Abstract
MUNE, a technique used in ALS clinical trials to quantitatively assess motor neuron loss, should also be valuable in assessing progression in spinal bulbar muscular atrophy (SBMA), an x-linked neuronopathy. In ALS, instability of single motor units (SMUP) prompted Shefner9 to modify the statistical MUNE method to exclude SMUPs ≤ 40μV. It is unknown if there is a similar SMUP instability in the more chronic degenerative disease of SBMA.
In this study, the standard parameter of excluding SMUP < 10 μV was compared with the exclusion of SMUP < 40 μV in the calculation of the statistical MUNE. The mean statistical MUNE, using the standard method and the Shefner method, was 60±21 to 47±23, respectively. Similar to ALS, SBMA showed an increased proportion (17%) of individual SMUPs ≤ 40 μV compared to normal controls.
In conclusion, excluding SMUPs ≤ 40 μV from the statistical MUNE calculations is appropriate for SBMA subjects because their SMUPs characteristics are similar to ALS. Exclusion of the low amplitude SMUPs reduces the calculated MUNE.
Keywords: Spinal bulbar muscular atrophy, motor unit number estimation, motor neuron disease
Introduction
Spinal and bulbar muscular atrophy (SBMA) or Kennedy’s Disease5 is an x-linked disorder associated with degeneration of the anterior horn motor neurons and dorsal root ganglion cells. The clinical features of motor neuron degeneration resemble amyotrophic lateral sclerosis (ALS) with morbidity due to weakness, but SBMA has greater chronicity with a fairly normal life span2. Motor unit number estimation (MUNE) has been found to be useful in monitoring motor neuron degeneration in clinical trials in ALS and may be more sensitive in detecting change than motor strength3. Likewise, MUNE may be useful in clinical trials in SBMA for detecting subtle responses to treatment.
Among the different techniques for obtaining MUNE, the statistical MUNE has been one of the suggested methods for clinical trials because of its ease in performance and reproducibility1. However, several limitations of the statistical MUNE method were noted in clinical trials of ALS. The statistical MUNE technique assumes a Poisson model of distribution in which the mean equals the variance and there is a low activation probability for calculating the single motor unit potential (SMUP) amplitudes and thus the MUNE8. Studies in ALS have noted that the calculated amplitude of SMUPs includes many small SMUPs, a finding at odds with the expectation for reinnervated motor units9. The variability in SMUP size in unstable reinnervated motor units, has been suggested to cause an erroneous small mean SMUP amplitude leading to a higher than expected MUNE. Because of this observation, it was proposed by Shefner to exclude SMUPs <40 μV in the statistical MUNE evaluation of ALS clinical trials9. Secondly, there are large gaps in the stimulus intensity scan obtained with the statistical MUNE in subjects with ALS that are not routinely observed in normal subjects. These are likely to represent large reinnervated motor units. The combination of small measured SMUP amplitudes motor units caused by motor unit instability and large reinnervated units are incompatible with the assumptions used in calculating the MUNE by the statistical method. Shefner and colleagues have suggested modifying the statistical MUNE to exclude SMUPs <40 μV and counting the very high amplitude SMUP separately would improve the reproducibility of MUNE values in clinical trials for subjects with ALS10. Therefore, we sought to evaluate the validity of applying Shefner’s modifications, particularly the exclusion of SMUPs <40 μV, on the statistical MUNE calculated for SBMA subjects. These calculations were carried out on baseline data obtained from a double-blind placebo-controlled treatment protocol using dutasteride, an anti-testosterone agent, in SBMA subjects.
Materials and Methods
Subjects
Fifty-four men with genetically confirmed SBMA gave consent for the protocol, which was approved by the IRB. As part of the baseline evaluations prior to an interventional trial (NCT 00303446), four sensory nerve conduction studies - median, ulnar, radial, and sural, and two motor nerve conduction studies – peroneal and median, were performed using standard techniques7. MUNE was evaluated in one hand muscle, generally the right abductor pollicis brevis (APB) muscle, unless the muscle was too atrophic and had a very low CMAP (< 1.5 mV). Alternative muscles included the left APB muscle or the abductor digiti minimi (ADM) muscle. Fourteen age-matched healthy male control subjects underwent MUNE testing in a separate IRB approved protocol. MUNEs on bilateral APB muscles were evaluated. Control subjects who were found to have evidence of median nerve entrapment in the carpal tunnel (distal latency >4.5 ms) or abnormal median CMAPS (amplitude <4.5 mV) were excluded.
MUNE Sampling
The MUNE was performed using the statistical MUNE program on the Nicolet Viking Select EMG machine. A stimulus-intensity scan was first performed to measure the range of CMAP values from threshold to maximal stimulus level. Optimally, four windows of the CMAP range (runs) were assessed with the aim of sampling about 40% of the total CMAP. The targets for the four runs were: R1: 10–20%, R2: 25–35%, R3: 40–50%, R4: 55–65%. In some SBMA subjects, these ranges needed to be altered because of the presence of a “true” gap, which signified the presence of a large SMUP. A true gap was a gap that included >10% of the stimulus intensity scan and confirmed by the absence of SMUPs on more selective sampling within the gap window9.
MUNE Calculations
The mean SMUP amplitudes obtained from the statistical MUNE program were calculated using a number-weighted equation6,1. The large SMUPs or gaps were subtracted from the original motor response (CMAPT) in the following equation to obtain an adjusted CMAP (CMAPadj): CMAPadj = CMAPT – Gap1 – Gap 2 – Gap 3, etc. The CMAPadj was then used for calculating the MUNE from the mean SMUP amplitude, which was then added to the number of individual high amplitude SMUPs or gaps to generate the final MUNE value: MUNE = (CMAPadj/SMUPmean) + number of gaps. The second MUNE calculation used the above MUNE calculation except that runs with SMUPs ≤ 40 μV were omitted from the calculation of the mean SMUP amplitude.
Results
Baseline Characteristics
The mean age of the fifty-four SBMA subjects was 53.0±10.0 (range 37–79). The mean age of the fourteen normal controls was 54.8±8.2 (range 43–69). The SBMA subjects had a mean CAG repeat length was 47 and a mean CPK 1089 (118–3181). Two SBMA subjects had a MUNE evaluated in the ADM muscle and were excluded from the MUNE evaluations in this paper. In SBMA subjects, the mean CMAP amplitude for the median (6.3±3.1 mV) and peroneal motor (2.7±2.0 mV) responses were normal though 28% of the median CMAPs and 52% of the peroneal CMAPs were below laboratory norms. There were low amplitude sensory responses in 98% of the studies with mean amplitudes of 5±4μV, 4±3μV, 5±3μV, and 2±2μV in the median, ulnar, radial, and sural sensory nerves, respectively. In most of the fourteen age-matched controls, right and left APB muscles were sampled for a total of 24 muscles evaluated by MUNE. The mean CMAP amplitude of the APB was 9.7±2.8 mV in the controls.
Distribution of Individual SMUP Amplitudes
SMUP amplitudes were calculated from each of approximately four runs. Each gap in the stimulation intensity curves was counted as a single high amplitude SMUP. In SBMA subjects, the mean of the individual SMUP amplitudes (figure 1a) was 378±504 μV (range 10–2580μV). SMUP ≤ 40μV accounted for 17% of all SMUPs. The number of SMUP ≤ 40μV runs ranged from zero to four with a significantly higher number of SMUP ≤ 40μV runs (p=0.0001) in SBMA subjects with low CMAP amplitudes (<4.5 mV) (data not shown).
Figure 1. Distribution of Individual SMUP Amplitudes.
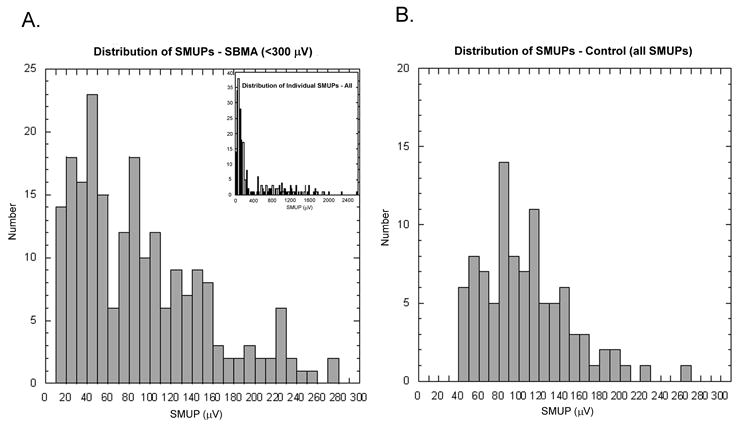
SMUPs were collected from four runs at different percentages of stimulation. In addition, high amplitude SMUPs, as determined by gaps in the distribution curve, were included. The individual SMUP amplitudes determine the mean SMUP amplitude used in the MUNE calculations.
A. Distribution of SMUP amplitudes in SBMA subjects. The large graph shows the individual SMUPs obtained directly from the runs and under 300μV. The smaller graph (inset) includes both SMUPs obtained from the individual runs and calculated from the gaps. The SBMA subjects have a mean individual SMUP amplitude of 378±504 μV (range 10–2580μV).
B. Distribution of SMUP in Age-matched Controls. The graph shows all the individual SMUPs obtained from the age-matched controls. The mean individual SMUP amplitude was 106 ± 44 μV (range of 41 to 269 μV)
The high amplitude SMUPs, as determined by presence of gaps, accounted for 31% of the individual SMUPs with a mean amplitude of 1012±475 μV (range 229 –2580μV) (figure 1a, inset). For the age-matched controls (figure 1b), the mean individual SMUP amplitude was 106 ± 44 μV (range of 41 to 269 μV). The controls did not have any SMUPs ≤ 40μV or gaps indicative of high amplitude SMUPs.
Distribution of mean SMUPs for MUNE calculations
For MUNE calculations, a mean SMUP amplitude was determined for each subject from SMUP amplitudes calculated from individual runs (figure 2a), but excluding those high amplitude SMUPs determined from the gaps. In the SBMA subjects, two mean SMUP amplitude values were calculated; first, using all the SMUPs, and the secondly excluding SMUPs ≤ 40 μV. In SBMA subjects, the resultant mean SMUP amplitude was 78±40μV and 130±156 μV, respectively. Expectedly, there was a significantly lower mean SMUP amplitude when all SMUPs were included because of the large number of less than 40μV SMUPs. The mean SMUP amplitude for the age-matched controls was 101±28μV, with no amplitudes less than 40 μV. The mean SMUP amplitude was significantly smaller in the SBMA subjects compared to the controls when all SMUPs were included (p<0.0001) but not when the low amplitude SMUPs (≤40μV) were excluded.
Figure 2. Distribution of Mean SMUP Amplitudes and MUNE.
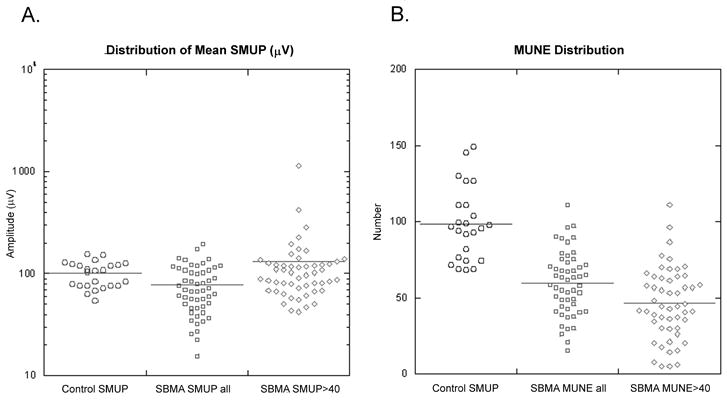
A. Distribution of mean SMUP amplitude in SBMA and Age-matched controls. The mean SMUP amplitudes were calculated using a number-weighted equation and excluded the very large SMUPs obtained from analyzing the gaps in the SBMA subjects. The control subjects (○) have a mean SMUP amplitude of 101±28 μV. The SBMA subjects had a mean SMUP amplitude of 78±40μV when all SMUP were included (□) and 130±156 μV when SMUPs ≤ 40 μV were excluded (◇).
B. Distribution of MUNE in SBMA and Age-matched Controls. The control subjects (◦) have a mean MUNE of 98±24 (range 68 – 149), compared to SBMA subjects with a mean MUNE of 60±21 (range 15–111) when all SMUPs were included (□) and 47±23 (range 5–111), when SMUPs ≤ 40 μV were excluded (◇).
Distribution of MUNE in SBMA and Normal Subjects
The MUNE was calculated from the mean SMUP amplitude and CMAPT, adding in the number of high amplitude SMUPs as determined from the gaps (figure 2b). SBMA subjects had a mean MUNE of 60±21 (range 15–111) when all SMUPs were included and 47±23 (range 5–111), when SMUPs ≤ 40 μV were excluded. The concordance between the two methods employed in the SBMA calculation was 0.52 (two-sided 95%CL for rc = 0.35). The MUNE was significantly different between the two groups (p<0.0001). The age-matched controls had a mean MUNE of 98±24 (range 68 –149). Compared to the controls, SBMA subjects only had 37%, when including all SMUPs, or 17%, when excluding SMUPs ≤ 40 μV, of MUNEs within the range of the control MUNE values.
Relationship of MUNE and mean SMUP with CMAP
We examined the relationship between the MUNE with the CMAP amplitudes (figure 3). Overall, larger CMAP amplitudes were associated with larger MUNE values. This was more evident in the normal subjects and SBMA subjects when SMUPs ≤ 40 μV were excluded (figure 3) but not when all SMUPs were included. The MUNE calculation in thirty-eight SBMA subjects with normal CMAP amplitudes had a mean MUNE of 63±20 (all SMUPs included) or 54±21 (SMUP ≤40 μV excluded). With either method of calculation, SBMA subjects had a significantly lower MUNE than the normal subjects (p<0.0001). It was rare for a SBMA subject to have a low CMAP with a MUNE in the range of age-matched controls. Only two SBMA subjects fulfilled these criteria if all SMUPs were included in the MUNE calculation. There was no significant difference in MUNE in SBMA subjects with normal CMAP amplitudes compared to all SBMA subjects.
Figure 3. Relationship of CMAP amplitude and MUNE.
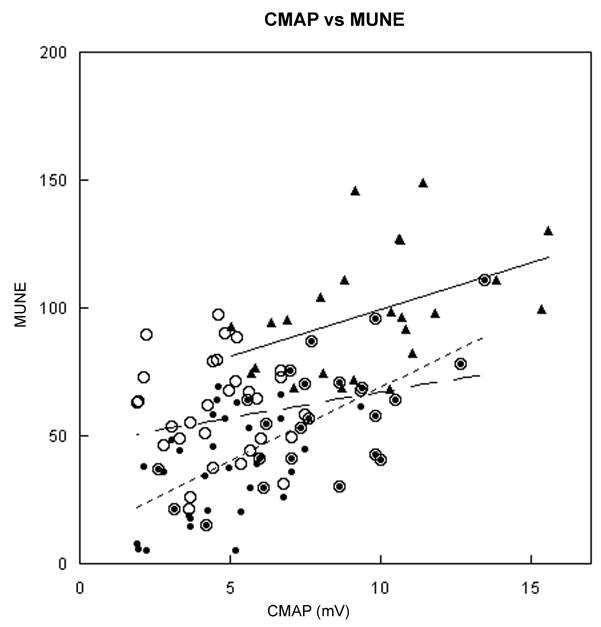
The CMAP amplitudes were compared to age-matched controls and the two SBMA calculations for MUNE. The control subjects and SBMA subjects trend towards higher MUNE values with higher CMAP amplitudes. The slope for control subjects was r2=0.19 and the SBMA subjects were r2 = 0.07 and r2 = 0.44, respectively, for all SMUP evaluated or SMUPs ≤ 40 μV excluded. Controls MUNE – (▲); SBMA MUNE (all SMUPs) – (○); SBMA MUNE (SMUPs ≤ 40 μV excluded) – (●).
We also evaluated the relationship between the number of gaps or very large amplitude SMUPs compared to the MUNE. Subjects with a low MUNE (<50) had a mean of 2.3±1 gaps compared to 1.1±.9 gaps in subjects with higher MUNEs (>50), and there was no significant difference between mean SMUP amplitudes ((p=0.13) with 0.97±.44 mV for the low MUNEs and 1.16±.35 mV for the higher MUNEs. There was a significant difference in the percentage of the CMAP composed by these very large SMUPs (p < 0.0001) with 43% in the low MUNE group and 17% in the higher MUNE group.
Discussion
As expected, the SBMA subjects had a lower MUNE compared to the normal subjects. As in ALS, the statistical method calculated SMUP sizes smaller than 40 μV in many SBMA subjects. These apparently small SMUPs, which become more prevalent as the CMAP amplitude decreases, similarly suggest an alternation sampling error and are more likely related to greater variability in neuromuscular transmission as a result of newly reinnervated motor units rather than a distinct population of small motor units. Excluding these small SMUPs as proposed by Shefner and colleagues9 resulted in a significant decrease in the calculated MUNE. The mean SMUP amplitude as calculated by this modification was larger than the control mean SMUP amplitude and would support the suggestion that motor unit remodeling had occurred with reinnervation and resultant larger individual motor units. This correlates with the EMG findings of chronic neurogenic changes of high amplitude, long duration motor units without a population of small motor units that was noted in two of our SBMA subjects (data not shown) and in the literature10. Including all the SMUPs in the calculation of MUNEs in SBMA subjects resulted in a significantly lower mean SMUP amplitude than the age-matched control mean SMUP amplitude, contradictory to the concept of reinnervation. Further evidence in support of motor unit remodeling as a prominent part of the SBMA pathophysiology was the large percentage of SBMA subjects with normal CMAP amplitudes and low MUNEs, independent of the methodology for calculating the mean SMUP amplitude. As well, the percentage of the CMAP composed of very large SMUPs significantly increased as the MUNE dropped below 50 suggesting a marked increase in compensation through the remaining motor neurons after a loss of a critical amount of motor neurons.
There are limitations to applying Shefner’s modification to the analysis of the statistical MUNE similar to the limitations observed in the ALS study11. First, removal of the individual SMUPs ≤ 40 mV decreases the number of runs and percent of the CMAP sampled. As the number of motor units declines, the ability to carry-out the statistical method also declines, and in some respects, makes the MUNE calculation resemble the incremental method for MUNE calculation10 The second issue is the presence of gaps frequently interspersed with the small SMUPs affects the choice of sampling windows used to acquire the individual SMUPs. This may decrease the probability of a Poisson sampling distribution and affect the assumptions inherent in the statistical MUNE4. Despite these limitations, we feel that determining the MUNE by the Shefner method is acceptable for evaluating the motor neuronopathy in SBMA subjects. It accounts for shortcomings of greater SMUP instability that are not accounted for in the statistical MUNE method.
Compared to the multicenter study of Celecoxib in an ALS population11, our study found that the modifications to the statistical MUNE were consistent with the expected pathophysiology of chronic motor neuron disease of an increased SMUP amplitude and low MUNE. Follow-up evaluations of the MUNE in this population are necessary to determine if there is continued increase in the SMUP amplitude as the disease progresses or the amplitude plateaus as the limits of reinervation are attained. Furthermore our study suggests that the MUNE abnormalities precede the loss of CMAP amplitude and may be useful in following disease progression in its early stages. For the purposes of clinical trials, clinical correlation between strength and MUNE is also warranted to determine if MUNE is an appropriate surrogate marker for motor neuron degeneration in SBMA.
Acknowledgments
This research was supported by the Intramural Research Program of the NINDS, NIH. We are grateful for all the technical assistance provided by Barbara Lear.
Abbreviations
- ADM
adductor digiti minimi, ALS, amyotrophic lateral sclerosis, APB, abductor pollicis brevis
- CMAP
compound muscle action potential
- MUNE
motor unit number estimation
- SBMA
Spinal-bulbar muscular atrophy
- SMUP
single motor unit potential
- mV
millivolt
- μV
microvolt
References
- 1.Bromberg MB. Updating motor unit number estimation (MUNE) Clin Neurophysiol. 2007;118:1–8. doi: 10.1016/j.clinph.2006.07.304. [DOI] [PubMed] [Google Scholar]
- 2.Chahin N, Klein C, Mandrekar J, Sorenson E. Natural history of spinal-bulbar muscular atrophy. Neurology. 2008;70:1967–1971. doi: 10.1212/01.wnl.0000312510.49768.eb. [DOI] [PubMed] [Google Scholar]
- 3.Gooch CL, Shefner JM. ALS surrogate markers. MUNE. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5 (Suppl 1):104–107. doi: 10.1080/17434470410019889. [DOI] [PubMed] [Google Scholar]
- 4.Henderson RD, McClelland R, Daube JR. Effect of changing data collection parameters on statistical motor unit number estimates. Muscle Nerve. 2003;27:320–331. doi: 10.1002/mus.10325. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18:671–680. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- 6.Kwon O, Lee KW. Reproducibility of statistical motor unit number estimates in amyotrophic lateral sclerosis: comparisons between size- and number-weighted modifications. Muscle Nerve. 2004;29:211–217. doi: 10.1002/mus.10530. [DOI] [PubMed] [Google Scholar]
- 7.Liveson JA, Ma DM. Laboratory reference for Clinical Neurophysiology. New York City: Oxford University Press; 1992. [Google Scholar]
- 8.Lomen-Hoerth C, Slawnych MP. Statistical motor unit number estimation: from theory to practice. Muscle Nerve. 2003;28:263–272. doi: 10.1002/mus.10351. [DOI] [PubMed] [Google Scholar]
- 9.Shefner JM, Cudkowicz ME, Zhang H, Schoenfeld D, Jillapalli D. The use of statistical MUNE in a multicenter clinical trial. Muscle Nerve. 2004;30:463–469. doi: 10.1002/mus.20120. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante MA, Wilbourn AJ. The characteristic electrodiagnostic features of Kennedy’s disease. Muscle Nerve. 1997;20:323–329. doi: 10.1002/(SICI)1097-4598(199703)20:3<323::AID-MUS9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Shefner JM, Cudkowicz ME, Zhang H, Schoenfeld D, Jillapalli D. Revised statistical motor unit number estimation in the Celecoxib/ALS trial. Muscle Nerve. 2007;35:228–234. doi: 10.1002/mus.20671. [DOI] [PubMed] [Google Scholar]


