Abstract
The goal was to test 14 nerve agent model compounds of soman, sarin, tabun, and cyclohexyl methylphosphonofluoridate (GF) for their suitability as substitutes for true nerve agents. We wanted to know whether the model compounds would form the identical covalent adduct with human butyrylcholinesterase that is produced by reaction with true nerve agents. Nerve agent model compounds containing thiocholine or thiomethyl in place of fluorine or cyanide were synthesized as Sp and Rp stereoisomers. Purified human butyrylcholinesterase was treated with a 45-fold molar excess of nerve agent analog at pH 7.4 for 17 h at 21°C. The protein was denatured by boiling and digested with trypsin. Aged and non-aged active site peptide adducts were quantified by MALDI-TOF mass spectrometry of the tryptic digest mixture. The active site peptides were isolated by HPLC and analyzed by MALDI-TOF-TOF mass spectrometry. Serine 198 of butyrylcholinesterase was covalently modified by all 14 compounds. Thiocholine was the leaving group in all compounds that had thiocholine in place of fluorine or cyanide. Thiomethyl was the leaving group in the GF thiomethyl compounds. However, sarin thiomethyl compounds released either thiomethyl or isopropyl, while soman thiomethyl compounds released either thiomethyl or pinacolyl. Thiocholine compounds reacted more rapidly with butyrylcholinesterase than thiomethyl compounds. Labeling with the model compounds resulted in aged adducts that had lost the O-alkyl group (O-ethyl for tabun, O-cyclohexyl for GF, isopropyl for sarin, and pinacolyl for soman) in addition to the thiocholine or thiomethyl group. The nerve agent model compounds containing thiocholine, and the GF thiomethyl analog were found to be suitable substitutes for true soman, sarin, tabun, and GF in terms of the adduct they produced with human butyrylcholinesterase. However, the soman and sarin thiomethyl compounds yielded two types of adducts, one of which was thiomethyl phosphonate, a modification not found after treatment with authentic soman and sarin.
Keywords: nerve agent analogs, mass spectrometry, butyrylcholinesterase, active site serine, dehydroalanine, beta-elimination
Introduction
The nerve agents soman, sarin, tabun, and GF are among the most toxic chemicals known (1). Minute quantities can be lethal to humans, as demonstrated in the Tokyo subway attack with sarin where 12 persons died and about 5000 were injured (2). The great toxicity of these agents has led to restriction of their use for investigational purposes, so that only military laboratories have access to these compounds. Non-military research laboratories must use nerve agent simulants such as diisopropylfluorophosphate, or nerve agent model compounds. We chose to use nerve agent model compounds, whose design suggested they would yield the same covalently modified protein as the true nerve agents. Stereoselective isomers were synthesized because it is known that the cholinesterases react preferentially with specific stereoisomers of nerve agents (3-6) . The present work tested the hypothesis that model compounds of soman, sarin, tabun, and GF would react with human butyrylcholinesterase to yield adducts identical to those produced by reaction with true nerve agents. This information will determine the choice of model compounds that will yield suitable nerve agent modified proteins for use in the evaluation of biological targets of nerve agents.
Chromogenic nerve agent analogs containing p-nitrophenol or the fluorescent 3-chloro-7-oxy-4-methylcoumarin as the leaving group have been synthesized (7, 8). They make adducts identical to those of authentic nerve agents. Therefore, these analogs are useful for screening large enzyme libraries for nerve agent hydrolase activity. Other fluorescent analogs have been synthesized to use in the search for variants of paraoxonase that detoxify nerve agents more rapidly than wild type paraoxonase (9).
Nerve agent simulants that have no possibility of making an adduct identical to that of a nerve agent, include demeton (S-2-ethylthioethyl O,O-dimethyl phosphorothioate) an analog of VX, diisopropylfluorophosphate, an analog of sarin, and dipinacolyl methylphosphonate, an analog of soman. The first two can be used as substitutes for nerve agents when characterizing organophosphorus hydrolases (10). The third, a non-toxic soman analog, is used for immunoassay screening of antibodies (11).
Methods
Materials
Nerve agent model compounds were synthesized at the Human BioMolecular Research Institute (San Diego, CA) (12). The model compounds were dissolved in dimethylsulfoxide to make 100 mM solutions and used immediately. Human butyrylcholinesterase was purified from outdated human plasma by ion exchange chromatography at pH 4.0 followed by affinity chromatography on procainamide-Sepharose, and anion exchange at pH 7 on a Protein-Pak DEAE 8HR 1000 Å, 10 × 100 mm HPLC column (Waters/Millipore) (13). The purified butyrylcholinesterase had an activity of 540 units/mL (with 1 mM butyrylthiocholine at 25°C, pH 7.0) and a protein concentration of 0.75 mg/mL. Sequencing grade modified trypsin (V5113, Promega, Madison, WI) in 50 mM acetic acid at a concentration of 0.4 μg/μl was stored at -80°C. Alpha-cyano-4-hydroxycinnamic acid (70990, Fluka, via Sigma, St. Louis, MO) was recrystallized from ethanol, dried, and stored at -20°C.
Inhibition of butyrylcholinesterase
A 0.25 mL aliquot of butyrylcholinesterase (0.19 mg = 2.2 nmoles) in pH 7.4 phosphate buffered saline was treated with 1 μl of 100 mM nerve agent analog at 21°C for 17 h. The molar ratio of butyrylcholinesterase to nerve agent was 1:45.
Butyrylcholinesterase activity assay
The assay contained 1 mM butyrylthiocholine, 0.5 mM 5,5-dithiobis(2-nitrobenzoic acid) in 2 mL of 0.1 M potassium phosphate pH 7.0, at 25°C and 1 μl of butyrylcholinesterase. The absorbance increase at 412 nm was recorded on a Gilford spectrophotometer interfaced to MacLab 200 (ADInstruments Pty Ltd., Castle Hill, Australia) and a Macintosh computer. Activity was calculated from the extinction coefficient of 13,600 M-1cm-1. Units of activity are micromoles substrate hydrolyzed per min.
Digestion with trypsin
The nerve agent treated BChE was denatured in a boiling water bath for 10 min. The cooled solution received 2 μl of 1 M ammonium bicarbonate to raise the pH to about 8.3, and 10 μl of 0.4 μg/μl trypsin. Digestion was overnight at 37°C.
HPLC
Digests were centrifuged to remove a pellet and injected into a Phenomenex C18 column, 100 × 4.6 mm, on a Waters 625 LC system. Peptides were eluted with a 60 min gradient starting with 100% buffer A (0.1% trifluoroacetic acid in water), and ending with 60% buffer B (acetonitrile containing 0.09% trifluoroacetic acid) at a flow rate of 1 mL per min. One mL fractions were collected.
MALDI-TOF-TOF mass spectrometer
The digest before HPLC separation, as well as each HPLC fraction was analyzed in the MS mode on the MALDI-TOF-TOF 4800 mass spectrometer (Applied Biosystems, Foster City, CA). A 0.5 μl aliquot was spotted on an Opti-TOF 384 Well Insert (P/N 1016629, Applied Biosystems), dried, and overlaid with 0.5 μl of alpha-cyano-4-hydroxycinnamic acid (10 mg/mL in 50% acetonitrile, 0.1% trifluoroacetic acid). MS spectra were acquired using delayed extraction in reflector mode with a laser intensity of 3500 volts. Each spectrum was the sum of 500 laser shots. All other settings on the instrument were default settings. Default settings include a bin size of 0.5 ns, final detector voltage 1.905, and delay extraction time 500 ns. The instrument was calibrated with Glu-Fibrinopeptide standards. Spectra were saved to DATA EXPLORER where an output window listed the cluster area for each peak.
The peptide sequence and the identity of the modified amino acid were determined by fragmenting the parent ions in the MS/MS mode of the MALDI-TOF-TOF mass spectrometer. The acquisition method was the factory method MSMS - 1 kv positive. Default settings included a precursor mass window of ±10 Da and a random pattern of shots. The metastable suppressor was ON and the timed ion selector was enabled. The y-ions and b-ions were assigned with the aid of the Proteomics Toolkit, a free online fragment ion calculator (http://db.systemsbiology.net).
Quantitation of phosphonylated peptides
Relative amounts of phosphonylated peptides before and after aging were calculated from cluster areas displayed in the output window of DATA EXPLORER. This method of quantitation assumes that the unaged and aged peptides ionize with similar efficiencies. Each sample served as its own internal control because the two peptides whose cluster areas were compared were in the same MALDI spot, in the same MALDI-TOF spectrum. They had the same amino acid sequence and the same net charge. The phosphonate in the aged sample, though negatively charged at neutral pH, has no charge in 0.1% trifluoroacetic acid, the solvent for the MALDI matrix. Thus the net charge on both peptides was the same. The validity of calculating labeled and unlabeled peptide quantities from cluster areas in the same MALDI spot was confirmed by amino acid composition analysis for peptides labeled by reaction with p-nitrophenyl acetate (14).
Safety consideration
Nerve agent model compounds are less toxic than authentic nerve agents. To ensure the safety of personnel, only small quantities that would not intoxicate a human were in one vial. Vials were opened in a fume hood. Empty vials and pipette tips were detoxified in 0.1 M sodium hydroxide. Personal protective equipment was worn.
Results
Inhibition of butyrylcholinesterase activity
Purified human butyrylcholinesterase (0.75 mg/mL) was treated with a 45-molar excess of the nerve agent model compounds listed in Table 1. A 1 μl aliquot was removed after various times to measure enzyme inhibition. After 3 hours incubation at 21°C, pH 7.4, inhibition levels were 90-99.9% for the thiocholine model compounds (1-8 in Table 1) and for the thiomethyl GF model compounds (9 and 10). However, inhibition levels for the thiomethyl sarin and soman model compounds (11-14) were about 80% for the Sp isomers and about 40% for the Rp model compounds. The incubations were continued for a total of 17 h at which time all samples were inhibited at least 85%. The least inhibited sample was the one treated with the thiomethyl soman Rp analog 14, which was inhibited about 85%. In summary, the most rapidly reacting inhibitors were the thiocholine model compounds. The most slowly reacting inhibitors were the thiomethyl Rp model compounds of sarin and soman.
Table 1.
Structures of nerve agent model compounds and masses of the butyrylcholinesterase tryptic peptide after labeling and aging
| # | Structure | analog of | isomer | adduct mass (abundance, %) |
mass of aged adduct (abundance, %) |
|---|---|---|---|---|---|
| 1 |  |
tabun | Sp | 3063.5 (50±5%) | 3036 (50±5%) |
| 2 | 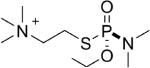 |
tabun | Rp | 3063.5 (54±2%) | 3036 (46±2%) |
| 3 | 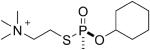 |
GF | Sp | 3088.5 (44±2%) | 3006.5 (56±2%) |
| 4 | GF | Rp | 3088.5 (86±3%) | 3006.5 (14±3%) | |
| 5 | sarin | Sp | 3048.5 (74±3%) | 3006.5 (26±3%) | |
| 6 | 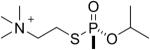 |
sarin | Rp | 3048.5 (99±1%) | 3006.5 (1±1%) |
| 7 |  |
soman | Sp | 3090.5 (26±4%) | 3006.5 (74±4%) |
| 8 | 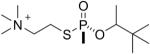 |
soman | Rp | 3090.5 (78±5%) | 3006.5 (22±5%) |
| 9 | 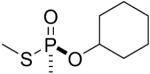 |
GF | Sp | 3088.5 (40±1%) | 3006.5 (60±1%) |
| 10 | 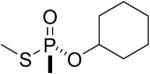 |
GF | Rp | 3088.5 (38±2%) | 3006.5 (62±2%) |
| 11 | 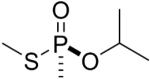 |
sarin | Sp | 3048.5 (79±3%) 3036.5 (5±1%) |
3006.5 (16±1%) |
| 12 | 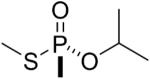 |
sarin | Rp | 3048.5 (76±2%) 3036.5 (13±2%) |
3006.5 (11±3%) |
| 13 | 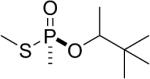 |
soman | Sp | 3090.5 (26±2%) 3036.5 (19±2%) |
3006.5 (55±1%) |
| 14 | 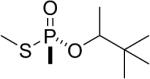 |
soman | Rp | 3090.5 (8±2%) 3036.5 (70±5%) |
3006.5 (22±3%) |
Numbers in parentheses (%) indicate the relative abundance of each type of adduct after 17 h reaction at pH 7.4, 21°C, for four replicates ± standard deviation. The monoisotopic mass of the singly charged unlabeled peptide is 2928.5 amu. Accession number P06276 for human butyrylcholinesterase.
Mass spectrometry of tryptic digests to identify nerve agent adducts on butyrylcholinesterase
After 17 h of treatment with nerve agent model compounds, the butyrylcholinesterase samples were denatured by boiling and digested with trypsin. The digests were spotted on a MALDI plate and the masses of the labeled active site peptides determined by MALDI-TOF mass spectrometry.
Table 1 shows the structures of the 14 nerve agent model compounds and the peptide masses observed for the labeled active site tryptic peptides of human butyrylcholinesterase. The amino acid sequence of the 29-residue active site tryptic peptide of human butyrylcholinesterase is SerValThrLeuPheGlyGluSerAlaGlyAlaAlaSerValSerLeuHisLeuLeuSerProGlySerHisSerLeuPhe ThrArg. The active site serine is residue 8 in the peptide, which corresponds to residue 198 in the mature protein and to residue 226 in accession #P06276 where numbering includes the 28 amino acid signal peptide. The active site serine was covalently modified by the nerve agent model compounds.
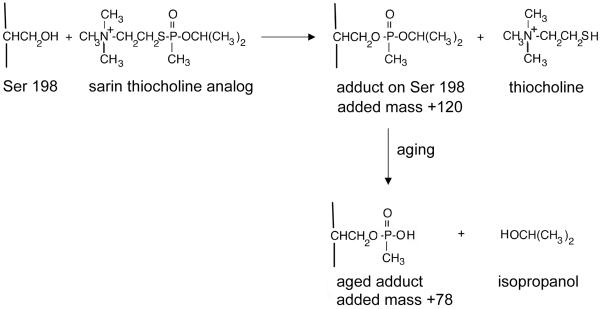
Nerve agent model compounds 1-2 have thiocholine in place of the cyanide in tabun, while model compounds 3-8 have thiocholine in place of the fluorine atom in GF, sarin, and soman. MALDI-TOF mass spectrometry showed that the reaction of butyrylcholinesterase with nerve agent model compounds 1-8 resulted in covalent binding of the nerve agent to Serine 198 and simultaneous release of thiocholine. This reaction is illustrated in Figure 1 with the sarin thiocholine analog.
Figure 1.
Covalent binding of the sarin nerve agent model compounds 5 or 6 to human butyrylcholinesterase. The active site serine (Ser198) forms an initial adduct with an added mass of +120 amu. Thiocholine is released in this step. This is followed by a dealkylation reaction called “aging” which releases isopropanol.
The mass of the tryptic peptide became 3063.5 after addition of 135 amu from the tabun analog (1 and 2 in Table 1), 3088.5 after addition of 160 amu from the GF analog (3 and 4), 3048.5 after addition of 120 amu from the sarin analog (5 and 6), and 3090.5 after addition of 162 amu from the soman analog (7 and 8). These masses are consistent with release of thiocholine from model compounds 1-8 upon covalent bond formation with butyrylcholinesterase. The same adducts form when butyrylcholinesterase is treated with authentic tabun, GF, sarin, and soman.
Mass spectrometry also showed that the adducts for compounds 3-8 in Table 1 underwent a dealkylation reaction in which sarin lost isopropyl, GF lost O-cyclohexyl, and soman lost pinacolyl alcohols. The aged products all had the same monoisotopic mass of 3006.5, consistent with the structure shown in Figure 1 for the methylphosphonyl adduct whose added mass is 78 amu. The result for aging of the tabun adduct was unclear (1 and 2 in Table 1). The aged product appeared to have a mass of 3036 amu which does not allow one to distinguish between 3036.5 amu for loss of the dimethylamine and 3035.5 amu for loss of the O-ethyl group. However, the crystal structure of the aged tabun adduct of human butyrylcholinesterase showed that aging resulted in loss of O-ethyl (15). Since the crystal structure is definitive, we conclude that aging of the tabun adduct results in loss of the O-ethyl group.
The thiomethyl model compounds of GF (9 and 10 in Table 1) made a butyrylcholinesterase adduct with an added mass of 160 amu, which means the thiomethyl group was released during covalent bond formation with Serine 198. Both isomers formed the same adduct, though the Sp isomer reacted more rapidly than the Rp isomer.
The thiomethyl model compounds of sarin (11 and 12 in Table 1) released either thiomethyl or isopropyl to form adducts with an added mass of +120 or +108. For example, for the thiomethyl sarin Rp isomer 12 it was estimated that about 76% had an added mass of 120 amu (to give 3048.5) representing loss of thiomethyl, while 13% had an added mass of 108 amu (to give 3036.5) representing loss of isopropyl. The remaining 11% was the aged adduct with a monoisotopic mass of 3006.5 amu.
Similarly, the thiomethyl model compounds of soman (13 and 14 in Table 1) formed adducts that released either the thiomethyl or the pinacolyl group. For the thiomethyl Sp soman isomer 13 it was estimated that 26% had a mass of 3090.5 representing loss of the thiomethyl group, 19% had a mass of 3036.5 representing loss of pinacolyl, and 55% had a mass of 3006.5 representing loss of both thiomethyl and pinacolyl groups. In contrast, the thiomethyl Rp soman isomer 14 formed a majority of 3036.5 amu adduct (70%), representing loss of pinacolyl. Only 22% of the thiomethyl Rp soman adduct had aged to 3006.5 amu.
We conclude that the initial adducts with the thiomethyl model compounds of sarin and soman differ from those produced by authentic sarin and soman because 5 to 70% retained the thiomethyl group on the phosphorus atom. In contrast, all of the thiocholine model compounds yield adducts that are indistinguishable from those produced by authentic nerve agents.
Quantitation of types of adducts
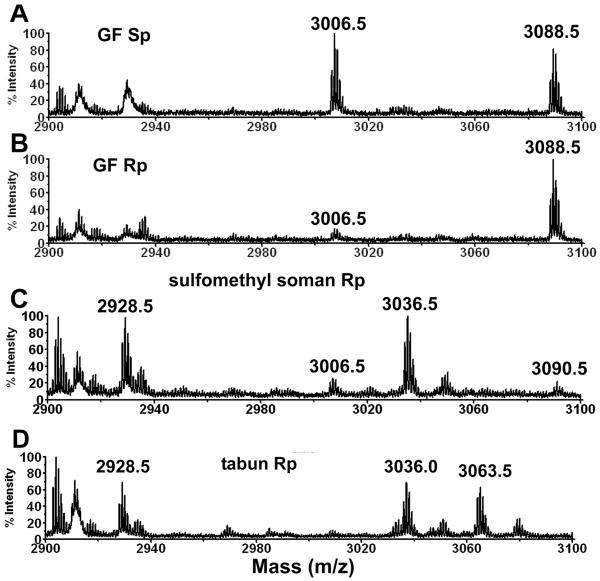
Values for the relative abundance of the adducts in Table 1 were calculated from cluster areas in MALDI-TOF spectra of trypsin-digested nerve agent analog-inhibited butyrylcholinesterase. The calculation assumes the peptides ionize with similar efficiencies when serine carries the non-aged or aged nerve agent. Example MALDI-TOF spectra are in Figure 2. Panels A and B compare the MALDI-TOF spectra of the tryptic digests of butyrylcholinesterase inhibited with the GF Sp thiocholine isomer and the GF Rp thiocholine isomer (3 and 4 in Table 1). The peak clusters at 3088.5 are isotopes of the active site peptide labeled on serine with cyclohexyl methylphosphonate. The peak clusters at 3006.5 are isotopes of the labeled active site peptide that has lost the cyclohexyl group as a result of aging. The peak areas for 3006.5 and 3088.5 are approximately equal for GF Sp in Figure 2A, showing that about 56% of the labeled butyrylcholinesterase has aged. In contrast 86% of the labeled peptide in Figure 2B has a mass of 3088.5 and 14% has a mass of 3006.5, indicating that the GF Rp isomer yielded low amounts of aged butyrylcholinesterase.
Figure 2.
MALDI-TOF spectra of tryptic digests of human butyrylcholinesterase inhibited with A) GF thiocholine Sp isomer, B) GF thiocholine Rp isomer, C) thiomethyl soman Rp isomer, D) tabun thiocholine Rp isomer. The inhibition reaction proceeded at 21°C for 17 h in pH 7.4 buffer with a 45-fold molar excess of nerve agent analog. Each MALDI spot contained 0.5 μl of 8.8 pmol/μl butyrylcholinesterase digest. The laser intensity was 3500 volts.
The MALDI-TOF spectrum of Figure 2C shows a prominent peak at 3036.5 for the tryptic peptide of butyrylcholinesterase labeled with the thiomethyl soman Rp isomer. About 70% of the labeled peptide is the thiomethyl methylphosphonate adduct with mass 3036.5. This is an unusual adduct that is not found by reaction with authentic soman. The peaks at 3090.5 for the pinacolyl methylphosphonate adduct, and at 3006.5 for the aged adduct represent 8% and 22% of the labeled peptide. The unlabeled active site peptide has a monoisotopic mass of 2928.5 amu for the singly charged ion.
Figure 2D shows peaks for the non-aged tabun adduct at 3063.5 and aged tabun adduct at 3036.0. About 46% of the labeled peptide is the non-aged adduct and 54% the aged adduct.
Verification of peptide identities
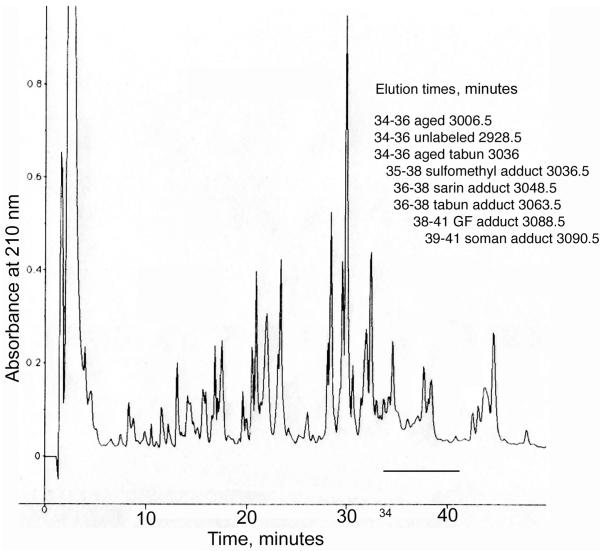
The masses in Table 1 are consistent with the interpretation that they are the nerve agent analog labeled tryptic peptides of human butyrylcholinesterase. However, these masses do not provide irrefutable proof of the peptide identities. Absolute proof was obtained from MS/MS spectra of peptides that had been purified by reverse phase HPLC. Figure 3 shows an HPLC trace monitored at 210 nm for a tryptic digest of butyrylcholinesterase labeled with thiomethyl sarin Rp (11 in Table 1). The aged peptide of mass 3006.5 amu and the unlabeled peptide of mass 2928.5 amu eluted between 34-35 min. The thiomethyl phosphonate adduct of mass 3036.5 eluted between 35-37 min. The isopropyl phosphonate adduct of mass 3048.5 eluted between 36-38 min.
Figure 3.
Reverse phase HPLC of trypsin-digested human butyrylcholinesterase labeled with thiomethyl sarin Rp. The 0.18 mg of labeled human butyrylcholinesterase (2.2 nanomoles) was digested with trypsin and loaded onto a 100 × 4.6 mm Phenomenex C18 column. Peptides were eluted with a 60 min gradient, from 0 to 60% acetonitrile, 0.1% trifluoroacetic acid at a flow rate of 1 mL/min. A 0.5 μl aliquot from each 1 mL fraction was examined in the MALDI-TOF mass spectrometer to identify the fraction that contained the active site peptide. The inset summarizes the elution times for unlabeled peptide and for peptides labeled with tabun, GF, sarin, or soman.
HPLC traces for the other labeled butyrylcholinesterase digests were nearly identical in appearance to the trace in Figure 3. There were no distinguishing peaks that could be assigned to labeling by a particular nerve agent analog. Though the labeled active site peptides eluted between 34-41 min, they co-eluted with other peptides. The presence of the other peptides explains why the HPLC traces are not unique for each type of label. The inset to Figure 3 shows that the heavier masses tended to elute later than the lighter masses.
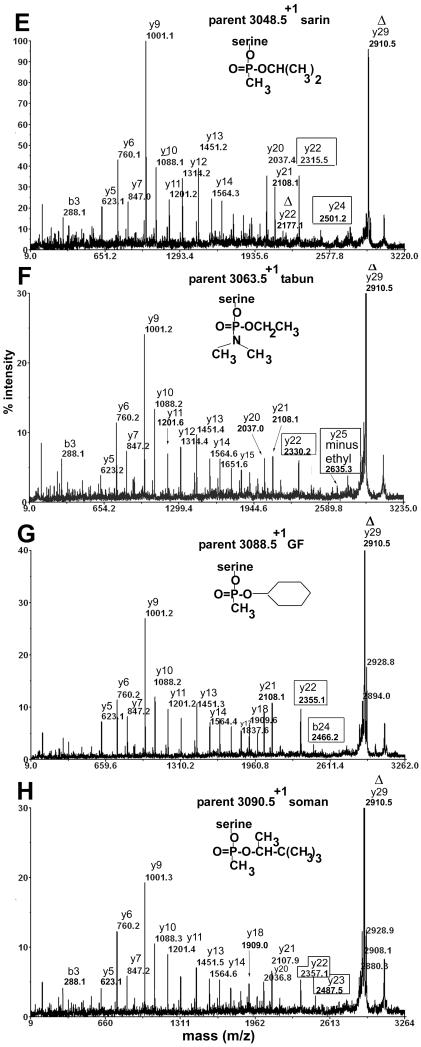
MS/MS spectra were acquired for 0.5 μl aliquots of the HPLC fractions by using the MS/MS mode of the MALDI-TOF-TOF mass spectrometer. The 8 spectra in Figure 4 are for the active site tryptic peptide of human butyrylcholinesterase. The spectrum in panel A is for the unlabeled peptide, while the spectra in panels B-H are for the peptides labeled on serine 198 (serine 8 in the peptide) by various phosphonates. No distinction is made between the adducts formed by Sp and Rp isomers because they yielded the same adduct masses.
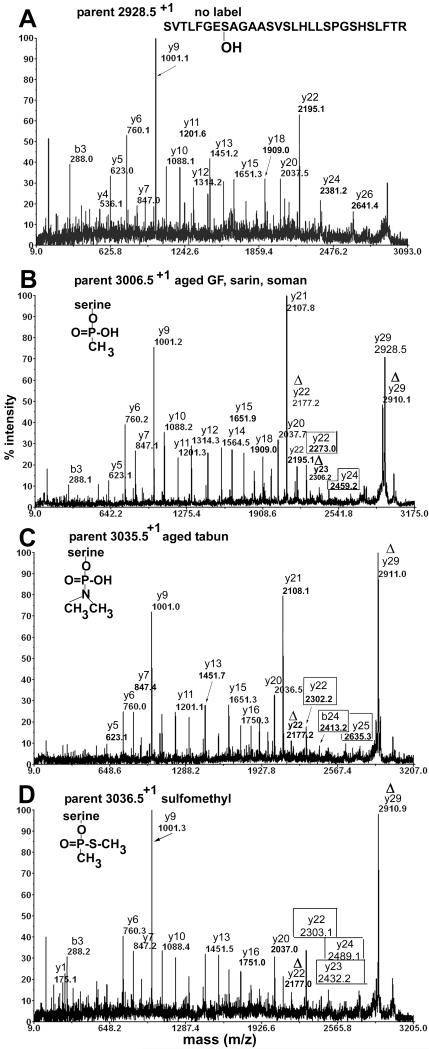
Figure 4.
MS/MS spectra acquired on the MALDI-TOF-TOF mass spectrometer for peptide SVTLFGESAGAASVSLHLLSPGSHSLFTR of human butyrylcholinesterase. A) no label, B) methylphosphonate adduct produced by aging of GF, sarin, and soman adducts, C) aged tabun adduct, D) thiomethyl methylphosphonate adduct produced by reaction with thiomethyl sarin and soman model compounds, E) non-aged sarin adduct, F) non-aged tabun adduct, G) non-aged GF adduct, H) non-aged soman adduct. The delta symbol Δ designates masses that are consistent with loss of the phosphonate and a molecule of water. The boxed masses designate fragments that retain the phosphonate.
The unlabeled peptide in Figure 4A has a singly charged parent ion of mass 2928.5 amu. The y-ion series supports the amino acid sequence SVTLFGESAGAASVSLHLLSPGSHSLFTR. The most prominent peak, y9 with mass 1001.1, represents cleavage between serine and proline to yield the y9 ion PGSHSLFTR, consistent with the fact that peptide bonds on the N-terminal side of proline are particularly susceptible to collision induced dissociation (16). The mass at 2195.1 amu represents cleavage on the N-terminal side of the active site serine to yield the y22 ion SAGAASVSLHLLSPGSHSLFTR.
Figure 4B shows the MS/MS spectrum for the singly charged parent ion of mass 3006.5 where the active site serine is labeled with methylphosphonate. The same derivative was produced by aging of butyrylcholinesterase inhibited by GF, sarin, and soman. The alkyl group that distinguishes GF, sarin, and soman has been enzymatically removed during the aging process to yield an adduct of mass 3006.5 amu. The peak at 2910.1 amu is the Δy29 ion. This ion is consistent with the loss of the organophosphorus agent and loss of a molecule of water from the parent ion to yield dehydroalanine in place of the phosphonylated active site serine. The Δy29 ion is prominent in Figures 4B-4H, showing that all organophosphorus agents, regardless of their identity, are easily released from serine in the MS/MS mode of the mass spectrometer. Similarly, the Δy23 ion at 2306.2 contains dehydroalanine in place of serine. Support for our assignment of dehydration at the active site serine and not at one of the other serine residues in the Δy23 ion comes from the fact that the masses of the y21 and smaller y-ions are consistent with peptides that contain normal serines. Figure 4B has two types of y22 ions. The y22 ion at 2195.1 has the same mass as the y22 ion in the spectrum for unlabeled peptide in Figure 4A. Its presence in Figure 4B suggests that some of the organophosphorus agent was released from serine without concomitant loss of a molecule of water, so that the serine remained serine. The other y22 ion at 2273.0 amu has the methylphosphonate intact on the active site serine. The y24 ion at 2459.2 amu also carries intact methylphosphonate. These ions provide additional proof for modification of the active site serine by methylphosphonate.
Figure 4C shows the MS/MS spectrum for the singly charged parent ion of mass 3035.5 where the active site serine is labeled with dimethylaminephosphonate. The modification is the result of aging of tabun-labeled butyrylcholinesterase with release of O-ethyl. Two types of y22 ions are present. The Δy22 ion at 2177.2 amu has lost the organophosphorus agent and a molecule of water and therefore contains dehydroalanine in place of the active site serine. The y22 ion at 2302.2 amu retains the organophosphorus agent. Similarly, the b24 ion at 2413.2 and the y25 ion at 2635.3 retain the organophosphorus agent. Ions that retain the organophosphorus agent provide additional proof of the mass of the modifying agent.
Figure 4D shows the MS/MS spectrum for the singly charged parent ion of mass 3036.5 where the active site serine is labeled with thiomethyl methylphosphonate. This unusual modification was produced by reaction of butyrylcholinesterase with thiomethyl sarin and thiomethyl soman model compounds. To create this adduct, thiomethyl sarin released the isopropyl group or thiomethyl soman released the pinacolyl group, leaving the thiomethyl group on phosphorus. The thiomethyl derivative is not observed with authentic nerve agents. The y22 ion at 2303.1, the y24 ion at 2489.1 and the y23 ion at 2432.2 amu retain the thiomethyl methylphosphonate on serine. The majority of the organophosphorus agent has been released from serine to yield the dehydroalanine ions Δy29 at 2910.9 and Δy22 at 2177.0. The thiomethyl sarin and soman model compounds also produced the alternative adducts shown in Figures 4E and 4H.
Figure 4E shows the MS/MS spectrum for the singly charged parent ion of mass 3048.5 where the active site serine is labeled with O-isopropyl methylphosphonate by thiomethyl sarin and thiocholine sarin model compounds, compounds 5, 6, 11 and 12. In this spectrum, most of the label has been released to produce the Δy29 dehydroalanine ion. However, the y22 ion at 2315.5 and the y24 ion at 2501.2 indicate that a portion of the peptides retain the O-isopropyl methylphosphonate on the active site serine.
Figure 4F shows the MS/MS spectrum for the singly charged parent ion of mass 3063.5 where the active site serine is labeled with O-ethyl, N-dimethylphosphonoamidate by tabun model compounds, compounds 1 and 2. The y22 ion at 2330.2 retains the phosphoamidate on the active site serine. The y25 ion at 2635.3 has lost O-ethyl but retains dimethylaminephosphonate.
Figure 4G shows the MS/MS spectrum for the singly charged parent ion of mass 3088.5 where the active site serine is labeled with O-cyclohexyl, methylphosphonate by thiocholine GF and thiomethyl GF model compounds, compounds 3, 4, 9, and 10. The y22 ion at 2355.1 and the b24 ion at 2466.2 retain phosphonate on the active site serine.
Figure 4H shows the MS/MS spectrum for the singly charged parent ion of mass 3090.5 where the active site serine is labeled with O-pinacolyl methyl phosphonate by thiocholine soman and thiomethyl soman model compounds, compounds 7, 8, 13, and 14. This spectrum was taken from a sample labeled with compound 8 (the Rp thiocholine analog of soman). The pinacolyl group is still present on this parent ion because the Rp analog did not undergo aging. The y22 ion at 2357.1 and the y23 ion at 2487.5 retain the phosphonate on the active site serine and thus support the parent ion mass in providing proof that the modifying agent was the soman analog.
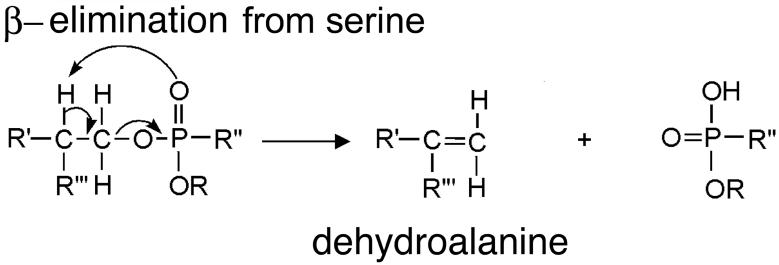
Beta-elimination to release organophosphorus agent
The Δy29 ion in Figures 4B-4H has 29 amino acids, but no active site serine and no organophosphorus agent. The masses are consisted with the interpretation that serine was converted to dehydroalanine during a beta-elimination reaction that released the organophosphorus agent. Sequence analysis of the modified active site peptide indicates that the conversion to dehydroalanine occurs only on the serine that had been modified with organophosphorus agent. None of the other 6 serines in this peptide was converted to dehydroalanine. The likely mechanism to explain the loss of organophosphorus agent plus loss of a molecule of water is illustrated in Figure 5. The mass spectrometer promotes beta-elimination by excitation of peptide ions during MS/MS acquisition. An analogous beta-elimination occurs in the test tube when the sample pH is raised to pH 11 or higher.
Figure 5.
Beta-elimination converts serine to dehydroalanine and releases the organophosphorus agent from serine.
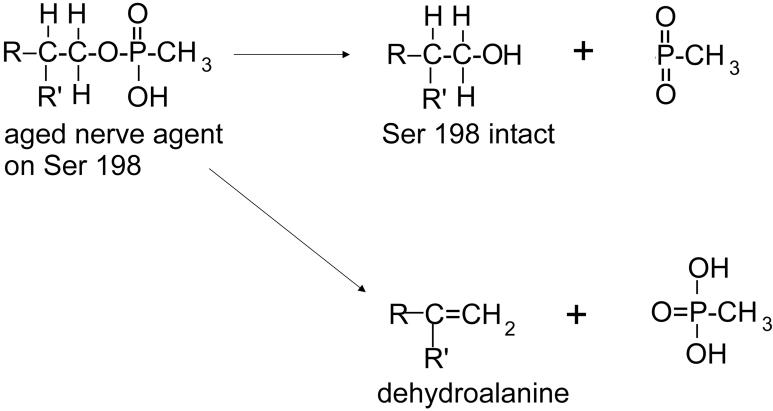
Two mechanisms to release organophosphorus agent in the mass spectrometer
Figure 4B shows one example of the MSMS spectrum of the aged adduct for GF, sarin, and soman. We have a total of 12 MSMS spectra for parent ion 3006.5 for compounds 3-14. Comparison of the 12 MSMS spectra shows a pattern of fragment ions suggesting two mechanisms of fragmentation for aged adducts. All MSMS spectra of parent ion 3006.5 have peaks at 2928.5 and 2910.5 amu, which are of comparable high intensity. The y29 ion at 2928.5 is the active site peptide from which the nerve agent has been released while leaving the serine structure intact. The Δy29 ion at 2910.5 is the dehydroalanine form of the active site peptide produced by beta-elimination. Another consistent feature is that all spectra have a y22 ion at 2195.2 in which the active site serine is intact but has lost the organophosphorus agent. The y22 ion at 2195.2 is always accompanied by a Δy22 ion at 2177.2, representing loss of water from the active site serine, though the intensity of this peak is always less than that of the 2195.2 peak. Figure 6 illustrates the two mechanisms of fragmentation for the aged butyrylcholinesterase adduct.
Figure 6.
Aged adducts fragment via two mechanisms. In one mechanism the aged phosphonate group is released from serine while leaving the serine structure intact. The competing pathway releases the organophosphonate and a molecule of water to yield dehydroalanine in place of serine.
The non aged adducts all fragment to give an intense Δy29 ion at 2910.5 indicative of formation of dehydroalanine in the process of losing the phosphonate (Figures 4C-4H). In some cases a weak Δy22 ion at 2177.2 is also observed (Figures 4C-4E), which supports formation of dehydroalanine. However, only weak y29 ions at 2928.5 amu are seen for the non aged adducts (Figures 4G, 4H). The 2928.5 ion is never as intense as the Δy29 ion indicating that the beta elimination mechanism is the dominant mechanism for release of non aged organophosphonate from serine. Consistent with the low intensity of the 2928.5 ion, a y22 ion at 2195.2 amu was never detected. It is concluded that the non aged adduct preferentially fragments by the beta-elimination mechanism, whereas the aged adduct fragments by two mechanisms. Dephosphonylation to yield serine rather than dehydroalanine is probably promoted by the hydroxyl group on the phosphorus atom in the aged adduct.
Loss of phosphate from phosphoserine-containing peptides also occurs via two routes: one involving loss of 98 amu (formation of dehydroalanine) and the other involving loss of 80 amu. Unlike the case for organophosphorus agents, the pathway leading to dehydroalanine is always much more active than the other. The greater intensity for the 98 amu pathway has, in fact, become diagnostic for the presence of phosphoserine (17).
Discussion
Adducts produced by authentic nerve agents
Fidder et al treated purified human butyrylcholinesterase with authentic soman-[methyl-14C] and reported the fragmentation spectrum of the tryptic peptide in Figure 1 of their paper (18). The parent ion was the aged soman adduct (3008.3 amu for the 14C methylphosphonate derivative). Their mass spectrum acquired on a Q-TOF Micromass instrument is consistent with our mass spectrum acquired on a MALDI-TOF-TOF mass spectrometer. Both mass spectrometers show a major peak at 2910 amu. This mass is consistent with the dehydroalanine form of the peptide, representing loss of the methylphosphonate and a molecule of water from the parent ion. Precedent for facile loss of phosphorus compounds from serine in the MALDI mass spectrometer comes from studies on phosphoserine containing peptides which readily lose 98 Da (phosphate plus water) (17, 19). In the case of nerve agent-labeled butyrylcholinesterase, a few y-ions retain methylphosphonic acid on the active site serine, yielding a set of parallel y-ions, some with the organophosphorus agent, and some in the dehydroalanine form. Both types of mass spectrometers yield these parallel sets of ions for the 29-residue tryptic peptide.
Fidder et al., used pepsin to digest sarin-treated butyrylcholinesterase and found a non-aged parent ion mass consistent with addition of sarin and loss of fluorine (18). Their MS/MS spectrum for the non-aged parent ion shows that beta-elimination occurs with the non-aged sarin adduct. A parallel set of ions retaining the methylphosphonate label was not seen with the 9-residue peptic peptide; the collision energy for the short peptide converted all of the organophosphorus labeled serine to dehydroalanine.
Tsuge et al., studied chymotryptic peptides of human butyrylcholinesterase treated with soman, sarin, and VX (20). The b-ions in their MS/MS spectra lost the organophosphorus agent and a molecule of water yielding identical sets of b-ions for non-aged sarin, non-aged VX, non-aged soman, and aged soman adducts. The nerve agent was identified from the mass of the parent ion, which is unique for each nerve agent. In conclusion, the adducts and MS/MS spectra we produced with nerve agent model compounds yielded results identical to those produced with authentic nerve agents.
A general principle for fragmentation of organophosphorus labeled serine adducts can be derived from these studies. The energy of fragmentation can produce a population of ions that has lost the organophosphorus agent and a molecule of water, yielding dehydroalanine in place of the active site serine. Aged as well as non-aged organophosphorus-labeled serine adducts degrade to dehydroalanine in the mass spectrometer. However, not all of the labeled serine will lose the label during fragmentation. This behavior is the same as the well established behavior of phosphoserine containing peptides (17, 19, 21).
Stereoselectivity
Rp and Sp nerve agent model compounds gave similar butyrylcholinesterase adducts. Because of the long incubation times between butyrylcholinesterase and the nerve agent model compounds, we did not expressly study the stereoselectivity of adduct formation. However, our data suggest that phosphonylation of butyrylcholinesterase is stereoselective, in agreement with the literature for related nerve agents (3-6). Stereo-selectivity is most clearly illustrated in the case of the slowly phosphonylating thiomethyl agents (i.e., 11-14). After 3 hours of reaction 80% of the butyrylcholinesterase was labeled by the Sp isomers, while only 40% was labeled by the Rp. The percentage of inhibition showed a clear preference for Sp > Rp. Presumably, evaluation of incubations run for a much shorter time would have shown stereoselective adduct formation for the faster reacting compounds. However, the object of the present study was to quantify the extent of protein adduction and thus, exhaustive covalent labeling was done. Kinetic studies are the best way to show stereoselectivity of inhibition and those studies have been done in an accompanying report (12).
Rp stereoisomers do not undergo aging
Crystal structure studies of organophosphorus adducts of butyrylcholinesterase and acetylcholinesterase have resulted in an understanding of the mechanism of aging (15, 22, 23). The mechanism requires the alkyl group that is to undergo aging to be positioned close to His 438 and Glu 199 in the choline pocket (22). These residues catalyze the dealkylation reaction called “aging”. Placement of the Sp and Rp isomers into the active site of butyrylcholinesterase shows that the alkyl group is correctly positioned for dealkylation in the Sp isomer, but not in the Rp isomer. In the Rp isomer the alkyl group projects toward the acyl-binding pocket, a location not accessible for interaction with His 438 and Glu 199. The Rp stereoisomer is therefore not capable of undergoing dealkylation. The crystal structure results are in conflict with the results in Table 1 where substantial aging is observed for all Rp isomers except sarin Rp 6. The following discussion rationalizes the mass spectrometry results.
The compounds can be divided into four functionally distinct groups.
1. The tabun adducts (1 and 2) constitute a special case because deamination might occur when the dimethylamino group projects toward the choline pocket. Thus aging could occur by removal of either dimethylamine (28 amu minus a proton to give a 27 amu loss) or ethyl (29 amu minus a proton to give a 28 amu loss). If deamination is taken as a valid aging pathway, then either the Rp or Sp isomer could age.
2. The Sp form of the thiocholine analog of sarin (5) showed 26% aging, while the Rp form (6) showed only 1% aging (well within experimental error of zero). Therefore, this enantiomeric pair behaved as predicted from the geometry of the active site of BChE. Namely, the Sp form aged while the Rp form did not.
3. Both the Sp and Rp forms of the thiomethyl analog of sarin (11 and 12) yielded two types of initial covalent adduct: one from the release of the thiomethyl moiety (3048.5 amu) and another from the release of the isopropoxy (3036.5 amu). For the Sp form, initial release of the thiomethyl would leave the isopropoxy moiety in the choline-binding site, suitably oriented to support aging. For the Rp form, initial release of the isopropoxy would leave the thiomethyl in the choline-binding site, suitably oriented to support aging. Therefore, aging of both the Sp and Rp forms can be rationalized. The behavior of the thiomethyl model compounds of soman (13 and 14) can be rationalized in the same manner.
4. That leaves only three of the seven enantiomeric pairs for which aging of the Rp form is unexplained. These are the thiocholine analog of cyclosarin (3 and 4), the thiocholine analog of soman (7 and 8), and the thiomethyl analog of cyclosarin (9 and 10). In all three cases, the thioalkyl moiety was released from both enantiomers in the initial covalent reaction. No masses were found that would be consistent with release of the alkoxy moiety in the initial covalent reaction. Therefore, the argument that can be made for enantiomeric pairs 11/12 and 13/14 is not applicable for enantiomeric pairs 3/4, 7/8 and 9/10. In these cases, the most logical explanation for aged masses is contamination of the Rp preparation by the Sp form. The contamination by the Sp isomer would need to be no more than 1%. This small amount of contamination could lead to high amounts of aged product because the Sp isomers reacts with butyrylcholinesterase much faster than the Rp isomers.
In conclusion, adducts of human butyrylcholinesterase with the Rp stereoisomer of cyclosarin, sarin, and soman do not age.
Rp stereoisomer useful for identifying the poison
The conclusion that adducts with the Rp stereoisomer do not age has application to mass spectral diagnosis of exposure to nerve agents. Nerve agents used in war are a mixture of stereoisomers. The Sp stereoisomer will yield an aged adduct that is identical for soman, sarin, and GF and is not distinguishable by mass spectrometry. In contrast, the Rp stereoisomer will produce a butyrylcholinesterase adduct that will not age. The non aged butyrylcholinesterase adduct will retain the pinacolyl group of soman, the isopropyl group of sarin or the cyclohexyl group of GF. The non aged masses allow identification of the poison to which a person was exposed. However, only a small percentage of the butyrylcholinesterase will have been inhibited by the Rp form. Because of the difference in rates of reaction, exposure to a nerve agent mixture of stereoisomers will result in preferential inhibition by the Sp form.
MALDI-TOF is useful for aging studies
A MALDI-TOF mass spectrum of a nerve agent labeled butyrylcholinesterase tryptic digest shows the unlabeled active site peptide as well as the nerve agent labeled peptide and the aged peptide, all in one spectrum. This allows quantitation of the relative cluster areas and therefore estimation of the amount of each type of adduct present. This approach has been successfully applied to aging studies on both butyrylcholinesterase and acetylcholinesterase, labeled with a variety of organophosphorus agents (24, 25). Previously, aging could be estimated only from enzyme activity assays where the proportion of enzyme that could not be reactivated with oximes was defined as aged enzyme (26, 27).
Conclusion
The thiocholine nerve agent model compounds react with human butyrylcholinesterase to yield adducts indistinguishable from those produced by authentic nerve agents.
The MALDI-TOF spectra of tryptic digests of nerve agent-labeled human butyrylcholinesterase show the presence of unmodified peptides, adducted peptides, and aged peptides. Two of the three thiomethyl compounds yielded unanticipated adducts. None of the thiocholine compounds yielded evidence of new or unanticipated adducts. Accordingly, the thiocholine-containing nerve agent model compounds appear to give the same adducts as authentic nerve agents. Therefore, they are suitable substitutes for nerve agents in work that aims to study biological effects of nerve-agent modified proteins.
Acknowledgement
Supported by NIH grant U01 NS058038-03 (to JC), U01 NS058056-03 (to OL), U54NS058183 (to JZ), NIH Cancer Center Support grant P30CA036727, US Army Medical Research & Materiel Command W81XWH-07-2-0034 (to OL), US Army Medical Research Institute of Chemical Defense V91ZLK-06-R-0029 (to JZ), French Procurement Agency DGA/PEA 08co501 (to FN) and Agence Nationale pour la Recherche ANR-06-BLAN-0163 (to FN).
References
- (1).Munro N. Toxicity of the Organophosphate Chemical Warfare Agents GA, GB, and VX: Implications for Public Protection. Environ Health Perspect. 1994;102:18–37. doi: 10.1289/ehp.9410218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ohbu S, Yamashina A, Takasu N, Yamaguchi T, Murai T, Nakano K, Matsui Y, Mikami R, Sakurai K, Hinohara S. Sarin poisoning on Tokyo subway. South Med J. 1997;90:587–593. doi: 10.1097/00007611-199706000-00002. [DOI] [PubMed] [Google Scholar]
- (3).Saxena A, Viragh C, Frazier DS, Kovach IM, Maxwell DM, Lockridge O, Doctor BP. The pH dependence of dealkylation in soman-inhibited cholinesterases and their mutants: further evidence for a push-pull mechanism. Biochemistry. 1998;37:15086–15096. doi: 10.1021/bi980917z. [DOI] [PubMed] [Google Scholar]
- (4).Hosea NA, Berman HA, Taylor P. Specificity and orientation of trigonal carboxyl esters and tetrahedral alkylphosphonyl esters in cholinesterases. Biochemistry. 1995;34:11528–11536. doi: 10.1021/bi00036a028. [DOI] [PubMed] [Google Scholar]
- (5).Millard CB, Lockridge O, Broomfield CA. Organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase: synergy results in a somanase. Biochemistry. 1998;37:237–247. doi: 10.1021/bi972057c. [DOI] [PubMed] [Google Scholar]
- (6).De Bisschop HC, Michiels KW, Vlaminck LB, Vansteenkiste SO, Schacht EH. Phosphonylation of purified human, canine and porcine cholinesterase by soman. Stereoselective aspects. Biochem Pharmacol. 1991;41:955–959. doi: 10.1016/0006-2952(91)90201-f. [DOI] [PubMed] [Google Scholar]
- (7).Briseno-Roa L, Hill J, Notman S, Sellers D, Smith AP, Timperley CM, Wetherell J, Williams NH, Williams GR, Fersht AR, Griffiths AD. Analogues with fluorescent leaving groups for screening and selection of enzymes that efficiently hydrolyze organophosphorus nerve agents. J Med Chem. 2006;49:246–255. doi: 10.1021/jm050518j. [DOI] [PubMed] [Google Scholar]
- (8).Li WS, Lum KT, Chen-Goodspeed M, Sogorb MA, Raushel FM. Stereoselective detoxification of chiral sarin and soman analogues by phosphotriesterase. Bioorg Med Chem. 2001;9:2083–2091. doi: 10.1016/s0968-0896(01)00113-4. [DOI] [PubMed] [Google Scholar]
- (9).Amitai G, Adani R, Yacov G, Yishay S, Teitlboim S, Tveria L, Limanovich O, Kushnir M, Meshulam H. Asymmetric fluorogenic organophosphates for the development of active organophosphate hydrolases with reversed stereoselectivity. Toxicology. 2007;233:187–198. doi: 10.1016/j.tox.2006.09.020. [DOI] [PubMed] [Google Scholar]
- (10).Grimsley JK, Calamini B, Wild JR, Mesecar AD. Structural and mutational studies of organophosphorus hydrolase reveal a cryptic and functional allosteric-binding site. Arch Biochem Biophys. 2005;442:169–179. doi: 10.1016/j.abb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- (11).Lenz DE, Brimfield AA, Hunter KW, Jr., Benschop HP, de Jong LP, van Dijk C, Clow TR. Studies using a monoclonal antibody against soman. Fundam Appl Toxicol. 1984;4:S156–164. doi: 10.1016/0272-0590(84)90148-9. [DOI] [PubMed] [Google Scholar]
- (12).Barakat N, Zheng X, MacDonald M, Gilley C, Okolotowicz K, Cashman J, Zhang J. Chemical synthesis of two series of methylphosphonothioate nerve agent analogs and their stereoselective interaction with human butyrylcholinesterase and human acetylcholinesterase. Chem Res Toxicol. 2009 doi: 10.1021/tx900096j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lockridge O, Schopfer LM, Winger G, Woods JH. Large scale purification of butyrylcholinesterase from human plasma suitable for injection into monkeys; a potential new therapeutic for protection against cocaine and nerve agent toxicity. J Med CBR Def. 2005;3 doi: 10.1901/jaba.2005.3-nihms5095. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lockridge O, Xue W, Gaydess A, Grigoryan H, Ding SJ, Schopfer LM, Hinrichs SH, Masson P. Pseudo-esterase activity of human albumin: slow turnover on tyrosine 411 and stable acetylation of 82 residues including 59 lysines. J Biol Chem. 2008;283:22582–22590. doi: 10.1074/jbc.M802555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Carletti E, Li H, Li B, Ekstrom F, Nicolet Y, Loiodice M, Gillon E, Froment MT, Lockridge O, Schopfer LM, Masson P, Nachon F. Aging of Cholinesterases Phosphylated by Tabun Proceeds through O-Dealkylation. J Am Chem Soc. 2008 doi: 10.1021/ja804941z. [DOI] [PubMed] [Google Scholar]
- (16).Breci LA, Tabb DL, Yates JR, 3rd, Wysocki VH. Cleavage N-terminal to proline: analysis of a database of peptide tandem mass spectra. Anal Chem. 2003;75:1963–1971. doi: 10.1021/ac026359i. [DOI] [PubMed] [Google Scholar]
- (17).Annan RS, Carr SA. Phosphopeptide analysis by matrix-assisted laser desorption time-of-flight mass spectrometry. Anal Chem. 1996;68:3413–3421. doi: 10.1021/ac960221g. [DOI] [PubMed] [Google Scholar]
- (18).Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- (19).Qin J, Chait BT. Identification and characterization of posttranslational modifications of proteins by MALDI ion trap mass spectrometry. Anal Chem. 1997;69:4002–4009. doi: 10.1021/ac970489n. [DOI] [PubMed] [Google Scholar]
- (20).Tsuge K, Seto Y. Detection of human butyrylcholinesterase-nerve gas adducts by liquid chromatography-mass spectrometric analysis after in gel chymotryptic digestion. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:21–30. doi: 10.1016/j.jchromb.2006.02.054. [DOI] [PubMed] [Google Scholar]
- (21).Tholey A, Reed J, Lehmann WD. Electrospray tandem mass spectrometric studies of phosphopeptides and phosphopeptide analogues. J Mass Spectrom. 1999;34:117–123. doi: 10.1002/(SICI)1096-9888(199902)34:2<117::AID-JMS769>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- (22).Nachon F, Asojo OA, Borgstahl GE, Masson P, Lockridge O. Role of water in aging of human butyrylcholinesterase inhibited by echothiophate: the crystal structure suggests two alternative mechanisms of aging. Biochemistry. 2005;44:1154–1162. doi: 10.1021/bi048238d. [DOI] [PubMed] [Google Scholar]
- (23).Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999;38:7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- (24).Li H, Schopfer LM, Nachon F, Froment MT, Masson P, Lockridge O. Aging pathways for organophosphate-inhibited human butyrylcholinesterase, including novel pathways for isomalathion, resolved by mass spectrometry. Toxicol Sci. 2007;100:136–145. doi: 10.1093/toxsci/kfm215. [DOI] [PubMed] [Google Scholar]
- (25).Jennings LL, Malecki M, Komives EA, Taylor P. Direct analysis of the kinetic profiles of organophosphate-acetylcholinesterase adducts by MALDI-TOF mass spectrometry. Biochemistry. 2003;42:11083–11091. doi: 10.1021/bi034756x. [DOI] [PubMed] [Google Scholar]
- (26).Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch Toxicol. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- (27).Masson P, Froment MT, Bartels CF, Lockridge O. Importance of aspartate-70 in organophosphate inhibition, oxime re-activation and aging of human butyrylcholinesterase. Biochem J. 1997;325(Pt 1):53–61. doi: 10.1042/bj3250053. [DOI] [PMC free article] [PubMed] [Google Scholar]