Abstract
Individuals with obesity frequently have an atherogenic lipid profile. It has been proposed that the insulin resistance observed in these individuals is involved in the development of these lipid abnormalities. However, most studies that have examined the relationship between insulin resistance and lipid abnormalities have included subjects who are either obese and/or glucose intolerant, two factors that may affect lipid levels independent of insulin resistance. We have therefore examined the impact of insulin resistance on plasma lipids in a healthy, lean (average BMI < 24), non-diabetic population (n =104). In our subjects we observed a wide range of values for insulin sensitivity index values (ISI) as calculated by the formula of Matsuda and DeFronzo. Lipid values ranged considerably in this population, but incidence of hypertriglyceridemia and hypercholesterolemia were low in the absence of obesity. We first examined the relationship between ISI and total and regional adipose stores as assessed by dual-energy X-ray absorptiometry (DXA). In men, we observed higher values for indices of total and central adipose stores that were significantly associated with decreased insulin sensitivity. In contrast, in women, ISI values were not associated with any variables related to either total or regional adiposity. In men, ISI was also significantly associated with higher triglycerides levels (P < 0.01) when adjusted for age and percent truncal fat. In women however, there was no significant association between ISI and triglycerides (P = 0.14). Instead, in women, the total and truncal fat were independent predictors of several lipid levels. These results both highlight gender differences in the associations between insulin resistance, regional adipose stores and lipids values, and emphasize the importance of adipose stores on the development of an individual’s lipid profile.
Introduction
It is well recognized that the obese state, and in particular excess fat stores in the abdomen, is associated with a more atherogenic lipid profile (higher triglycerides (TG), small dense LDL cholesterol, lower HDL cholesterol and higher remnant cholesterol)(1–6). Increased total and abdominal fat stores are also associated with the development of insulin resistance, and it has been proposed that the insulin resistance is pathophysiologically involved in the development of the lipid abnormalities (7). Both insulin resistance and lipid abnormalities are observed in the non-obese population (8;9). Whether insulin resistance can significantly impact serum lipids in the absence of overt obesity is unclear, as most studies that have examined the association between insulin resistance and the lipid abnormalities have included individuals with obesity and/or glucose intolerance (10–20). A dysregulation of blood glucose, while also associated with insulin resistance can independently affect lipid values as well. To assess the independent contribution of insulin resistance to lipid values, and to determine whether small differences in total and regional adipose stores impacted the atherogenic profile of non-obese subjects, we studied these variables in a lean population with normal glucose tolerance. The present studies identify different relative influences of insulin sensitivity and adipose stores on lipid levels between men and women in the absence of obesity and glucose intolerance.
Methods
Healthy non-obese (BMI < 27) nondiabetic sedentary subjects between the ages of 20 to 50 were recruited from the local population. Women were premenopausal. Individuals with diabetes, cardiovascular diseases, HIV and other active infections, thyroid disorders, epilepsy, cancer, hepatitis, cystic fibrosis, sickle cell disease, asthma or renal disease were excluded. Subjects were not taking medications known to affect either insulin sensitivity, carbohydrate metabolism, or lipid metabolism. These medications included glucocorticoids, adrenergic agonists, psychotropic drugs, diuretics, beta blockers, and HMG CoA reductase inhibitors. Individuals regularly participating in vigorous physical activity were not enrolled in the study. A body mass index (BMI) cutoff of less than 27 was chosen because it has been reported that in BMI values of 27 and below, there is a wide range of insulin sensitivity values and no correlation between BMI and insulin action (21). The inclusion cutoff for Asian Americans was set lower at ≤ 25 because of the increased susceptibility for insulin resistance and type 2 diabetes at lower BMI values in this population (22).
Ethical considerations
All subjects gave informed consent. The protocols and consent forms were approved by the University of California, San Francisco institutional review board and Clinical Research Center where the study was conducted.
Measurements of total and regional adipose stores
Height was measured with a research center stadiometer. Body weight was recorded. Waist and hip circumferences were measured by a standardized protocol. Body composition was assessed by dual-energy X-ray absorptiometry (DXA). In a subset of subjects (24 women, 7 men), we also measured abdominal fat stores by MRI. In these subjects (N=22), truncal fat was more predictive of abdominal fat stores as determined by MRI than either waist circumference or WHR in men and women (data shown).
Insulin sensitivity
In the morning following an overnight fast, subjects underwent a 75 g oral glucose tolerance test, with blood samples collected at −15, 0, 30, 60, 90 and 120 minutes for determination of glucose and insulin concentrations. Glucose was determined in whole blood by the glucose oxidase technique (Sigma). No patient was diabetic, and none had impaired glucose tolerance. Separate analysis of the subjects with fasting glucose > 100mg/dl indicated that serum lipid values in these 2 females and 6 males were not different than for those individuals of the same sex with normal fasting glucose. Thus, inclusion of these subjects did not alter the results of the study or our analysis of the data. Insulin levels were measured using a Linco ELISA assay. Insulin sensitivity index (ISI) was calculated according to the formula by Matsuda and DeFronzo [(ISI = 10,000/√(fasting glucose × fasting insulin) × (mean glucose × mean insulin)] (23).
A hyperinsulinemic euglycemic clamp (24) was performed in a subset of 28 subjects. Insulin was infused at a rate of 80mU/m2/min. Bedside blood glucose was measured at 5 minutes intervals to ensure it remained in the same range as the fasting glucose. The steady-state period for calculating of insulin sensitivity was between 90 and 120 minutes. In a subset of subjects (N = 28), insulin sensitivity was quantified by a hyperinsulinemic, euglycemic clamp. ISI values were highly correlated with these measurements of insulin-stimulated glucose disposal (r2 = 0.57, P = 0.001).
Laboratory tests
A fasting lipid profile including LDL pattern size, intermittent density lipoproteins was measured using a vertical ultracentrifugation technique (VAP panel – Atherotech, Birmingham, AL). Highly sensitive C-reactive protein (hsCRP) and fasting homocysteine levels were also measured.
Statistical analysis
Analyses were conducted using Stata Version 9.2 (StataCorp, College Station, TX). Multivariate linear regressions models were fit for each of the blood lipid outcome variables after examination of their distributions. Since the distributions of TG and homocysteine levels were right skewed, these outcomes were log transformed.
The distributions of the ISI measurements and the adiposity measurements were also explored. A 3-category ISI variable was derived from the ISI measurements categories corresponding to the first quartile, a combination of the second and third quartiles, and the fourth quartile. Data from men and women were pooled in order to determine cut point values for quartiles across the full population. To have a more parsimonious model, the middle quartiles were combined after preliminary analyses indicated no difference between the two middle quartiles. Adiposity measurements were similarly categorized to 3-level variables. Note that Percent Total Fat and Waist Circumference were derived from gender-specific quartiles since distributions for these two adiposity variables were significantly different between men and women.
Multivariate linear regression models were fit for each outcome with predictors including age, the ISI categorical variable, an adiposity categorical variable, gender, and interaction terms for ISI by gender and adiposity by gender. Lincom statements were used to assess specific effects, for example, to evaluate the difference in the age and percent truncal fat adjusted TG levels between men in the lowest ISI quartile and men in the highest ISI quartile.
Results
1. Clinical Characteristics
Subject characteristics are shown in Table 1. There were significant differences between the male and female subjects for age and all anthropometric variables. Thus, the impact of these indices of insulin sensitivity, body weight and total and regional adiposity on serum lipids were analyzed separately for men and women.
Table 1.
Clinical characteristics of enrolled subjects.
| Female (N = 60) | Male (N = 44) | |||
|---|---|---|---|---|
| Ethnicity | Caucasian African American Asian Hispanic Native American |
35 5 11 7 2 |
Caucasian African American Asian Hispanic Native American |
23 8 5 4 4 |
| Age (years) | 33 ± 1 | 19 – 50 | 40 ± 1† | 20 – 50 |
| BMI (kg/m2) | 22.5 ± 0.3 | 17.9 – 27.0 | 24.1 ± 3 * | 20.3 – 26.7 |
| Body fat (%) | 31.9± 0.9 | 17.7 – 43.0 | 21.0 ± 1.2† | 6.3 – 35.9 |
| Trunk Fat (%) | 31.5 ± 1.0 | 15.5 – 44.9 | 24.3 ± 1.5† | 7.0 – 42.9 |
| Waist (cm) | 72.4 ± 0.8 | 61.2 – 88.4 | 84.7 ± 0.9† | 73.2 – 96.8 |
| WHR | 0.753 ± 0.8 | 0.673 – 0.887 | 0.873 ± 0.006† | 0.785 – 0.943 |
| Fasting Glucose | 83 ± 1 | 60 – 106 | 92 ± 1† | 74 – 114 |
| Fasting Insulin | 4.3 ± 0.3 | 1.1 – 19.0 | 4.3 ± 0.3 | 1.7 – 9.8 |
| ISI | 11.6 ± 0.6 | 3.8 – 29.1 | 10.5 ± 0.7 | 4.0 – 27.9 |
| Triglycerides (mg/dl) | 69 ± 3 | 31 – 140 | 98 ± 6† | 41 – 265 |
| Cholesterol (mg/dl) | 174 ± 4 | 118 – 268 | 180 ± 5 | 89 – 268 |
| LDL Cholesterol (mg/dl) | 95 ± 3 | 53 – 189 | 111 ± 5 * | 34 – 183 |
| HDL Cholesterol (mg/dl) | 63 ± 2 | 37 – 88 | 51 ± 2† | 30 – 84 |
| Cholesterol Remnant (mg/dl) | 13 ± 1 | 3 – 44 | 18 ± 1* | 4 – 39 |
| Homocysteine | 7.0 ± 0.2 | 4.1 – 9.8 | 9.1 ± 0.4† | 5.9 – 23.2 |
Values shown are Mean ± SEM.
P < 0.001
P < 0.0001. All other values are not significant.
2. Insulin Sensitivity Distribution
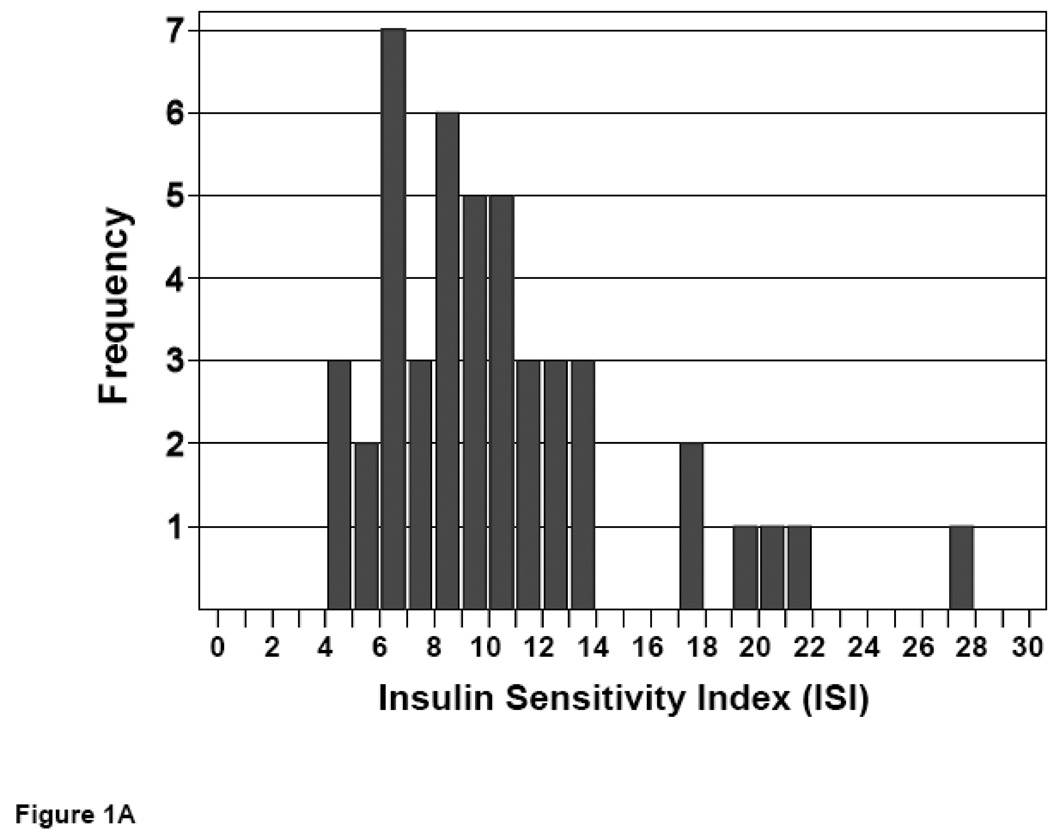
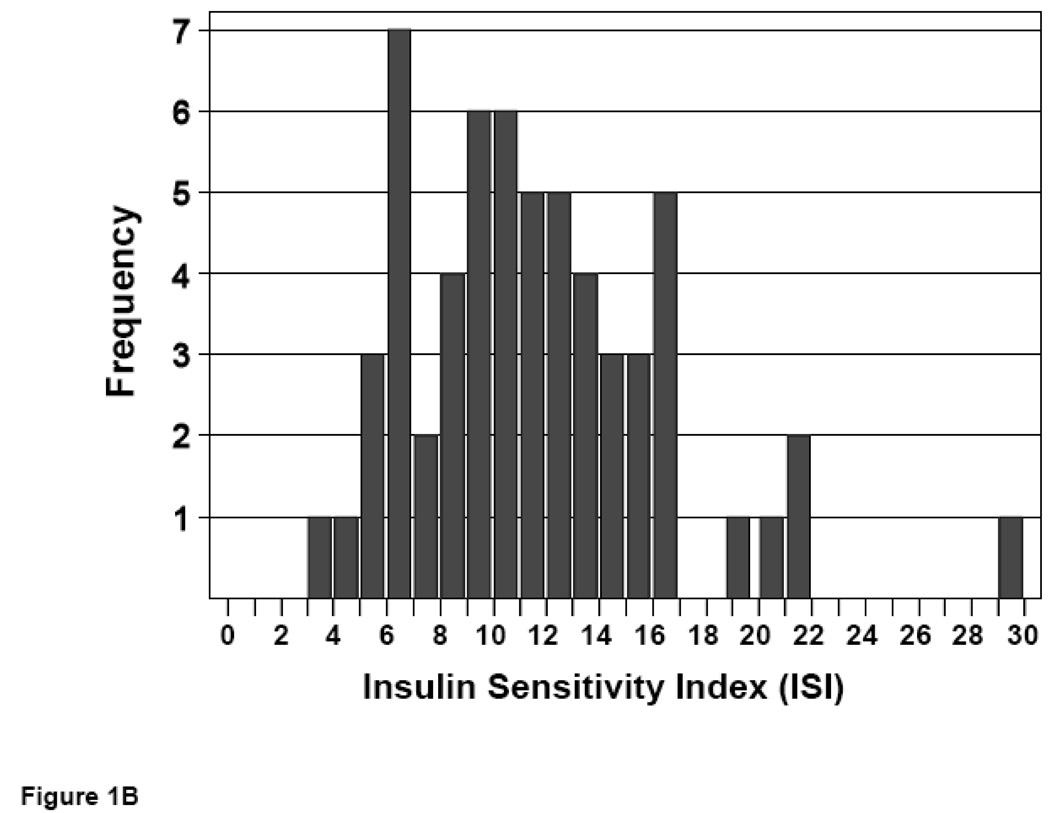
Following an overnight fast, an oral glucose tolerance test (OGTT) was performed. Blood glucose and serum insulin values at 0, 30, 60, 90, and 120 minutes following glucose challenge were employed to calculate an insulin sensitivity index (ISI) value for each subject (23). ISI values distributed over a 7-fold range, with no significant difference in mean ISI values for men vs. women (10.6 ± 0.7 vs. 11.6 ± 0.6, P = NS) (Figure 1).
Figure 1. Distribution of insulin sensitivity in non-obese subjects.
ISI values were calculated from glucose and insulin responses to an OGTT. Frequencies of ISI values are shown for male (A) and female (B) subjects.
3. Relationship between ISI and total and regional adiposity
To explore the impact of adiposity on the serum lipid profile, we assessed total and regional fat stores by several different methods. We selected percent total fat (total fat) and percent truncal fat (truncal fat) measurements obtained by DXA as the primary indices for total body fat and central fat stores. Truncal fat determined by DXA was highly correlated with total abdominal fat as measured by MRI (r = 0.875, P < 0.0001) and subcutaneous abdominal fat volume (r=0.866, p<0.0001). Truncal fat was significantly less predictive of visceral fat stores (r = 0.282, P = 0.20), suggesting that truncal fat is a more appropriate marker of total central fat than visceral fat. These relationships were not different between men and women. These data therefore support the use of truncal fat as an appropriate index of central adiposity in both sexes that is more accurate than common anthropometric measures. There were significant differences between the men and women subjects for age and measurements of generalized and regional stores (Table 1).
We assessed the contribution of total and regional adipose stores on insulin sensitivity by correlational analysis (Table 2). We observed that in men, higher values for indices of total and central adipose stores were associated with decreasing insulin sensitivity. ISI was negatively correlated with BMI (r = −0.39, P < 0.05), total fat (r = −0.41, P < 0.05), waist circumference (r = −0.48, P < 0.05), and truncal fat mass (r = −0.40, P < 0.05). In contrast, ISI values in women were not associated with any variables related to total or regional adiposity.
Table 2.
Correlations between indices of adiposity and ISI
| Females | Males | |
|---|---|---|
| BMI | −0.105 | −0.395* |
| % Body Fat | −0.062 | −0.395† |
| Waist Circumference | 0.022 | −0.414† |
| WHR | −0.106 | −0.279 |
| Truncal Fat | −.110 | −0.519† |
Values are Pearson correlations coefficients.
P < 0.01
P < 0.005. All other values are not significant.
4. The relationship between insulin sensitivity and serum lipid values adjusted for total and central adiposity
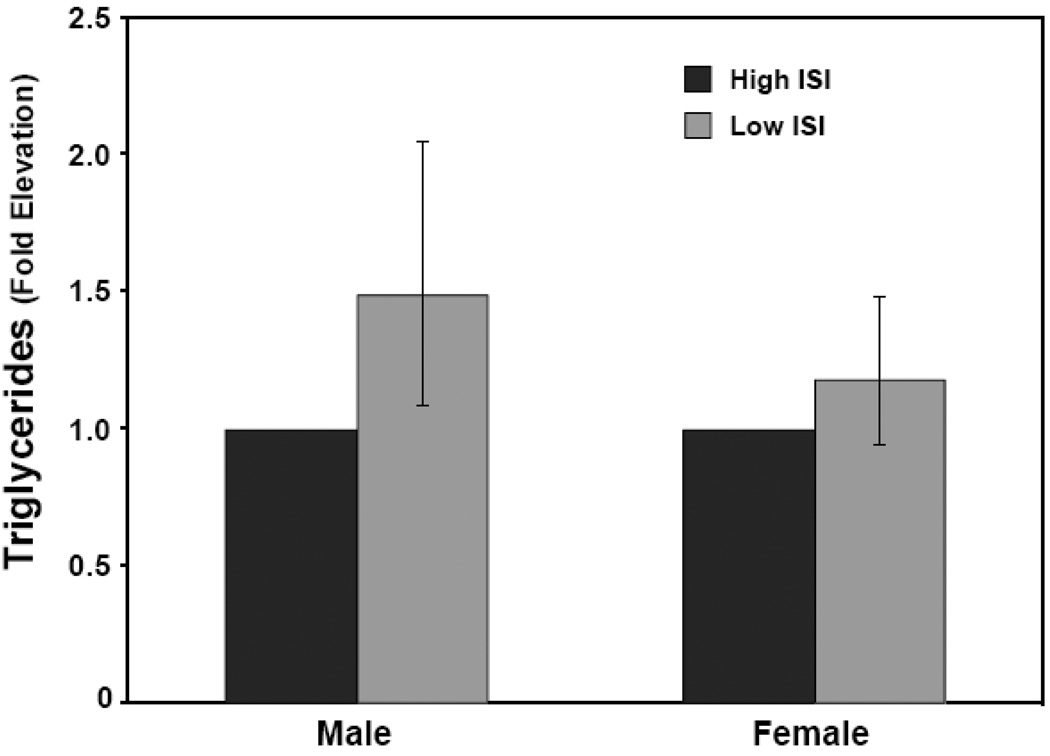
Due to the non-linear relationship between these variables, the effects of ISI and adiposity on serum lipids were examined across quartiles for these parameters. In men, ISI was significantly associated with TG (P < 0.01) when adjusted for age and truncal fat. Adjusted TG levels in the most resistant quartile were 1.49 fold higher than in the most sensitive quartile (P = 0.01) (Figure 2). The relationship between ISI and TG was similar when adjusting for age and total fat, rather than truncal fat (P = 0.01).
Figure 2. Serum triglycerides are elevated in insulin resistant men but not women.
Serum TG values were logged and adjusted for age and truncal fat. Subjects were grouped into quartiles of ISI. Male subjects in the lowest ISI quartile had adjusted TG values 1.49 fold higher than those in the highest ISI quartile (95% CI: 1.09–2.05, P = 0.01). Adjusted TG values for females in the lowest ISI quartile were 1.18-fold higher than those in the highest ISI quartile, a difference that was not statistically significant (95% CI: 0.94–1.48, P = 0.15).
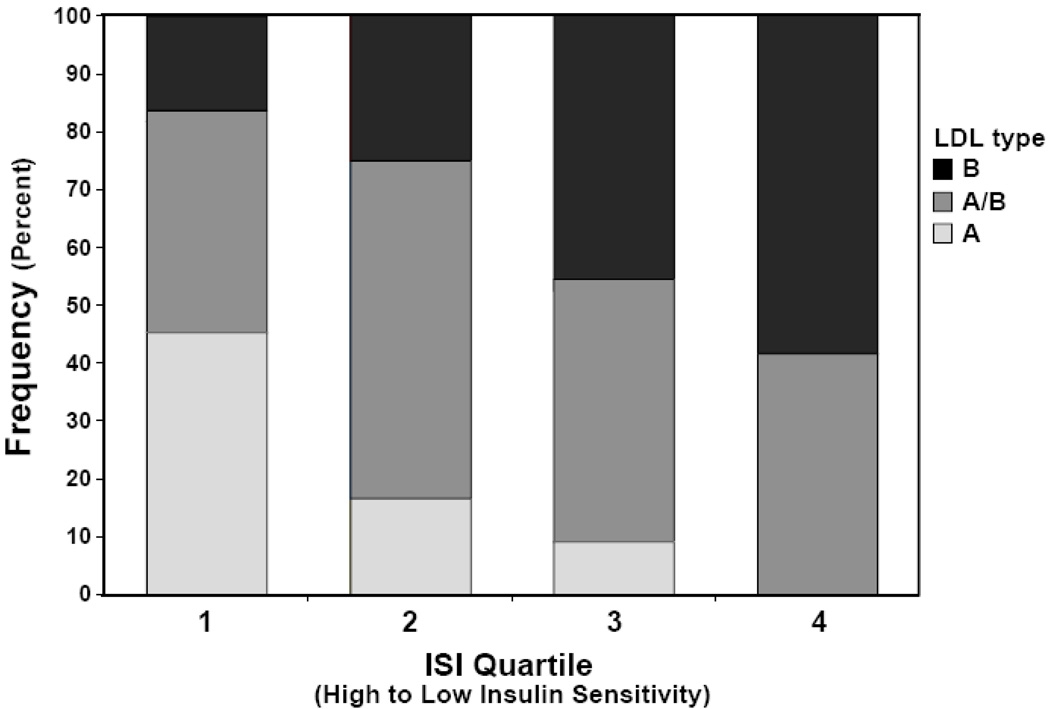
In contrast, in women the association between ISI and TG levels was less pronounced. There was no significant effect of ISI on TG when adjusted for age and truncal fat (P = 0.14). Adjusted triglyceride levels were 1.18 fold increased in the lowest vs. the highest quartile of ISI, but this difference did not reach statistical significance (P = 0.15) (Figure 2). While ISI had a smaller absolute influence on triglyceride levels in women compared to men, the interaction effect between gender and ISI on TG levels did not reach statistical significance (P = 0.08) LDL subclass pattern B identifies small dense LDL particles. Pattern A is predominant large LDL particles and A/B is intermediate (25). Low ISI values were associated with a more atherogenic LDL subtype in men but not women (Figure 3). However, neither LDL, HDL, remnant, nor total cholesterol levels were significantly associated with ISI in either sex. Similarly, homocysteine, hsCRP levels were not significantly associated with differences in insulin sensitivity in either sex (Table 1).
Figure 3. Insulin resistance associated with more atherogenic LDL subtypes in men.
LDL subtype (A, A/B, and B) distribution is presented across quartiles of unadjusted ISI values. Reductions in ISI were associated with a significant change in LDL composition toward a more atherogenic B subtype as determined by ANOVA (P < 0.05).
5. The relationship between central and total adiposity and lipid values adjusted for insulin sensitivity
In contrast to ISI, there was a significant independent effect of adiposity on multiple serum lipid parameters in women when adjusted for age and ISI that was not observed in men. In men, adjusted triglyceride levels were not associated with total or truncal percent fat (P=0.59, P=0.68, respectively). Accordingly, adjusted triglyceride values were not different between the lowest and highest quartiles of truncal percent fat (1.17 fold increase, 95% CI: 0.81, 1.69, P = 0.40) (Figure 4). Similarly, age and ISI-adjusted values for LDL cholesterol, HDL cholesterol, cholesterol remnants, total cholesterol, homocysteine, hs-CRP were not significantly associated with difference in total or truncal percent fat (data not shown). In women, however total and truncal percent fat were independent predictors of several serum lipid parameters. Quartile analysis by ANOVA indicated that, when adjusted for age and ISI, there was a significant association between truncal fat and TG (p=0.02), total cholesterol (P=0.04), LDL cholesterol (p=0.02), and cholesterol remnants (P= 0.01). Age and ISI-adjusted triglyceride levels were 1.42 fold increased in the highest truncal percent fat quartile compared to the lowest quartile, an effect that just missed statistical significance (95% CI: 0.99, 2.05) (Figure 4).
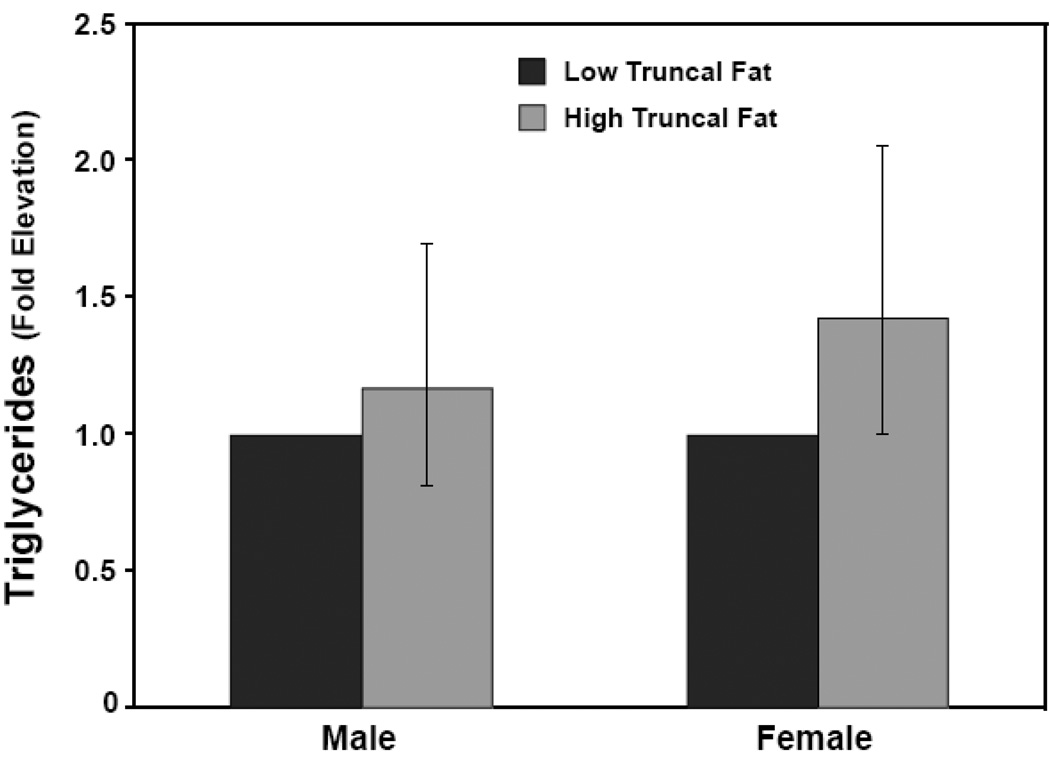
Figure 4. Serum triglycerides not independently associated with truncal fat in men.
Serum TG values were logged and adjusted for age and ISI. Subjects were grouped into quartiles of truncal fat. Adjusted TG values were not different in male subjects in the highest quartile for truncal fat compared to the lowest quartile (1.17 fold; 95% CI: 0.81–1.69, P = 0.40). Adjusted TG values for females in the highest truncal fat quartile were 1.42-fold higher than those in the lowest truncal fat quartile, a difference that just missed statistical significance (95% CI: 0.99–2.05, P = 0.06).
Similarly, women in the highest quartile for truncal fat had age and ISI-adjusted LDL cholesterol levels 29 mg/dl higher than those in the lowest quartile (95% CI: 2.3, 55.7 mg/dl, P= 0.03). Interestingly, the increased LDL content accompanying increased truncal fat was associated with an increased prevalence of less atherogenic LDL subtypes (data not shown). However, age and ISI-adjusted levels of the atherogenic cholesterol remnants were 9 mg/dl higher in the highest truncal fat quartile (95% CI: 2.2, 16.4 mg/dl, P = 0.01).
The association of age and ISI-adjusted serum lipids with percent total fat in women followed a similar trend (data not shown), but was less marked; only cholesterol remnants were significantly independently associated with the quartile of total body fat (P=0.04).
6. Prevalence and determinants of cardiovascular risk in the non-obese population
We examined the prevalence of our cases where the lipid values exceeded thresholds for cardiovascular risk as defined by the World Health Organization (26); Adult Treatment Panel III (27) and International Diabetes Federation (28). We found that these markers of CV risk were relatively rare in this population. In women, there were no cases of hypertriglyceridemia (>150mg/dl) or elevated homocysteine levels (> 10.4 µmol/L). There were two cases (3%) of high LDL (>160 mg/dl) and six cases (10%) each of low HDL (< 50mg/dl) and high hs-CRP (>3 mg/L). Chi square analysis of ISI quartiles indicates that the prevalence of these risk markers does not increase with insulin resistance in women. The prevalence of high LDL levels was significantly (P = 0.05) associated with being in the highest quartile for truncal fat in women. The association of truncal fat with LDL risk, and the general low prevalence of cardiac risk markers in this these women with BMI < 27, underscores the critical role of adiposity in the incidence of hyperlipidemia in women.
In men, there was greater prevalence of several risk markers compared to women. There were 4 cases (9% incidence rate) of TG over 150 and elevated hs-CRP, 5 cases (11%) of elevated LDL cholesterol, 9 cases (20%) of elevated homocysteine and 10 (22%) cases of low HDL cholesterol.
In general high TG, and low HDL cholesterol incidence in men tended to be more prevalent in the insulin resistant group, but these trends did not meet statistical significance. The prevalence of LDL values >160 mg/dl was significantly greater in the most insulin resistant quartile (2/12) compared to the other quartiles combined (3/34) (P < 0.05). The highest quartile of truncal fat was associated with increased incidence of elevated hs-CRP (3/11 cases) (P < 0.05) but not hypertriglyceridemia, elevated LDL or homocysteine, or low HDL cholesterol levels.
Overall, in the absence of obesity there was minimal evidence for clustering of CV risk factors in this population. In men with HDL cholesterol levels below 40 mg/dl, the prevalence of hypertriglyceridemia was increased (3/4) compared to the men with normal HDL cholesterol (7/42) (P < 0.05). Low HDL was also associated with increased incidence of CRP > 3 mg/L (3/10 vs. 1/36) (P < 0.05).
Discussion
In order to determine the impact of insulin resistance on serum lipid variables associated with cardiac risk and the metabolic syndrome without the confounding effects of obesity, we calculated ISI values from OGTT data on healthy subjects with BMI values ≤ 27. We observed that this relationship between insulin sensitivity and serum lipids was apparently different between men and women. However, much of this gender effect could be attributed to the finding that the relatively small variance in adipose stores seen in this population differently effected insulin sensitivity in men compared to women.
When we examined the relationship between fat stores and insulin sensitivity, we found that in men higher values of total and truncal fat were associated with reduced insulin sensitivity. Surprisingly in women, the truncal fat and total fat were not associated with differences in insulin sensitivity. This relationship was observed whether we employed anthropomorphic measurements as indices for adipose stores, or values derived from DXA or in some subjects, MRI, and whether ISI values were used to estimate whole body insulin action or insulin-mediated glucose disposal was directly assessed by hyperinsulinemic, euglycemic clamp. It is possible that there is some threshold for adiposity past which the well-documented association between fat stores and insulin sensitivity is observed. This lack of an association between adiposity and insulin sensitivity below a BMI of 27 had been established previously (21), and is the reason we selected this apparent threshold as the cutoff for enrollment in this study. In males, however, ISI values were not independent of adiposity across this range of BMI. It is clear that, in this population of females, some physiologic factors other than central or total adipose stores influence insulin action sufficiently to produce the range of ISI values observed. We selected individuals that did not regularly participate in vigorous physical activity in order to remove the impact of exercise training on insulin sensitivity from this study. It is likely that factors such as intramyocellular lipids which were not measured in the present study but have previously been reported to be associated with insulin resistance (29–31) may explain the range of ISI in this population of women.
We also observed a gender difference in the associations of insulin resistance and regional adipose stores with the serum lipid profiles. While overall interaction effects between gender and ISI on these parameters did not meet statistical significance, the magnitude whereby ISI influenced serum lipids was much greater in males than females. In men, insulin resistance (after adjustment for total or truncal adiposity) was significantly associated with elevated triglyceride levels and higher levels of small dense LDL particles (the atherogenic phenotype). In women, however, ISI had little impact on the lipid parameters. Instead, in women it was the total and truncal fat stores (adjusted for ISI) that were significantly associated with elevated TG, total cholesterol, LDL cholesterol and remnants, while in males ISI-adjusted values for serum lipids did not vary as a function of total or truncal fat. An explanation for this gender difference in the impact of insulin resistance and adiposity on lipid measures may lie in the above observation, that in men, insulin resistance is closely associated with truncal and total fat stores. It may not therefore be possible in men to distinguish an independent association between adipose measure and lipid parameters.
For women the lack of association between adiposity and insulin sensitivity do allow for a cleaner examination of the relationship between insulin resistance and serum lipids that are traditionally linked to cardiovascular risk and the metabolic syndrome. The fact that we observed no association between insulin resistance and serum lipids in women does not support the hypothesis that insulin resistance is pathophysiologically involved in the development of the atherogenic lipid abnormalities.
We observed that even though there were a range of lipid values and insulin sensitivities in lean obese non diabetic individuals, very few patients had lipid values that exceeded threshold risk as defined by the WHO, ATPIII and IDF (26–28). These results further highlight the importance of accumulated fat on the determination of cardiovascular risk markers. Even in a normal weight population, the relatively small variations in adipose stores exert an influence on serum lipids in women and on insulin action in men. Still, the relatively high incidence of cardiovascular risk markers is seemingly dependent on acquiring additional fat stores beyond those seen in this population with a BMI cutoff of ≤ 27. The absence of clinically defined hypertriglyceridemia and other atherogenic markers in insulin resistant women suggests that obesity is a more significant causative agent in the metabolic syndrome, which explains the lack of symptom clustering seen in this population.
The results of this study are limited primarily by sample size. While we employed ISI calculations to quantify whole body insulin action, the results were similar when insulin-mediated glucose disposal values generated by glucose clamp were used in the subset of subjects undergoing that procedure. Similarly, MRI-determined abdominal fat volume values did not produce different results than those obtained by DXA determination of truncal fat. It is possible that results would have been different had the study included subjects with impaired glucose tolerance. While this may have introduced subjects with a more severe form of insulin resistance, any impact of hyperglycemia and related complications on serum lipids would have confounded the ability to determine the singular effects of insulin resistance on these parameters.
In conclusion our results highlight the gender differences in the associations between insulin resistance, adipose measures and lipid parameters. Studies that investigate mechanisms of insulin resistance in the non-obese population should therefore consider these gender differences in their analyses.
Acknowledgements
The authors wish to acknowledge the staff of the Clinical Research Center at UCSF for their assistance in conducting these studies, and Drs. Su-Chun Cheng, and Barbara Grimes for their assistance with biostatistical analysis.
This work was supported in part by NIH grant R01DK59358 and CTSI NIH/NCRR UCSF-CTSI UL1 RR024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Committee on Human Research of the University of California, San Francisco, approved the study protocols and they were in accordance with the Helsinki Declaration.
The authors have no conflict of interest.
Reference List
- 1.Abate N, Garg A, Peshock RM, Straygundersen J, Adamshuet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45:1684–1693. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990 Jul;10(4):497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 3.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. 2004 Sep;89(9):4206–4210. doi: 10.1210/jc.2004-0631. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;Vol 46(Iss 10):1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 5.Meigs JB, Dagostino RB, Wilson PWF, Cupples LA, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome: The Framingham Offspring Study. Diabetes. 1997;46:1594–1600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- 6.Tai ES, Lau TN, Ho SC, Fok AC, Tan CE. Body fat distribution and cardiovascular risk in normal weight women. Associations with insulin resistance, lipids and plasma leptin. Int J Obes Relat Metab Disord. 2000 Jun;24(6):751–757. doi: 10.1038/sj.ijo.0801220. [DOI] [PubMed] [Google Scholar]
- 7.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 8.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008 Aug 11;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004 Apr;53(4):495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosch A, Muhlen I, Kopf D, Augustin W, Dierkes J, Konig W, et al. LDL size distribution in relation to insulin sensitivity and lipoprotein pattern in young and healthy subjects. Diabetes Care. 1998;Vol 21(Iss 12):2077–2084. doi: 10.2337/diacare.21.12.2077. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992 Jun;41(6):715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, Mayerdavis EJ, Goff D, Zaccaro DJ, Laws A, Robbins DC, et al. Relationships between insulin resistance and lipoproteins in nondiabetic African Americans, Hispanics, and non-Hispanic whites: The Insulin Resistance Atherosclerosis Study. Metabolism. 1998;Vol 47(Iss 10):1174–1179. doi: 10.1016/s0026-0495(98)90319-5. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005 Aug 1;96(3):399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 14.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005 Feb;54(2):333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 15.Abbott WG, Lillioja S, Young AA, Zawadzki JK, Yki-Jarvinen H, Christin L, et al. Relationships between plasma lipoprotein concentrations and insulin action in an obese hyperinsulinemic population. Diabetes. 1987 Aug;36(8):897–904. doi: 10.2337/diab.36.8.897. [DOI] [PubMed] [Google Scholar]
- 16.Garg A, Helderman JH, Koffler M, Ayuso R, Rosenstock J, Raskin P. Relationship between lipoprotein levels and in vivo insulin action in normal young white men. Metabolism. 1988 Oct;37(10):982–987. doi: 10.1016/0026-0495(88)90157-6. [DOI] [PubMed] [Google Scholar]
- 17.Laakso M, Pyorala K, Voutilainen E, Marniemi J. Plasma insulin and serum lipids and lipoproteins in middle-aged non-insulin-dependent diabetic and non-diabetic subjects. Am J Epidemiol. 1987 Apr;125(4):611–621. doi: 10.1093/oxfordjournals.aje.a114574. [DOI] [PubMed] [Google Scholar]
- 18.Godsland IF, Crook D, Walton C, Wynn V, Oliver MF. Influence of insulin resistance, secretion, and clearance on serum cholesterol, triglycerides, lipoprotein cholesterol, and blood pressure in healthy men. Arterioscler Thromb. 1992 Sep;12(9):1030–1035. doi: 10.1161/01.atv.12.9.1030. [DOI] [PubMed] [Google Scholar]
- 19.Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med. 1992 Jan;231(1):25–30. doi: 10.1111/j.1365-2796.1992.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 20.Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest. 1993 Jul;92(1):141–146. doi: 10.1172/JCI116541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clausen JO, Borchjohnsen K, Ibsen H, Bergman RN, Hougaard P, Winther K, et al. Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians - Analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest. 1996;98:1195–1209. doi: 10.1172/JCI118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell-Morris LL, Treder RP, Shuman WP, Fujimoto WY. Fatness, fat distribution, and glucose tolerance in second-generation Japanese-American (Nisei) men. Am J Clin Nutr. 1989 Jul;50:9–18. doi: 10.1093/ajcn/50.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999 Sep;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 25.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. J Am Med Assoc. 1988 Oct 7;260(13):1917–1921. [PubMed] [Google Scholar]
- 26.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998 Jul;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) J Am Med Assoc. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed]
- 28.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005 Sep 24;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 29.Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsle U, et al. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia. 1999 Aug;42:932–935. doi: 10.1007/s001250051250. [DOI] [PubMed] [Google Scholar]
- 30.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999 Aug;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 31.Phillips DIW, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, et al. Intramuscular triglyceride and muscle insulin sensitivity: Evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–950. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]