Abstract
Erythrocyte formation involves the elimination of mitochondria at the reticulocyte stage of development. Nix−/− reticulocytes fail to eliminate their mitochondria at this step due to a defect in the targeting of mitochondria to autophagosomes. To determine the role of autophagy in this process, we generated Atg7−/− transplant mice. Atg7−/− reticulocytes exhibit a partial defect in mitochondrial clearance, demonstrating that there are both autophagy-dependent and -independent mechanisms of mitochondrial clearance. We used Atg7−/− autophagy-defective reticulocytes to study temporal events in mitochondrial clearance. Mitochondrial depolarization precedes elimination, but in Atg7−/− reticulocytes the depolarization event is markedly delayed. Since Atg7 regulates autophagosome formation, we infer that mitochondrial depolarization occurs downstream of autophagosome formation in reticulocytes. We propose that there are two mechanisms of mitochondrial clearance: one that is triggered by mitochondrial depolarization, and a second NIX-dependent mechanism, which is not. The NIX-dependent mechanism remains to be elucidated.
Keywords: reticulocyte, autophagy, mitophagy, mitochondria, NIX, Atg7, mitochondrial depolarization, mitochondrial clearance
Mitochondrial clearance is a well-recognized but poorly understood biological phenomenon. In mammalian cells, it serves as a quality control mechanism, facilitating the elimination of damaged, depolarized mitochondria. Mitochondria are also eliminated during certain developmental processes. Ubiquitinated sperm mitochondria are eliminated by the egg after fertilization, and mitochondria are cleared from lens and erythroid cells at the final stages of their maturation. We are studying reticulocytes to gain insight into the mechanisms of mitochondrial clearance. Reticulocytes are formed from late-stage erythroblasts in the bone marrow, by nuclear expulsion. After several days in the bone marrow, reticulocytes are released into the circulation where they complete their development into erythrocytes. The transformation from reticulocyte to erythrocyte involves cytoskeletal remodeling, a decrease in surface area and volume, and the elimination of endoplasmic reticulum, mitochondria, and ribosomes.
While it is generally accepted that autophagy is involved in the elimination of damaged or compromised mitochondria, the role of autophagy in mitochondrial clearance during development is less clear. Ultrastructurally, the process of mitochondrial clearance in erythroid cells resembles autophagy. On the other hand, in Atg5−/− neonates, Atg5 was found to be dispensable for organellar clearance in lens and erythroid cells.
NIX is an atypical BH3-only protein, which localizes to the mitochondrial outer membrane. Nix−/− reticulocytes exhibit a selective defect in mitochondrial clearance. Autophagy is activated during terminal erythroid differentiation, but is unaffected by the loss of NIX. Thus, NIX is not generally required for autophagy in erythroid cells. However, mitochondria are not properly targeted to degradative vacuoles in Nix−/− reticulocytes, and tend to accumulate on the cytoplasmic face of undigested autophagosomes. The underlying defect appears to be in the sequestration of individual mitochondria by double-layered membranes, without which fusion with degradative vacuoles and subsequent elimination is not possible.
The presence of undigested autophagosomes in Nix−/− reticulocytes led us to critically address the role of autophagy in mitochondrial clearance in an autophagy-deficient model. Atg7 is an E1-like enzyme, which is required for the conjugation of Atg12 to Atg5, and Atg8 to phosphatidylethanolamine. Atg7 plays a central role in autophagy, and deficiency of Atg7 causes perinatal lethality due to an inability to utilize essential and branched-chain amino acids. To obtain Atg7−/− reticulocytes, we transplanted Atg7−/− fetal liver cells into lethally-irradiated adult recipients. Engrafted Atg7−/− transplant recipients exhibit lymphopenia, mild anemia, and reticulocytosis. In contrast to Nix−/− mice, Atg7−/− transplant recipients do not possess an abnormal population of circulating erythrocytes with retained mitochondria. On the other hand, mitochondrial clearance from Atg7−/− reticulocytes is impaired. These results indicate that mitochondrial clearance is partially defective in the absence of Atg7.
Mitochondria are eliminated from reticulocytes by exocytosis, singly in double-membraned structures without cytoplasm, or several at a time in degradative vacuoles. If these structures were mechanistically distinct in their generation, for example if autophagy were dispensable for the former, it could explain the partial effect of Atg7 deficiency on mitochondrial clearance. However, ultrastucturally, we were unable to make this distinction. Mitochondria in degradative vacuoles are still observed in Atg7−/− reticulocytes, albeit in reduced numbers. We conclude that autophagy promotes NIX-dependent mitochondrial clearance in reticulocytes, but is not essential for this process.
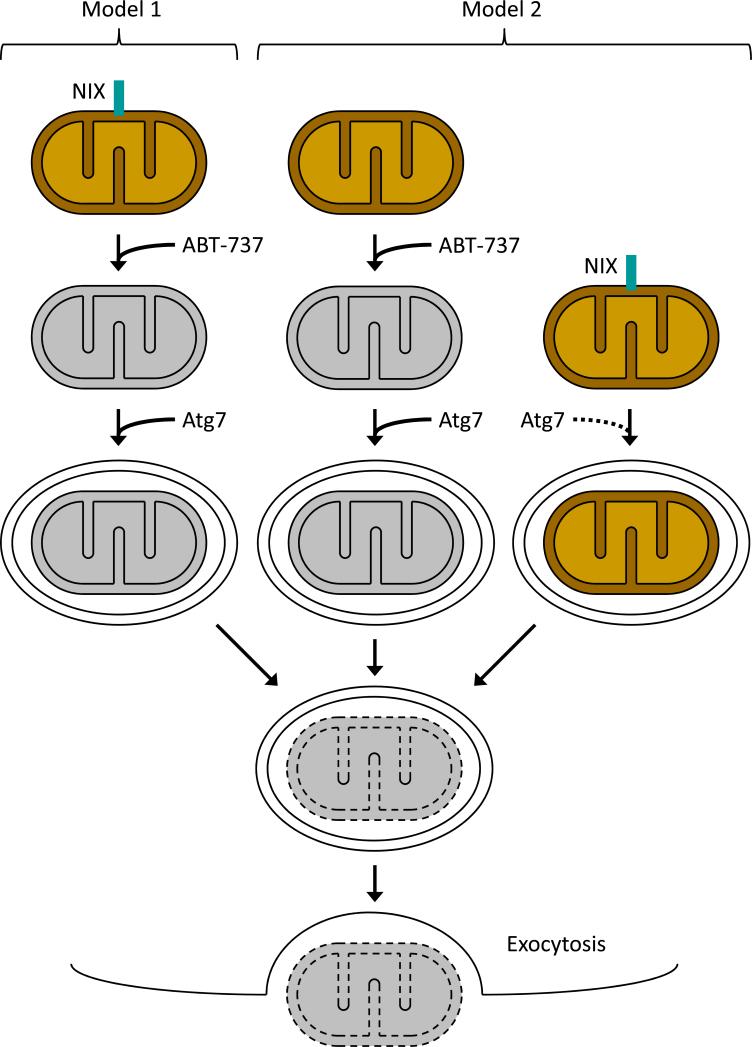
Deficiency of NIX causes a striking defect in mitochondrial clearance, but its mechanism of action is not known. One suggestion is that NIX triggers clearance by causing mitochondrial depolarization. However, if this is the case, then it does not occur through typical proapoptotic pathways, since BAX, BAK, and activation of the mitochondrial permeability transition pore are dispensable for mitochondrial clearance. Along the same line, the BH3-mimetic ABT-737, which bypasses the requirement for NIX, must function differently, since in contrast to NIX it requires BAX or BAK. The ability of ABT-737 and other depolarizing compounds to rescue mitochondrial clearance in Nix−/− reticulocytes is consistent with the suggestion that NIX causes depolarization (Fig. 1, model 1); however, it is also consistent with a model that places NIX on a separate pathway (Fig. 1, model 2). To distinguish between these possibilities, we examined the polarization status of mitochondria in autophagy-defective Atg7−/− reticulocytes. If NIX causes mitochondrial depolarization, then depolarized mitochondria should accumulate in autophagy-defective Atg7−/− reticulocytes. Instead, many mitochondria remain polarized, even after several days in culture. Based on this result, we conclude that NIX is insufficient to cause depolarization, and that depolarization occurs after autophagosome formation, but prior to elimination. Therefore, the mechanism by which NIX mediates mitochondrial clearance remains unsolved.
Figure 1.
Two models of NIX-dependent mitochondrial clearance in reticulocytes. In model 1, NIX causes mitochondrial depolarization (represented as gray color), which in turn triggers autophagosome formation and clearance. ABT-737 bypasses the requirement for NIX by causing depolarization. In model 2, the depolarization-induced pathway is latent, and NIX promotes the formation of double-membraned structures around mitochondria. In this case, Atg7 contributes through its role in autophagosome formation, but is not essential for mitochondrial clearance (dashed arrow). In the absence of Atg7, mitochondrial clearance is impaired, and model 1 predicts that depolarized mitochondria will accumulate, whereas model 2 predicts that polarized mitochondria will persist (see text for details). In both cases, NIX (teal box) is displayed at the NIX-dependent step. Once enclosed in a double-membraned structure, mitochondria are degraded (dashed lines) and eliminated by exocytosis.
The main implication of these studies is that there are at least two mechanisms of mitochondrial clearance. The first is triggered by depolarization, and serves as a mitochondrial quality control mechanism. This mechanism involves the recruitment of Parkin, or another E3-ligase, to depolarized mitochondria. The second is controlled by NIX in reticulocytes, or the related protein BNIP3 in MEF cells. Given the role of NIX and BNIP3 in mitochondrial clearance, this mechanism may serve to regulate mitochondrial mass. Both mechanisms utilize autophagy, to some extent. An important question is whether the mechanisms functionally overlap, because if they overlap to a significant degree, then it may be necessary to inactivate both to determine the role of mitochondrial clearance in development and homeostasis.
Footnotes
Autophagic punctum to: Zhang J, Randall MS, Loyd MR, Dorsey FC, Kundu M, Cleveland JL, and Ney PA. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood 114:157-164, 2009.