Abstract
To date, there are no effective pharmacotherapies for treating psychostimulant abuse. Previous preclinical and clinical studies have shown that continuous treatment with the monoamine releaser amphetamine reduces cocaine self-administration, but amphetamine selectively targets the dopamine system and is reinforcing. In the present study, we examined the consequences of administration of amphetamine and three structurally-related analogs that vary in their potencies for releasing dopamine and serotonin on behavioral-stimulant effects and nucleus accumbens dopamine levels in squirrel monkeys. Amphetamine and PAL-353, which have relatively high selectivity for releasing dopamine vs. serotonin, increased accumbens dopamine levels and induced stimulant effects on behavior maintained by a fixed-interval schedule of reinforcement. PAL-313, which has relatively low selectivity for releasing dopamine vs. serotonin, increased dopamine levels, but did not induce behavioral-stimulant effects. PAL-287, which is relatively nonselective in releasing dopamine and serotonin, did not increase dopamine levels or induce behavioral-stimulant effects. These results demonstrate that increasing serotonergic activity attenuates dopamine release and dopamine-mediated behavioral effects of monoamine releasers. In addition, these results support further investigation of PAL-313 and similar compounds as a potential medication for treating psychostimulant abuse.
Keywords: amphetamine, dopamine, locomotor activity, microdialysis, nonhuman primate, nucleus accumbens, psychostimulants, serotonin, squirrel monkey
Psychostimulant abuse is a significant public health problem, with 2.1 million Americans reporting cocaine use and 1.9 million reporting methamphetamine use in 2006 (Substance Abuse and Mental Health Services Administration (SAMHSA), 2007). Unfortunately, there are no currently FDA-approved pharmacotherapies to treat stimulant dependence (Volkow and Li, 2004). Agonist substitution therapies have been successful in treating patients dependent on opioids (Kreek, 2000) or nicotine (Henningfield, 1995). Thus, drugs that have pharmacological and behavioral effects similar to those of psychostimulants have the potential to be effective medications for psychostimulant abuse. In order to identify these potential medications, there needs to be a better understanding of how psychostimulants produce their behavioral and neurochemical effects.
Psychostimulants interact with monoamine (dopamine, norepinephrine, and serotonin) neurons in the central nervous system. These neurons express specialized plasma membrane proteins that transport monoamines from the extracellular space back into the cytoplasm. Binding to these transporter proteins [dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT)] is the principal mechanism for inactivation of monoamine signaling (Howell and Kimmel, 2008). Drugs that interact with these transporters can be categorized as either reuptake inhibitors or substrate-type releasers, based on their mechanism of action. Reuptake inhibitors bind to transporters without being taken up into the cell. This binding blocks the reuptake of released neurotransmitter molecules, thereby elevating extracellular neurotransmitter levels in an impulse-dependent fashion. In contrast, substrate-type releasers bind to the transporter proteins and are then transported into the cytoplasm of nerve terminals. These releasers elevate extracellular neurotransmitter levels in two ways: by promoting efflux of the transmitter through the transporter protein and by increasing cytoplasmic transmitter levels by disrupting transmitter storage in vesicles (Rudnick, 1997; Rudnick and Clark, 1993; Sulzer et al., 2005).
Although cocaine is a nonselective inhibitor of all three monoamine transporters (Madras et al., 1989; Reith et al., 1986), the behavioral effects of cocaine associated with its abuse liability have been attributed primarily to its actions at DAT (Ritz et al., 1987). This has been substantiated in rodent, nonhuman primate, and clinical studies. A relationship between the potency of cocaine analogs at binding to the DAT in vitro and the potency of these analogs in vivo has been demonstrated by their locomotor-stimulant effects in rodents (Cline et al., 1992; Kuhar, 1993) and their cocaine-like behavioral effects in squirrel monkeys (Bergman et al., 1989; Madras et al., 1989; Spealman et al., 1989). The relevance of the DAT in the abuse liability of cocaine has been supported further by neuroimaging studies. In human cocaine users, a significant correlation was observed between the level of DAT occupancy and the magnitude of the subjective high following administration of cocaine (Volkow et al., 1997) or the behavioral stimulant methylphenidate (Volkow et al., 1999). Similarly, the abuse-related behavioral effects of monoamine releasers, such as amphetamine and methamphetamine, have been attributed to their effects on dopamine (Hanson et al., 2004; Koob and Bloom, 1988; Wise, 1996; Wise and Bozarth, 1987).
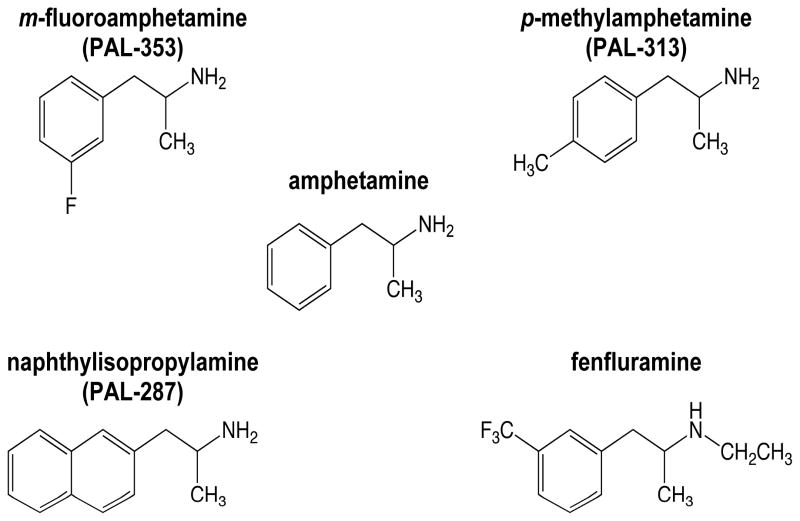
The purpose of the present study was to investigate the neurochemical and behavioral effects of mixed-action monoamine releasers in squirrel monkeys. Amphetamine and three structurally related analogs (Figure 1) were selected for comparison in these studies. While these drugs are equipotent in releasing dopamine in in vitro studies conducted in rodent tissue (Table 1), they vary in their potency for releasing serotonin. Within the group of selected compounds, amphetamine and PAL-353 are similar in their DA/5-HT releasing potency ratio, in that both are very selective for releasing dopamine. PAL-313 is less selective for dopamine, and PAL-287 even less so. The serotonin selective releaser fenfluramine was added to the behavioral studies for comparison. Our hypothesis was that, as the selectivity for serotonin release increased, drug-induced increases in extracelluar dopamine would decrease and drug-induced increases in locomotor activity would decrease accordingly.
Figure 1.
Chemical structures of amphetamine and the four structurally related drugs used in these studies.
TABLE 1.
In vitro potency as releasers of monoamine neurotransmitters in rodent brain tissue.
| EC50 (nM) |
|||
|---|---|---|---|
| Drug | [3H]DA | [3H]5-HT | DA/5-HT |
| d-Amphetaminea | 8.0 | 1756 | 0.004 |
| PAL-353a | 24.2 | 1937 | 0.01 |
| PAL-313a | 44.1 | 53.4 | 0.83 |
| PAL-287b | 12.6 | 3.4 | 3.7 |
| Fenfluramineb | >10,000 | 79.3 | 126.1 |
Modified from Wee et al., 2005 and
Rothman et al, 2007.
METHODS
Subjects
Ten adult male squirrel monkeys (Samiri sciureus) weighing 700–1200 g served as subjects. Animals lived in individual home cages and had daily access to food (Harlan Teklad monkey chow; Harlan Teklad, Madison, WI; fresh fruit and vegetables) and unlimited access to water. All monkeys had prior exposure to cocaine and other drugs with selective dopaminergic or glutamatergic activity in various behavioral studies. Animal use procedures were in strict accordance with the National Institutes of Health “Guide for Care and Use of Laboratory Animals” (Publication No. 85-23, revised 1985) and were approved by the Institutional Animal Care and Use Committee of Emory University.
Apparatus
During daily behavioral test sessions, each of six animals was seated in a Plexiglas chair within a ventilated, sound-attenuating chamber (MED Associates, Georgia, VT). The chair was equipped with stimulus lights, a response lever, and a tail stock for delivering a mild electrical stimulus. Behavioral test sessions lasted approximately 90 min each day, five days per week. During microdialysis experiments in a separate group of four animals, subjects were seated in a chair and fitted with an adjustable Lexan neckplate that was positioned perpendicular to the medial plane of the body just above the shoulder. These subjects had been acclimated to the chair and neckplate over several months prior to the start of these experiments. At least two weeks elapsed between microdialysis experiments.
Guide Cannulae Implantation
A stereotaxic apparatus was used to implant CMA/11 guide cannulae (CMA/Microdialysis, Acton, MA) bilaterally to target the nucleus accumbens of four monkeys in a procedure described previously (Czoty et al., 2000). Anesthesia was initiated with Telazol (tiletamine hydrochloride and zolazepam hydrochloride, 3.0 mg) and atropine. Inhaled isoflurane (1.0–2.0%) was administered to maintain depth of anesthesia during the procedure. A stainless steel stylet was placed in each guide cannulae when not in use. Analgesics [Banamine (flunixin meglumine)] and antibiotics [Rocephin (ceftriaxone)] were prescribed as necessary by veterinary staff. Animals were closely monitored during recovery from anesthesia, and a minimum of two weeks elapsed before microdialysis experiments were performed.
Stimulus Termination Procedures
Six animals were trained under a fixed-interval (FI) 300-s schedule of stimulus termination. At the beginning of the testing session, the behavioral chamber was illuminated with a red light for 300 s. When this interval had elapsed, the monkey had 3 s to press the lever one time to terminate the red light, which was associated with an impending electrical stimulus. Upon termination of the red light, a white light was illuminated for 15 s, followed by a 60-s timeout. If the lever was not pressed during the 3-s period, the animal received a 3-mA stimulus for approximately 200 ms to the tail, followed by a 60-s timeout. Responding during timeout periods had no scheduled consequences. Animals were tested each day, five days per week, and each daily session consisted of fifteen FI components. When rates and patterns of responding had stabilized such that there was less than 10% variability in response rate for five consecutive test days, drug experiments were initiated.
A single dose of drug or saline was administered i.m. in the thigh 5 min before the beginning of the experimental session. Animals were tested, but did not receive drug on Mondays and Wednesdays. They received a single dose of test drug on Tuesdays and Fridays, and saline was administered prior to the session on Thursdays, as a control for the injection procedure. Animals received each dose of each drug in ascending order. The animals used in this study had a response rate of 0.36 ± 0.03 (group mean ± SEM) presses per second when given saline.
Microdialysis procedures
CMA/11 dialysis probes with a shaft length of 20 mm and active dialysis membrane measuring 2 mm long and 0.24 mm diameter were flushed with artificial cerebrospinal fluid (1.0 mM Na2HPO4, 150 mM NaCl, 3 mM KCl, 1.3 mM CaCl2, 1.0 mM MgS04 and 0.15 mM ascorbic acid, final pH=7.4–7.56) for at least 20 min. Probes were inserted into the guide cannulae and connected to a Harvard PicoPlus microinfusion pump via FEP Teflon tubing. Probes were perfused with artificial cerebrospinal fluid at 2.0 μl/min for the duration of the experiment. Samples were collected every 6 min in microcentrifuge tubes and immediately refrigerated. Following a 60-min equilibration, four consecutive 6-min samples were collected for determination of baseline dopamine concentration. Following collection of baseline samples, saline or a dose of a test drug was administered i.m. and 6-min samples were collected for an additional 90 min. Animals were tested a maximum of one time every other week, and both sites were accessed in each study. This regimen of repeated access has produced consistent responses to drug treatment without significant gliosis (Czoty et al., 2000).
High-performance liquid chromatography (HPLC) and electrochemical detection were used to quantify levels of dopamine. The HPLC system consisted of a small bore (3.2 mm × 150 mm, 3 micron) column (ESA, Inc., Chelmsford, MA) with a commercially available mobile phase (MD-TM, ESA, Inc.) delivered by an ESA 582 solvent delivery pump at a flow rate of 0.6 ml/min. After loading onto the refrigerated sample tray, samples (12 μl) were automatically mixed with 3 μl of ascorbate oxidase, and 5 μl of this mixture was injected into the HPLC system by an ESA Model 542 autosampler. Samples were analyzed within 12 hours of collection, remaining either in a refrigerator or in the refrigerated autosampler tray during this time. Electrochemical analyses were performed using an ESA dual-channel analytical cell (model 5040) and guard cell (model 5020, potential = 350 mV) and an ESA Coulochem II detector. The potential of channel 1 was set to −150 mV for oxidation, while the potential of channel 2 was set to 275 mV for reduction. A full range of dopamine standards (0.5–25 nM) was analyzed both before and after each set of samples to evaluate possible degradation of dopamine. Levels of dopamine below 0.1 nM were considered below the limit of detection. A desktop computer collected data and chromatograms were generated by EZChrom Elite software (version 3.1, Scientific Software, Pleasanton, CA). The chromatograms were analyzed using the EZChrom software, comparing the area under the curve of the experimental samples with that of the standards. The neurochemical effects of the drugs were compared with the neurochemical effects of saline and cocaine. Basal levels of dopamine were between 3–5 nM, unadjusted for probe recovery, as reported in earlier studies (Czoty et al., 2000). Before and after each in vivo experiment, probes were tested in vitro to determine suitability of the probes. Percent recovery was similar for all probes (10–20%).
Drugs
Amphetamine (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% saline, while PAL-287, PAL-313, and PAL-353 (Research Triangle Institute, Research Triangle, NC), and fenfluramine (Sigma Aldrich, St. Louis, MO) were dissolved in sterile water. Drug doses were determined as salts. Drug injections were administered into the thigh muscle in a volume of 0.4 to 0.8 ml in both the behavioral and microdialysis studies.
Data analysis and statistics
Each microdialysis time course curve was analyzed using a one-way ANOVA. When a significant main effect was detected, the time points following drug administration were compared to the zero time point using Dunnett’s post hoc test. The overall rate data for each drug in the behavioral-stimulant dose-effect curves (Figure 3) was analyzed using a repeated-measures one-way ANOVA. When a significant main effect was detected, each dose was compared to vehicle using Dunnett’s post hoc test. The time-course behavioral-stimulant data (Figure 4) were analyzed such that each dose of each drug was compared to vehicle using a repeated-measures two-way ANOVA. When a significant main effect was detected, each time point following drug administration was compared to the corresponding time point following vehicle administration using Bonferroni post-hoc tests.
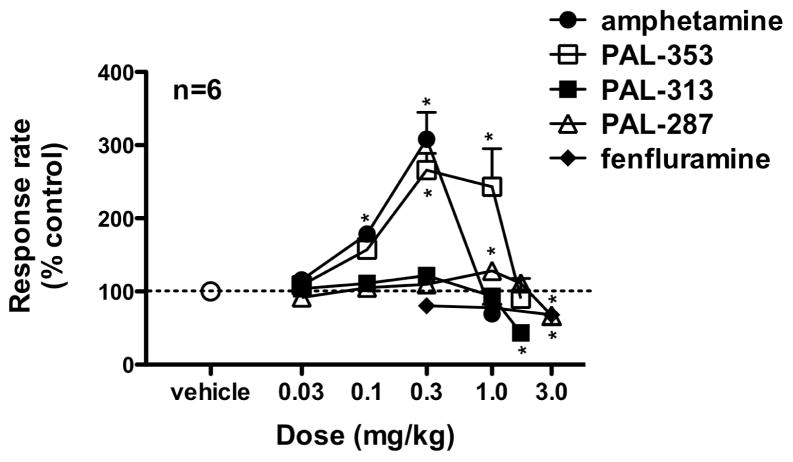
Figure 3.
Dose-effect curve of increases in rates of responding following i.m. administration of each drugs. Data (mean ± SEM) are presented as a percent of the rate of lever pressing following i.m. administration of vehicle. These rates were averaged across the entire 90-min session, resulting in one data point for each dose administered. The dotted line represents baseline responding rates following i.m. administration of saline. *p < 0.05, as compared to vehicle using Dunnett’s post-hoc tests.
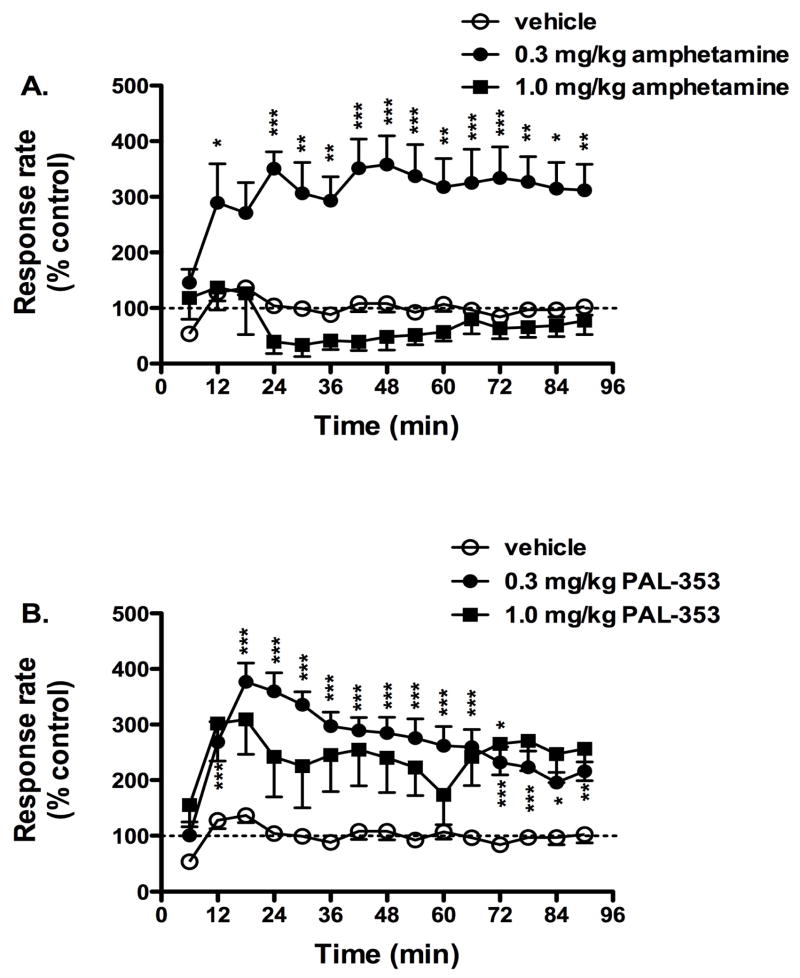
Figure 4.
Time course of increases in rates of responding following i.m. administration of 0.3 mg/kg or 1.0 mg/kg amphetamine (panel A) or 0.3 mg/kg or 1.0 mg/kg PAL-353 (panel B). Data (mean ± SEM) are presented as a percent of the rate of lever pressing following i.m. administration of saline. The dotted line represents baseline responding rates following i.m. administration of saline. *** p < 0.001, ** p < 0.01, * p < 0.05, as compared to vehicle at that time point using Bonferroni post-hoc tests.
RESULTS
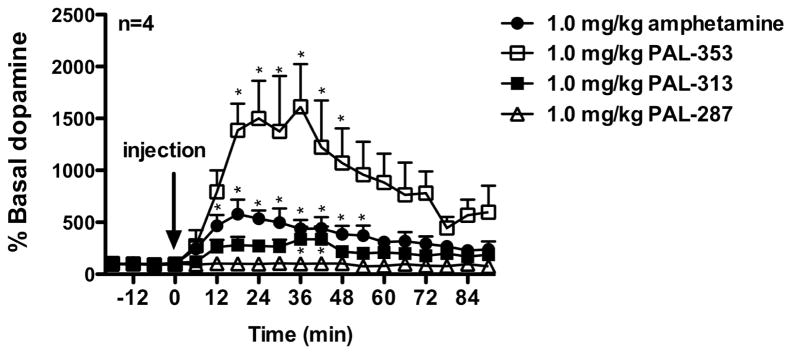
For the in vivo microdialysis studies, the same dose for each drug was selected (1.0 mg/kg, i.m.), as they are approximately equipotent in releasing dopamine as measured by in vitro assays using rodent tissue (Table 1). Administration of PAL-353 had a significant effect (F(18,54) = 5.343, p < 0.001) and produced the greatest increase in extracellular dopamine in the nucleus accumbens (Figure 2), with a peak of 1612% of basal dopamine levels 36 min after drug injection. There was a significant main effect of amphetamine administration (F(18,54) = 7.017, p < 0.001), which produced a peak increase of 579% of basal dopamine levels at 18 min after drug injection. Administration of PAL-313 produced a peak increase of 442% of basal dopamine levels at 42 min after drug injection, and there was a significant main effect of this drug (F(18,54) = 3.217, p < 0.001). Administration of PAL-287 produced a peak increase of 106% of basal dopamine levels at 30 min after drug injection. In contrast to the other three drugs, there was not a significant main effect of PAL-287 (F(18,54) = 1.230, NS).
Figure 2.
Increases in extracelluar dopamine following i.m. administration of each of the four drugs. Data (mean ± SEM) are presented as a percent of baseline dopamine levels prior to drug injection in each study. *p < 0.05, as compared to baseline using Dunnett’s post-hoc test.
The effects of a single i.m. administration of each of a range of doses of each drug on the rate of lever-pressing behavior was examined in a group of six squirrel monkeys that was trained on a fixed-interval schedule (Figure 3). The data are expressed as an average of the response rate as a percent of baseline responding following saline administration over the entire 90-min session. This baseline response following saline administration was reassessed between each set of dose-response determinations. Administration of amphetamine (0.03–1.0 mg/kg) significantly increased response rates above baseline levels (F(4,20) = 23.265, p < 0.0001) in an inverted U-shaped manner, with a peak at 0.3 mg/kg. Administration of PAL-353 (0.03–1.7 mg/kg) also significantly increased response rates above baseline levels (F(5,25) = 9.806, p < 0.001) in an inverted U-shaped manner, with a peak at 0.3 mg/kg. Administration of PAL-313 (0.03–1.7 mg/kg) significantly altered response rates, as compared to baseline levels (F(5,25) = 15.427, p < 0.001), such that 1.7 mg/kg significantly decreased responding to below baseline levels. Administration of PAL-287 (0.03–3.0 mg/kg) also significantly altered response rates (F(5,25) = 8.408, p < 0.001), such that 1.0 mg/kg significantly increased responding above baseline levels, but 3.0 mg/kg significantly decreased responding to below baseline levels. Lastly, administration of fenfluramine (0.3–3.0 mg/kg) significantly altered response rates (F(3,15) = 4.474, p = 0.020), such that 3.0 mg/kg significantly decreased responding below baseline levels.
The full time course of behavioral-stimulant effects of 0.3 and 1.0 mg/kg amphetamine and PAL-353 are presented in Figure 4, along with the effects of vehicle administration. In general, 0.3 mg/kg amphetamine increased response rates, while 1.0 mg/kg suppressed response rates (Figure 4A). Administration of 0.3 mg/kg amphetamine resulted in a peak increase of 358% basal activity 48 min after administration. There was a significant main effect of drug (F(1,5) = 32.227, p = 0.002) and of time (F(14,70) = 2.584, p = 0.005), but not of the interaction (F(14,70) = 1.466, NS). Administration of 1.0 mg/kg amphetamine resulted in a peak increase of 136% basal activity 12 min after administration, as well as a peak decrease of 39% basal activity 24 min. The main effects of both drug and time for this dose of amphetamine just missed statistical significance (F(1,5) = 5.269, p = 0.07; F(14,70) = 1.783, p = 0.059).
In general, both doses of PAL-353 increased response rates, although this effect was not dose-related (Figure 4B). Administration of 0.3 mg/kg PAL-353 resulted in a peak increase of 377% basal activity 18 min after administration. There was a significant main effect of drug (F(1,5) = 53.380, p < 0.0001) and of time (F(14,70) = 14.0875, p < 0.0001), as well as a significant interaction (F(14,70) = 7.04, p < 0.001). Administration of 1.0 mg/kg PAL-353 resulted in a peak increase of 306% basal activity 18 min after administration. There was a main effect of drug (F(1,5) = 7.687, p = 0.039 and of time (F(14,70) = 2.895, p = 0.002), but not of the interaction (F(14,70) = 0.843, NS).
DISCUSSION
The in vivo microdialysis studies described here show that acute systemic administration of amphetamine, PAL-353, and PAL-313 significantly increased dopamine levels in the nucleus accumbens in squirrel monkeys. The magnitude of this effect varied, such that the largest increase was observed following administration of PAL-353, followed by amphetamine and PAL-313. In contrast, administration of PAL-287 did not increase dopamine levels above baseline. In a separate group of squirrel monkeys, administration of amphetamine and PAL-353 induced behavioral-stimulant effects, while administration of PAL-313, PAL-287, and fenfluramine decreased response rates. In vitro assays using rodent tissue indicate that the four PAL drugs are nearly equipotent in releasing dopamine and norepinephrine, but their potencies in releasing serotonin vary (Negus et al., 2007; Rothman et al., 2001; Wee et al., 2005). Based on the literature, the rank order for releasing dopamine vs. serotonin of these drugs is amphetamine = PAL-353 > PAL-313 > PAL-287, and the current microdialysis and behavioral data reflect this order.
Earlier studies in rodents indicated that administration of amphetamine and PAL-353 increased locomotor activity, but PAL-313 and PAL-287 did not (Rothman et al., 2005; Wellman et al., 2009). The behavioral-stimulant effect of drugs in rodents is often associated with abuse liability in humans (Wise and Bozarth, 1987), although a dissociation between these two characteristics has been noted (Donovan et al., 1999; Rocha et al., 1998). Rhesus monkeys trained on a fixed-ratio schedule self-administered PAL-353, as did those trained on a progressive-ratio schedule (Wee et al., 2005). While PAL-313 maintained self-administration behavior in these same animals, the animals did not take as many infusions of PAL-313 as they did of PAL-353. In a separate study, rhesus monkeys trained on a fixed-ratio schedule did not self-administer PAL-287 across a range of doses (0.01–0.3 mg/kg/inf), although they readily self-administered cocaine (Rothman et al., 2005). These results suggested that the increased potency for releasing serotonin of PAL-313 and PAL-287 decreased the reinforcing effectiveness of these drugs, and the current results suggest that this mechanism is also involved in the blunted effect on extracellular dopamine release and operant behavior observed after drug administration. The current data also support earlier evidence that increasing serotonin release can attenuate drug-induced increases in dopamine levels in rodents and nonhuman primates (Czoty et al., 2002; Di Matteo et al., 2008). However, PAL-287 is also a partial agonist at the 5HT2C receptor (Rothman et al., 2005), which may also contribute to the decreased behavioral stimulant and rewarding effects of this drug (Bubar and Cunningham, 2008, 2006). In contrast, PAL-313 and PAL-353 are not active at this receptor (unreported observations, Blough).
One surprising result of these studies was that the magnitude of drug-induced dopamine increase in the microdialysis study was much larger for PAL-353 than for amphetamine, although these two drugs are chemically very similar and have very similar ratios of dopamine to serotonin release. In contrast to the large difference in neurochemical effects, the maximal increase in locomotor activity was similar for PAL-353 and amphetamine. The similarity in the magnitude of the behavioral-stimulant effect of these two drugs has also been observed in rats following i.p. administration (Wellman et al., 2009). In the present study, the 1.0 mg/kg dose of each of these drugs was on the descending limb of the dose-effect curve for behavioral effects, but 1.0 mg/kg PAL-353 still had a significant behavioral-stimulant effect, while 1.0 mg/kg amphetamine did not. Initially, this difference appears to be explained by the microdialysis data, as administration of 1.0 mg/kg PAL-353 produced a larger increase in dopamine than did the administration of 1.0 mg/kg amphetamine. However, the descending limb of behavioral effects of psychostimulants is generally attributed to stereotypy or unconditioned behavior (Katz, 1989; Skjoldager et al., 1991), not to decreases in dopamine release. Previous studies have shown that psychostimulants increase extracellular dopamine levels in a dose-dependent manner and do not result in an inverted U-shaped curve (Chen and Reith, 1994; Church et al., 1987; Hemby et al., 1995). Therefore, the decrease in behavioral output following 1.0 mg/kg amphetamine may not be due to a decrease in extracellular dopamine levels but could be a result of unconditioned behaviors resulting from increased dopamine levels. Alternatively, this relatively high dose of amphetamine may have increased extracellular serotonin to a level that augmented serotonin receptor activation, thus suppressing the observed behavioral output. In support of this hypothesis, PAL-313 did not alter response rates in the current study, although this drug increased dopamine levels to a maximum of 340% baseline at a dose of 1.0 mg/kg. Previous studies have shown that drugs that increase dopamine levels to 150–300% above baseline in the striatum, such as cocaine and tropane analogs of cocaine, also produce significant increases in operant behavior (Ginsburg et al., 2005; Kimmel et al., 2007). That neither 1.0 mg/kg amphetamine nor PAL-313 increased response rates despite increasing dopamine levels suggests that increased serotonin release also alters post-synaptic events, resulting in behavioral-stimulant effects that are lower than what one would predict based on the observed increases in dopamine levels. To test this hypothesis, future studies should determine changes in both extracellular dopamine and serotonin levels in this brain region following administration of a range of doses of these two drugs.
The current studies are the first to report in vivo neurochemical effects of PAL-353, PAL-313, and PAL-287 in nonhuman primates. Earlier studies examined the effect of PAL-287 on altering basal dopamine and serotonin levels in the prefrontal cortex of rodents. Administration of PAL-287 increased extracellular dopamine levels in the prefrontal cortex to the same extent as administration of amphetamine did (about 700% baseline) (Rothman et al., 2005), which contrasts with our current results in the nucleus accumbens of squirrel monkeys. In rodents, serotonin levels were increased following PAL-287 administration (about 900% baseline), but not following amphetamine administration (Rothman et al., 2005). Moreover, 3,4-methylenedioxymethamphetamine (MDMA)-induced locomotor activity was positively correlated with dopamine levels in the striatum, nucleus accumbens, and prefrontal cortex, as well as with serotonin levels in the striatum and prefrontal cortex of rodents. In the same study, stereotypy was positively correlated with dopamine levels in the striatum and nucleus accumbens and with serotonin levels in all three brain regions (Baumann et al., 2008). While the rodent data suggest that behavioral-stimulant effects of monoamine releasers are positively associated with increases in both dopamine and serotonin, nonhuman primate data suggest that increases in these behavioral effects are positively associated with increases in dopamine and are negatively associated with increases in serotonin.
In addition, these studies are the first reported microdialysis studies conducted in the nucleus accumbens of squirrel monkeys. Other groups have conducted studies in this brain region in rodents (Hooks et al., 1992; Steketee et al., 1992) and rhesus macaques (Bradberry et al., 2000). To date, most microdialysis studies in squirrel monkeys have focused on neurochemical changes in the caudate (Czoty et al., 2002; Czoty et al., 2000; Czoty et al., 2004; Ginsburg et al., 2005; Kimmel et al., 2005; Kimmel et al., 2007), although the putamen (Davis et al., 1997) and hippocampus (Ludvig et al., 2000) in this species have also been targeted. The present study confirms that the nucleus accumbens is an accessible and viable region for assessing experimentally-induced changes in neurotransmitter levels in this species.
Significant efforts have been directed toward the development of substitute agonists to treat cocaine abuse. For example, continuous treatment with amphetamine, which selectively releases dopamine and norepinephrine, dose-dependently decreased cocaine self-administration in rhesus monkeys under both progressive ratio and choice schedules (Negus, 2003; Negus and Mello, 2003a, b). In humans, studies show that treatment with amphetamine reduced cocaine use with little or no toxicity (Grabowski et al., 2001; Grabowski et al., 2004). Although amphetamine may appear to be an effective treatment for cocaine abuse and dependence, it has a high abuse liability, which may not be ideal for an effective medication. Accordingly, drugs that are structurally similar to amphetamine are being considered as potential medications and have been administered to rhesus monkeys that were initially trained to self-administer cocaine in order to assess the reinforcing effects of these amphetamine analogs. The potencies of four amphetamine analogs as a reinforcer in both fixed-ratio and progressive-ratio schedules did not correlate with the in vitro potencies of these analogs to release dopamine or serotonin. However, there was a very strong correlation between the ratio of the in vitro potencies for releasing dopamine versus serotonin and their reinforcing potency in both schedules of self-administration (Wee et al., 2005). These results indicate that increasing the selectivity for releasing dopamine versus serotonin increases the reinforcing effects of these drugs. As the drug becomes relatively more potent in releasing serotonin, the reinforcing effect decreases. These data support earlier studies that show increasing serotonergic tone decreases cocaine self-administration in rodents (Peltier and Schenk, 1993; Richardson and Roberts, 1991) and nonhuman primates (Czoty et al., 2002; Kleven and Woolverton, 1993). Neurochemical studies also show that increasing serotonergic tone attenuates cocaine-induced increases in dopamine in the caudate of squirrel monkeys (Czoty et al., 2002).
Studies with monoamine transporter inhibitors have shown that the behavioral profile of cocaine is influenced by actions on multiple neurotransmitters. Inhibition of SERT decreased cocaine self-administration in both rodents and nonhuman primates (Carroll et al., 1990; Czoty et al., 2002; Kleven and Woolverton, 1993). These SERT inhibitors also attenuated the behavioral-stimulant effects of cocaine in squirrel monkeys (Howell and Byrd, 1995; Spealman, 1993). Furthermore, inhibition of both DAT and SERT produced more robust reductions in cocaine self-administration in rhesus monkeys than did inhibition of DAT alone (Howell et al., 2007), providing additional support for targeting both of these neurotransmitters. Human patients receiving the combination of the dopamine releaser phentermine and the serotonin releaser fenfluramine as medications showed that the concurrent dopamine and serotonin release can ameliorate the symptoms of cocaine withdrawal and reduce illicit cocaine use (Kampman et al., 2000; Rothman et al., 1994). These preclinical and clinical findings support the rationale for the development of compounds that release both dopamine and serotonin as potential treatment medications for stimulant addiction.
The data presented here suggest that PAL-313 should be considered as a potential pharmacotherapeutic treatment for cocaine addiction, as it significantly elevates dopamine levels but does not have appreciable behavioral-stimulant effects in nonhuman primates. Furthermore, this compound maintained very low self-administration behavior in rhesus monkeys trained under a fixed-ratio schedule or those trained in a progressive-ratio schedule (Wee et al., 2005), suggesting that this drug is not reinforcing. Thus, this drug has several characteristics that render it favorable as a medication for treating psychostimulant addiction (Vocci et al., 2005).
Acknowledgments
This research was supported by U.S. Public Health Service grants DA00517 (LLH), DA12514 (LLH), DA12970 (BEB), and RR00165 (Division of Research Resources, National Institutes of Health). The authors would like to thank Mi Zhou, Julius T. Oatts, Michael A. Lowe, and Matthew E. Pontell for their expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Heather L. Kimmel, Email: heather.kimmel@emory.edu.
Daniel F. Manvich, Email: dmanvic@emory.edu.
Bruce E. Blough, Email: beb@rti.org.
S. Stevens Negus, Email: ssnegus@vcu.edu.
Leonard L. Howell, Email: lhowell@emory.edu.
References
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–17. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–5. [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–83. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–46. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–85. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990;35:237–44. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME. Effects of locally applied cocaine, lidocaine, and various uptake blockers on monoamine transmission in the ventral tegmental area of freely moving rats: a microdialysis study on monoamine interrelationships. J Neurochem. 1994;63:1701–13. doi: 10.1046/j.1471-4159.1994.63051701.x. [DOI] [PubMed] [Google Scholar]
- Church WH, Justice JB, Jr, Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur J Pharmacol. 1987;139:345–8. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- Cline EJ, Scheffel U, Boja JW, Carroll FI, Katz JL, Kuhar MJ. Behavioral effects of novel cocaine analogs: a comparison with in vivo receptor binding potency. J Pharmacol Exp Ther. 1992;260:1174–9. [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–7. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology. 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology (Berl) 2004;175:170–8. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- Davis MD, Heffner TG, Cooke LW. Dopamine agonist-induced inhibition of neurotransmitter release from the awake squirrel monkey putamen as measured by microdialysis. J Neurochem. 1997;68:659–66. doi: 10.1046/j.1471-4159.1997.68020659.x. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog Brain Res. 2008;172:7–44. doi: 10.1016/S0079-6123(08)00902-3. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, et al. Cocaine reward and MPTP toxicity: alteration by regional variant dopamine transporter overexpression. Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav. 2005;80:481–91. doi: 10.1016/j.pbb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–6. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–64. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Rau KS, Fleckenstein AE. The methamphetamine experience: a NIDA partnership. Neuropharmacology. 2004;47 (Suppl 1):92–100. doi: 10.1016/j.neuropharm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Reboussin D, Davies HM, Dworkin SI, Smith JE. Comparison of a novel tropane analog of cocaine, 2 beta-propanoyl-3 beta-(4-tolyl) tropane with cocaine HCl in rats: nucleus accumbens extracellular dopamine concentration and motor activity. J Pharmacol Exp Ther. 1995;273:656–66. [PubMed] [Google Scholar]
- Henningfield JE. Nicotine medications for smoking cessation. N Engl J Med. 1995;333:1196–203. doi: 10.1056/NEJM199511023331807. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Colvin AC, Juncos JL, Justice JB., Jr Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Res. 1992;587:306–12. doi: 10.1016/0006-8993(92)91012-4. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–9. [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–65. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Rukstalis M, Pettinati H, Muller E, Acosta T, Gariti P, et al. The combination of phentermine and fenfluramine reduced cocaine withdrawal symptoms in an open trial. J Subst Abuse Treat. 2000;19:77–9. doi: 10.1016/s0740-5472(99)00076-8. [DOI] [PubMed] [Google Scholar]
- Katz JL. Drugs as reinforcers: pharmacological and behavioral factors. In: Leibman JM, Cooper SJ, editors. The neuropharmacological basis of reward. Oxford: Oxford University Press; 1989. pp. 164–213. [Google Scholar]
- Kimmel HL, Ginsburg BC, Howell LL. Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse. 2005;56:129–34. doi: 10.1002/syn.20135. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of three monoamine uptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug Alcohol Depend. 1993;31:149–58. doi: 10.1016/0376-8716(93)90067-z. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–23. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann N Y Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ. Neurotransmitter transporters as drug targets: recent research with a focus on the dopamine transporter. The Pharmacologist. 1993;35:28–33. [Google Scholar]
- Ludvig N, Nguyen MC, Botero JM, Tang HM, Scalia F, Scharf BA, et al. Delivering drugs, via microdialysis, into the environment of extracellularly recorded hippocampal neurons in behaving primates. Brain Res Brain Res Protoc. 2000;5:75–84. doi: 10.1016/s1385-299x(99)00058-6. [DOI] [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. I. [3H]cocaine binding sites in caudate-putamen. J Pharmacol Exp Ther. 1989;251:131–41. [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003a;167:324–32. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine Releasers with Varying Selectivity for Dopamine/Norepinephrine versus Serotonin Release as Candidate “Agonist” Medications for Cocaine Dependence: Studies in Assays of Cocaine Discrimination and Cocaine Self-Administration in Rhesus Monkeys. J Pharmacol Exp Ther. 2007;320:627–36. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Peltier R, Schenk S. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology. 1993;110:390–4. doi: 10.1007/BF02244643. [DOI] [PubMed] [Google Scholar]
- Reith MEA, Meisler BE, Sershen H, Lajtha A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol. 1986;35:1123–9. doi: 10.1016/0006-2952(86)90148-6. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci. 1991;49:833–40. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, et al. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–7. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, et al. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005;313:1361–9. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Gendron T, Hitzig P. Combined use of fenfluramine and phentermine in the treatment of cocaine addiction: a pilot case series. Journal of Substance Abuse Treatment. 1994;11:273–5. doi: 10.1016/0740-5472(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Mechanisms of biogenic amine transporters. In: Reith M, editor. Neurotransmitter Transporters: Structure, Function, and Regulation. Totowa, NY: Humana Press; 1997. pp. 73–100. [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–63. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Skjoldager P, Winger G, Woods JH. Analysis of fixed-ratio behavior maintained by drug reinforcers. J Exp Anal Behav. 1991;56:331–43. doi: 10.1901/jeab.1991.56-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD. Modification of behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacology (Berl) 1993;112:93–9. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Madras BK, Bergman J. Effects of cocaine and related drugs in nonhuman primates. II. Stimulant effects on scheduled-controlled behavior. J Pharmacol Exp Ther. 1989;251:142–9. [PubMed] [Google Scholar]
- Steketee JD, Sorg BA, Kalivas PW. The role of the nucleus accumbens in sensitization to drugs of abuse. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:237–46. doi: 10.1016/0278-5846(92)90075-p. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), USPHS. Results from the 2006 National Survey on Drug Use and Health: National Findings. NSDUH Series H-32. Rockville, MD: Office of Applied Studies; 2007. [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–40. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–70. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–30. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. J Pharmacol Exp Ther. 1999;288:14–20. [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–54. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Davis KW, Clifford PS, Rothman RB, Blough BE. Changes in feeding and locomotion induced by amphetamine analogs in rats. Drug Alcohol Depend. 2009;100:234–9. doi: 10.1016/j.drugalcdep.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–40. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]