochemical injury.
Other genetic modifications alter distribution of arsenic and affect susceptibility to its toxic effects. Metallothionein (Mt) I/II knockout mice have been reported to be more sensitive than wild-type mice to liver and kidney injury induced by exposure to inorganic arsenic (42). Multidrug-resistance (Mdr) 1a/1b double knockout mice that received a single subcutaneous dose of arsenite retained more arsenic in liver, kidney, small intestine, and brain than did wild-type mice (43). Exposure to arsenite in drinking water for 10 weeks also resulted to higher tissue levels of arsenic in Mdr 1a/1b double knockout mice than in wild-type mice (41). Biochemical alterations and changes in gene expression patterns also indicated Mdr 1a/1b mice were more sensitive than wild-type mice to adverse effects of arsenic exposure. These findings suggest that genetic alterations that affect cellular distribution, binding, and retention can affect the systemic toxicity of inorganic arsenic and its metabolites. Addition of the As3mt knockout mice to the research armamentarium will help elucidate the role of metabolism in the control of the kinetic behavior of inorganic arsenic. Production of mice with different combinations of null genotypes for As3mt, Mt I/II, and Mdr 1a/1b will provide new insights into the connections between toxicokinetics and toxicodynamics of arsenic.
Supplementary Material
Acknowledgments
We gratefully acknowledge the advice and assistance of our colleagues at the U.S. Environmental Protection Agency. This research was supported in part by NIH grant 1 R01 010845-01A2 to M.S. and the US EPA Cooperative Agreement CR829522 (MS).
Footnotes
Publisher's Disclaimer: Disclaimer - This manuscript has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- 1.Challenger F. Biological methylation. Adv Enzymol. 1951;12:432–491. doi: 10.1002/9780470122570.ch8. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Waters SB, Drobna Z, Devesa V, Styblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase and the inorganic arsenic methylation phenotype. Toxicol Appl Pharmacol. 2005;204:164–169. doi: 10.1016/j.taap.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DJ, Hernandez-Zavala A, Cai SY, Boyer JL, Nava GM, Gaskins HR. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals in the invertebrate chordate. Ciona intestinalis. doi: 10.1093/toxsci/kfp250. Submitted to Toxicological Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fomenko DE, Xing W, Adair BM, Thomas DJ, Gladyshev VN. High-throughput identification of catalytic redox-active cysteine residues. Science. 2007;315:387–389. doi: 10.1126/science.1133114. [DOI] [PubMed] [Google Scholar]
- 7.Drobna Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Styblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol Appl Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drobna Z, Xing W, Thomas DJ, Styblo M. shRNA silencing of AS3MT expression minimizes arsenic methylation capacity of HepG2 cells. Chem Res Toxicol. 2006;19:894–898. doi: 10.1021/tx060076u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Thomas DJ. Strain-dependent disposition of inorganic arsenic in the mouse. Toxicology. 1999;137:95–108. doi: 10.1016/s0300-483x(99)00068-2. [DOI] [PubMed] [Google Scholar]
- 10.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76a rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 11.Hughes MF, Devesa V, Adair BM, Conklin SD, Creed JT, Styblo M, Kenyon EM, Thomas DJ. Tissue dosimetry, metabolism and excretion of pentavalent and trivalent dimethylated arsenic in mice after oral administration. Toxicol Appl Pharmacol. 2008;227:26–35. doi: 10.1016/j.taap.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows GJ, Turner EE. A new type of compound containing arsenic. J Chem Soc Trans. 1920;117:1373–1383. [Google Scholar]
- 13.Cullen WR, McBridge BB, Manji H, Pickett AW, Reglinski J. The metabolism of methylarsine oxide and sulfide. Appl Organomet Chem. 1989;3:71–78. [Google Scholar]
- 14.Naranmandura H, Suzuki N, Iwata K, Hirano K, Suzuki KT. Arsenic metabolism and thioarsenicals in hamsters and rats. Chem Res Toxicol. 2007;20:616–624. doi: 10.1021/tx700038x. [DOI] [PubMed] [Google Scholar]
- 15.Fricke MW, Zeller M, Sun H, Lai VW, Cullen WR, Shoemaker JA, Witkowski MR, Creed JT. Chromatographic separation and identification of products from the reaction of dimethylarsinic acid with hydrogen sulfide. Chem Res Toxicol. 2005;18:1821–1829. doi: 10.1021/tx050227d. [DOI] [PubMed] [Google Scholar]
- 16.Fricke M, Zeller M, Cullen W, Witkowski M, Creed J. Dimethylthioarsinic anhydride: a standard for arsenic speciation. Anal Chim Acta. 2007;583:78–83. doi: 10.1016/j.aca.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 17.Schwedt G, Rieckhoff M. Separation of thio- and oxothioarsenates by capillary zone electrophoresis and ion chromatography. J Chromatogr A. 1996;736:341–350. [Google Scholar]
- 18.Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 19.Gebel TW. Arsenic methylation is a process of detoxification through accelerated excretion. Int J Hyg Environ Health. 2002;205:505–508. doi: 10.1078/1438-4639-00177. [DOI] [PubMed] [Google Scholar]
- 20.Hughes MF, Kenyon EM. Dose-dependent effects on the disposition of monomethylarsonic acid and dimethylarsinic acid in the mouse after intravenous administration. J Toxicol Environ Health. 1998;53:95–112. doi: 10.1080/009841098159385. [DOI] [PubMed] [Google Scholar]
- 21.Vahter M, Marafante E. Reduction and binding of arsenate in marmoset monkeys. Arch Toxicol. 1984;57:119–124. doi: 10.1007/BF00343121. [DOI] [PubMed] [Google Scholar]
- 22.Vahter M, Couch R, Nermell B, Nilsson R. Lack of methylation of inorganic arsenic in the chimpanzee. Toxicol Appl Pharmacol. 1995;133:262–268. doi: 10.1006/taap.1995.1150. [DOI] [PubMed] [Google Scholar]
- 23.Vahter M, Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987;37:41–46. doi: 10.1016/0378-4274(87)90165-2. [DOI] [PubMed] [Google Scholar]
- 24.Marafante E, Vahter M. The effect of methyltransferase inhibition on the metabolism of [74As] arsenite in mice and rabbits. Chem Biol Interact. 1984;50:49–57. doi: 10.1016/0009-2797(84)90131-5. [DOI] [PubMed] [Google Scholar]
- 25.Marafante E, Vahter M, Envall J. The role of the methylation in the detoxication of arsenate in the rabbit. Chem Biol Interact. 1985;56:225–238. doi: 10.1016/0009-2797(85)90008-0. [DOI] [PubMed] [Google Scholar]
- 26.Csanaky I, Gregus Z. Effect of selenite on the disposition of arsenate and arsenite in rats. Toxicology. 2003;186:33–50. doi: 10.1016/s0300-483x(02)00604-2. [DOI] [PubMed] [Google Scholar]
- 27.Bentley R, Chasteen TG. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol Mol Biol Rev. 2002;66:250–271. doi: 10.1128/MMBR.66.2.250-271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Karamychev VN, Kozyavkin SA, Mills D, Pavlov AR, Pavlova NV, Polouchine NN, Richardson PM, Shakhova VV, Slesarev AI, Weimer B, O'Sullivan DJ. Comparative genomic analysis of the gut bacterium Bifidobacterium longum reveals loci susceptible to deletion during pure culture growth. BMC Genomics. 2008 May 27;9:247. doi: 10.1186/1471-2164-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall LL, George E, Kohan MJ, Styblo M, Thomas DJ. In vitro methylation of inorganic arsenic in mouse intestinal cecum. Toxicol Appl Pharmacol. 1997;147:101–109. doi: 10.1006/taap.1997.8269. [DOI] [PubMed] [Google Scholar]
- 30.Kubachka KM, Kohan MC, Herbin-Davis K, Creed JT, Thomas DJ. Exploring the in vitro formation of trimethylarsine sulfide from dimethylthioarsinic acid in anaerobic microflora of mouse cecum using HPLC-ICP-MS and HPLC-ESI-MS. Toxicol Appl Pharmacol. 2009 doi: 10.1016/j.taap.2008.12.008. published online December 28, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Kubachka KM, Kohan MC, Conklin SD, Herbin-Davis KM, Creed JT, Thomas DJ. In Vitro biotransformation of dimethylarsinic acid and trimethylarsine oxide by anaerobic microflora of mouse cecum analyzed by HPLC-ICP-MS and HPLC-ESI-MS. J Anal At Spectrom. 2009 published online May 22, 2009, doi: 10.1039=b817820h. [Google Scholar]
- 32.Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol Appl Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 33.Shen J, Wanibuchi H, Waalkes MP, Salim EI, Kinoshita A, Yoshida K, Endo G, Fukushima S. A comparative study of the sub-chronic toxic effects of three organic arsenical compounds on the urothelium in F344 rats; gender-baseddifferences in response. Toxicol Appl Pharmacol. 2006;210:171–180. doi: 10.1016/j.taap.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996;104:620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsueh YM, Huang YL, Huang CC, Wu WL, Chen HM, Yang MH, Lue LC, Chen CJ. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J Toxicol Environ Health A. 1998;54:431–444. doi: 10.1080/009841098158728. [DOI] [PubMed] [Google Scholar]
- 36.Chen YC, Guo YL, Su HJ, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Chao SC, Lee JY, Christiani DC. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45:241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, Rudnai P, Leonardi G, Fletcher T, Vahter M. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect. 2007;115:1081–1086. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Concha G, Vogler G, Nermell B, Vahter M. Low-level arsenic excretion in breast milk of native Andean women exposed to high levels of arsenic in the drinking water. Int Arch Occup Environ Health. 1998;71:42–46. doi: 10.1007/s004200050248. [DOI] [PubMed] [Google Scholar]
- 39.Hopenhayn C, Huang B, Christian J, Peralta C, Ferreccio C, Atallah R, Kalman D. Profile of urinary arsenic metabolites during pregnancy. Environ Health Perspect. 2003;111:1888–1891. doi: 10.1289/ehp.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 41.Xie Y, Liu J, Liu Y, Klaassen CD, Waalkes MP. Toxicokinetic and genomic analysis of chronic arsenic exposure in multidrug-resistance mdr1a/1b(-/-) double knockout mice. Mol Cell Biochem. 2004;255:11–18. doi: 10.1023/b:mcbi.0000007256.44450.8c. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 43.iu J, Liu Y, Powell DA, Waalkes MP, Klaassen CD. Multidrug-resistance mdr1a/1b double knockout mice are more sensitive than wild type mice to acute arsenic toxicity, with higher arsenic accumulation in tissues. Toxicology. 2002;170:55–62. doi: 10.1016/s0300-483x(01)00532-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


