Abstract
Repeated-acquisition procedures that include performance controls for effects not specific to acquisition permit the assessment of drug effects on learning on a within-subject, within-session basis. Despite the advantages of this methodology, few studies have examined effects of psychomotor stimulants on repeated acquisition in rodents. The purpose of the present study was to investigate the effects of methylenedioxymethamphetamine (MDMA, 0.3-10 mg/kg), methamphetamine (MA, 0.1-3 mg/kg) and methylphenidate (MPD,1-17 mg/kg) using repeated-acquisition procedures with performance controls in rats using a touch-screen apparatus. Rats were presented a 2 × 3 array of stimuli using a computer touch-screen and nose-pokes to target locations within the array were reinforced. In the acquisition component, the correct location changed across sessions, whereas during the performance component, the correct location was constant across sessions. All three drugs reduced accuracy of responding to target locations in a dose-dependent fashion. None of the compounds enhanced learning at any dose. MPD and MA produced significant disruptions of acquisition accuracy only at doses that also disrupted performance, but the 3 mg/kg dose of MDMA impaired acquisition of target responding without affecting performance. The selective impairment of acquisition found in the present study adds to the evidence of learning and memory disruption produced by acute MDMA administration and raise questions about the mechanisms for these actions.
Keywords: Repeated acquisition, Learning, Methylenedioxymethamphetamine, Methamphetamine, Methylphenidate, Touchscreen, Rats
Introduction
High rates of abuse of stimulant drugs such as methylenedioxymethamphetamine (MDMA), methamphetamine (MA) and methylphenidate (MPD) emphasize the importance of determining the acute and long-term effects of these and other drugs of abuse on user's health and psychological functioning. One concern is the possibility of adverse effects on cognitive processes such as learning and memory. There have been numerous clinical reports of learning, memory and other cognitive deficits in stimulant drug abusers (e.g., Homer et al., 2008; McCann et. al., 2008; Morton, 2005). On the other hand, some of these same drugs (e.g., some amphetamines and MPD) are used clinically to enhance cognitive abilities in ADHD patients, and there is evidence that healthy human participants may also show enhanced cognitive function following acute administration of these compounds (Barch and Carter, 2005; Hart et al., 2008).
Ethical and practical difficulties in determining the effects of potentially hazardous drugs in humans point to the importance of developing animal models that assess their effects on learning and memory. A number of studies have investigated the effects of psychomotor stimulants on learning, but the results have been mixed. For example, some studies have found only disruption of learning by amphetamines or MPD (Braida et al., 2002; Chuhan and Taukulis, 2006; Mayorga et al., 2000; Nagai, et al., 2007), while others have found enhancement of learning at some doses of amphetamines or MPD (Calhoun and Jones, 1974; Handley and Calhoun, 1978; Zhu et al., 2007).
A number of methodological differences among the above studies (including dosage, method of administration, species, and type of learning task, to name a few) complicate their interpretation. However, one critical problem involves the difficulty of distinguishing between drug effects on learning processes from influences on other aspects of performance (e.g., sensorimotor effects, motivation, etc.). One procedure that has been effective in assessing both learning and performance within the same session is a multiple schedule of repeated acquisition and performance (Thompson and Moerschbaecher, 1979a). Two components alternate within the session; both require a particular sequence of responses for reinforcement, but in one component the sequence changes each session (acquisition), whereas in the other component the sequence remains the same throughout the experiment (performance). The repeated acquisition/performance procedure (RAP) thus provides a stringent control for drug effects on processes unrelated to learning (see Cohn and Paule, 1995).
Relatively few studies have evaluated the effects of MA, MDMA, MPD or similar compounds with RAP procedures. Early studies showed that d-amphetamine produced selective impairments of learning response chains in monkeys (Thompson and Moerschbaecher, 1979b) and pigeons (Moerschbaecher et al., 1979). That is, responding in the acquisition component was generally disrupted at lower doses of d-amphetamine than those that affected responding in the performance component. Thompson (1976) found similar selective impairment of learning produced by MPD in pigeons. The effects of MDMA have been assessed using the RAP procedure in monkeys (Thompson et al., 1987) and rats (Winsauer et. al, 2004) learning response chains. MDMA did not produce selective effects in monkeys as there were no effects on accuracy in either the acquisition or performance components until doses were reached that produced marked decreases in overall responding. Effects were also non-selective in rats, although only one dose of MDMA (10 mg/kg) was used. There are studies of d-amphetamine and MPD in rats that have studied repeated acquisition of response sequences without a performance control. Paule and McMillan (1984) found that low doses of d-amphetamine improved accuracy and increased response rate, and that high doses decreased both accuracy and rate. An intermediate dose (1.0 mg/kg) decreased accuracy without affecting response rate, suggesting a selective effect. However, Mayorga et al. (2000) using a similar procedure found fairly close correspondence between impairment of accuracy and rate-decreasing effects of d-amphetamine and MPD.
Winsauer et al. (2004) provided the only experiment to evaluate MDMA in rats using the RAP procedure but studied only a single dose, and no published reports of the effects of MA and MPD in rodents using the full RAP procedure appear to be available. Thus, the present study was conducted to provide the first determination of dose-effect functions with MDMA, MA and MPD in rats using a RAP procedure. The specific procedures differed somewhat from more traditional RAP studies in that rats were trained using a computer touch-screen apparatus in which nose-pokes to target locations within a 2 × 3 stimulus display could be reinforced. In the presence of one stimulus array, the target location changed across sessions (the acquisition component), whereas in the presence of a different array, the target location remained the same across sessions (the performance component). Previous research suggests that this type of apparatus can be useful for investigating behavioral processes in rats. For example, Keller et al. (2000) has shown excellent visual discrimination in rats using a similar apparatus to the one used here. More importantly for the present study, Pitts et al. (2006) found selective effects of certain drugs (e.g., chlordiazepoxide) in a RAP procedure identical to the one used here. In the present study, the effects of MA, MPD and MDMA were determined.
Materials and methods
Subjects
Subjects were six male Holtzman Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) between 90-120 days old at the start of the study and from 264-410 days old by the study's completion. Rats were housed individually in a colony room maintained under a reversed 12:12 hr light-dark cycle (lights off from 0600-1800). Access to food (Purina® Lab Diet) was limited to 1 hr beginning 15-min after each session.
Apparatus
A 25 cm × 20 cm × 18 cm operant-conditioning chamber was used. The chamber was modified by cutting six 4.5 cm × 4.5 cm squares, arranged in a 2-row by 3-column matrix and spaced 1-cm apart, into the metal front wall of the chamber (see Figure 1). The squares provided access to a transparent touch-screen that was mounted on a 38 cm color computer monitor. Two stimulus sets, each consisting of six identical filled geometric shapes (either 3.0 cm white squares or 3.0 cm diameter green circles), could be displayed on the computer screen such that each shape was centered within one of the squares in the chamber wall. Responses consisted of nose-pokes that interrupted photobeams on the touch-screen corresponding to the locations of the geometric shapes.
Fig. 1.
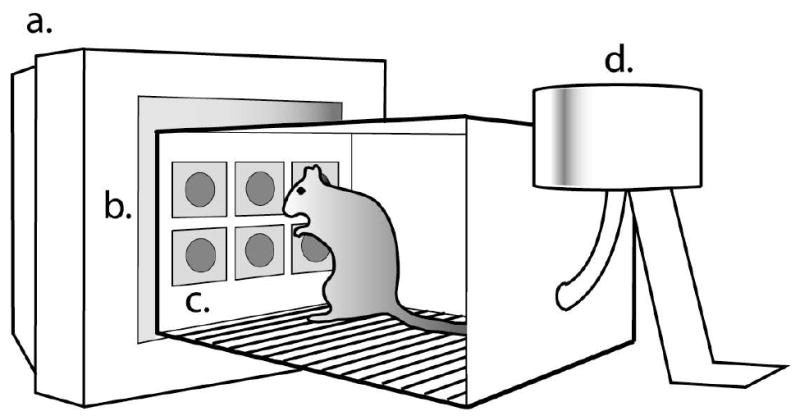
Schematic representation of the apparatus. Stimuli were presented on the computer monitor (a); nose-poke responses to stimuli were recorded by an IR touch screen (b); response locations were constrained by a 2 × 3 array of openings on one end of the chamber (c); and correct responses were reinforced be delivery of food pellets in a cup (not shown) at the back of the chamber (d).
A food cup, into which 45 mg sucrose pellets were dispensed as reinforcement, was located on the rear wall of the chamber. A 28-v DC light was located 10 cm directly above the cup. The two sidewalls of the chamber were made of clear Plexiglas and the floor consisted of stainless steel rods. The chamber was enclosed within a sound-attenuating box, along with a speaker that provided white noise (70 dB). Events were controlled and data collected using custom software on a computer located in an adjacent room.
Behavioral procedures
Initial Training
Rats were first adapted to the chamber and the food delivery mechanism. Once they began eating reliably from the food cup, the geometric shapes were projected onto the screen and nose-pokes were recorded; for three of the rats these were white squares and for the other three rats they were green circles. During these sessions, nose-pokes to any of the six locations were reinforced. After each nose poke, the computer screen was darkened, the feeder light was illuminated, and a pellet was delivered. After 1 s, the feeder light went off and the touch-screen was re-illuminated with the stimuli. Sessions continued until 40 pellet deliveries had been made or 60 min passed, whichever occurred first. When rats were nose poking reliably with short latencies, acquisition training began.
Acquisition Training
For each of these sessions one of the six locations was selected randomly, without replacement, as correct (S+); only pokes to that spot were reinforced. Once a cycle of all six locations had been completed a new cycle was randomly generated with the constraint that no location could be used on consecutive sessions. Pokes to the other locations (errors) initiated a 1.5-s timeout, during which the touch-screen darkened and pokes had no programmed consequences. The geometric shapes, either white squares or green circles, illuminated all six locations on each trial and, for each rat, were the same as those used in preliminary training. Training continued in this phase until errors showed a decreasing trend within each session after a minimum of 15 sessions.
Multiple-Component RAP Procedure
This procedure involved the addition of a performance component; thus, in this phase, sessions consisted of alternating acquisition and performance components. Each component occurred in the presence of one set of shapes (white squares or green circles); the stimulus set used for the acquisition component remained the same as in the previous phase and the other stimulus set was used for the performance component. In this phase, one of the six locations was randomly selected to be correct in the performance component (performance S+) for each rat, and this location remained constant across all sessions. The correct location for the acquisition component (acquisition S+) was selected randomly, without replacement, before each session from the remaining five locations (i.e., the performance S+ location was not used in the acquisition component). The remaining characteristics of the procedure were as described above. Table 1 indicates the component stimuli and the performance S+ location for each rat.
Table 1.
For each rat, the stimulus set present in each component, the correct location in the performance component, the number of sessions required to reach stability in the multiple-component RAP procedure, and the drug(s) received (numbers indicated order of drug experiments). WS = white squares and GC = green circles. The number indicating location of the performance component corresponds to the spot within a 2-row × 3-column matrix (1 = top left, 2 = top center, 3 = top right, and so on).
| Rat | ACQ Stimulus | PERF Stimulus | PERF Location | Sess to Stability | MDMA | MA | MPD |
|---|---|---|---|---|---|---|---|
| B1 | GC | WS | 5 | 89 | - | 1 | - |
| B2 | GC | WS | 2 | 134 | 3 | 2 | 1 |
| U6 | WS | GC | 6 | 59 | 2 | 1 | 3 |
| W7 | WS | GC | 3 | 74 | 3 | 2 | 1 |
| W11 | WS | GC | 3 | 55 | 1 | 3 | 2 |
| X36 | GC | WS | 6 | 103 | 1 | - | 2 |
An error-correction procedure was used such that a given component remained in effect until a correct response occurred and components alternated after each correct response. Sessions were conducted five days per week and lasted until 40 pellets in each component (i.e., a total of 80 pellets) were earned, 160 total responses occurred, or 1 hr elapsed, whichever occurred first.
Once responding was stable under this multiple-component RAP procedure, drug testing began. Stability criteria required that the following conditions were met for 10 consecutive sessions: a) errors per reinforcer in the performance component averaged less than 0.5, b) errors per reinforcer following delivery of the first reinforcer in the acquisition component averaged less than 2.0, and c) the number of errors in the acquisition component decreased across the session (Table 1 shows the number of sessions required to reach stability for each rat under the multiple-component RAP procedure).
Drug administration
Individual dose-effect curves were obtained for methamphetamine hydrochloride (HCL) (0.03-3.0 mg/kg, Sigma), methylenedioxymethamphetamine HCL (0.3-17.0 mg/kg; gift from the National Institute on Drug Abuse), and methylphenidate HCL (1.0-30.0 mg/kg, Sigma). Doses are expressed as the total salt. The range of doses was selected to include a) at least one dose that was not behaviorally active, b) doses that were comparable to those used therapeutically and recreationally by humans, and c) at least one dose that suppressed overall responding. Drugs were dissolved in physiological saline and injected ip 15 min prior to sessions conducted on Tuesdays and Fridays. During the regimen for a particular drug, data from sessions conducted on Thursdays served as the non-injection control. The effects of each dose and saline were determined two to five times in each subject. Doses were tested in ascending order during the initial determination; doses in subsequent determinations were tested in random order. Table 1 indicates the specific drug(s) received by each rat.
Data Analysis
Measures of accuracy and response speed were obtained for each component. Accuracy was expressed as the percentage of the total responses in a given component that were made to the S+ location (i.e., percent correct). Once stable performances had been reached, responding in the acquisition component generally became accurate by the tenth reinforcer presentation. In order to focus on this transition, the number of errors per reinforcer and percent correct were calculated for the first 10 reinforcer presentations in both acquisition and performance components. Drug effects on overall responding were assessed by response speed (reciprocal of the nose-poke latency for both correct and incorrect responses). Data for each rat in each component were averaged across the multiple determinations for each dose and these means (one data point per component and dose from each rat) were used for two-factor (component × dose), repeated-measures analysis of variance (ANOVA) with baseline (Thursday sessions conducted without injections) and saline conditions serving as controls. For each drug, the ANOVAs included all of the doses at which all rats obtained at least 10 reinforcers in each component; several of the rats failed to complete 10 reinforcers following the highest doses of each drug. Thus, this dose was eliminated from the statistical analysis for each drug; in such cases, however, a trial without a response was treated as an error with a speed of zero in order to illustrate the debilitating effects of these doses (see Figure 2). Whenever a significant main effect of dose and/or a significant interaction was obtained, effects of each dose were compared against those of saline within each component using repeated-measures LSD tests. The pattern of errors within sessions for saline and key doses was characterized by plotting mean errors per reinforcer as a function of ordinal position for the first 10 reinforcer presentations in each component.
Fig. 2.
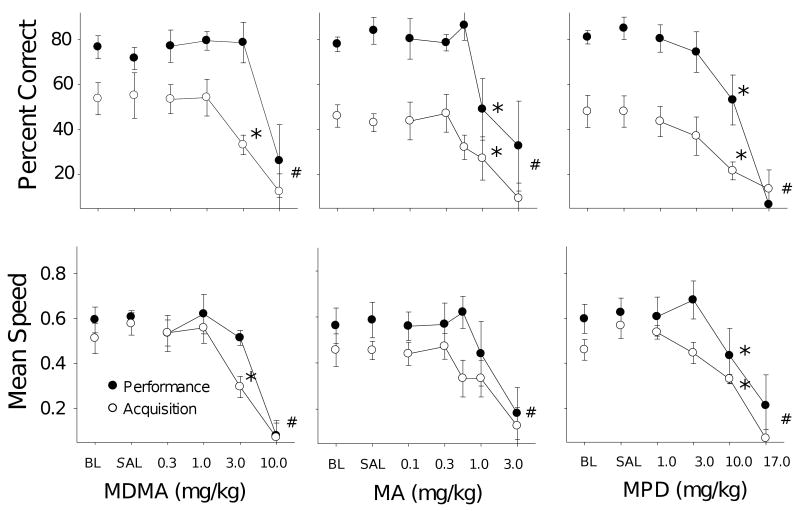
Dose-effect functions for MDMA (n=5; left panels), MA (n=5; middle panels), and MPD (n=5; right panels) on accuracy (upper row) and response speed (lower row), measured as percent correct responses for first ten reinforcer presentations in rats responding under a multiple-component repeated-acquisition and performance procedure (note that responses prior to the first reinforcer are omitted from this analysis as acquisition cannot be said to begin until the first response is reinforced). Unfilled symbols show effects in the acquisition component and filled symbols show effects in the performance component. Asterisks (*) indicate doses with effects that were significantly different from the effects of saline (p<0.05). Number signs (#) indicate effects of doses that were omitted from statistical analyses of accuracy because of their effects on overall responding. Error bars show SEs.
Results
Extensive training was required in the various phases of the study before stability criteria were met on the multiple-component procedure. Average number of sessions required for the six rats was 85.7 with a range of 55 to 134 sessions. However, by the end of the training phases, stable and accurate responding was obtained for each of the animals. As evident in Fig. 2 (upper panels), baseline levels of accuracy in the performance component were consistently near 80% correct, while in the acquisition component lower levels of accuracy near 50% correct were maintained (note that randomly selecting one of the locations had a 17% chance of being correct). While Fig. 2 presents mean data for the first ten reinforcers, Fig. 3 shows a trial by trial analysis, and illustrates that under control conditions errors declined rapidly across the first three or four reinforcers before accuracy reached asymptote (filled circles in the upper panels of Fig. 3). In contrast, errors were few in the performance component and were fairly evenly distributed across trials (filled circles in the lower panels of Fig. 3).
Fig. 3.
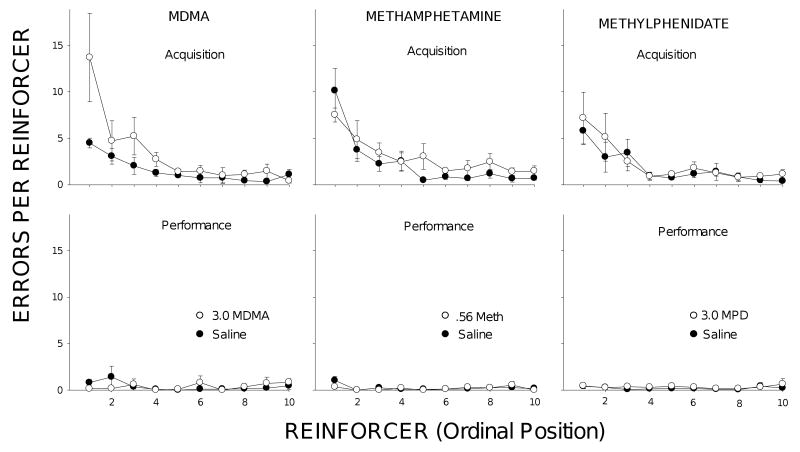
Effects of saline (filled symbols) and selected doses (unfilled symbols) of MDMA (3.0 mg/kg), MA (0.56 mg/kg), and MPD (3.0 mg/kg) on within-session error patterns in acquisition (top row) and performance (bottom row). Plotted are mean errors per reinforcer (and SEs) as a function of ordinal reinforcer for the first 10 reinforcer deliveries in each component.
Fig. 2 shows dose-effect functions for MDMA (left column), MA (center column), and MPD (right column) on percent correct (upper row) and response speed (lower row). Lower doses of MDMA had no effect on either speed or accuracy of responding in either component. At the 3.0 mg/kg dose, decreases in both speed and accuracy were evident in the acquisition component, but responding in the performance component was unaffected. At the highest dose (10.0 mg/kg), speed and accuracy were substantially reduced in both components. A component × dose (2 × 5) ANOVA on percent correct (omitting the 10 mg/kg dose which frequently resulted in a complete suppression of responding) revealed a significant main effect for component [F(1,4)=26.49, p=.007], no significant effect of dose [F(4,16)=1.005, p=.434] and a significant interaction [F(4,16)=3.42, p=.033]. The response speed analysis also resulted in a significant component × dose interaction [F(4,16)=5.53, p=.005], but the main effect for component failed to reach significance [F(1,4)=4.32, p=.106], while the main effect of dose was significant [F(4,16)=4.93, p=.009]. Post hoc tests for both accuracy and speed revealed significant differences between the 3.0 mg/kg dose and saline only in the acquisition component. In sum, MDMA decreased accuracy and speed in a dose-dependent fashion, but the effects were selective in that acquisition was impaired at a lower dose than was required to decrease performance.
In contrast to MDMA, effects of MA and MPD were generally less selective; doses that impaired acquisition were associated with comparable effects on performance. MA (middle panels) produced dose-dependent decreases in accuracy in both components. A component × dose ANOVA (2 × 6) on percent correct revealed a significant main effect for component [F(1,4)=72.41, p=.001], and a significant main effect of dose [F(5,20)=4.85, p=.005] but no interaction (F<1). Thus, the trend toward a selective effect on accuracy that can be seen at the 0.56 mg/kg dose was not sufficient to produce a significant interaction. Response speed was not affected by MA at the doses included in the analysis (note that the 3.0 mg/kg dose was excluded from the statistical analysis). There was a main effect of component [F(1,4)=18.11, p=.01] with respect to response speed for MA with more rapid responding in the performance component, but neither the main effect of dose [F(5,20)=1.61, p=.20], nor the dose × component interaction (F<1) was significant for speed. MA decreased accuracy in a non-selective fashion at doses that did not affect overall response speed, at least until the 3.0 mg/kg dose was reached when both accuracy and speed were substantially reduced and several animals stopped responding.
Dose × component (2 × 5) analyses of MPD effects also showed statistically significant, dose-dependent decreases in accuracy [F(4,16)=14.8, p <.001], but there was no significant interaction (F<1). As was the case in the other analyses, accuracy was higher in the performance component [F(1,4)=110.91, p<.001]. Outcomes of the response speed analyses showed only a significant main effect of dose [F(4,16)=14.35, p<.001). Neither the main effect of component [F(1,4)=5.14, p=.09], nor the interaction [F(4,16)=1.70, p=.20] reached significance. Thus, MPD affected acquisition only at doses that also disrupted performance accuracy.
Fig. 3 shows within-session error plots for acquisition (top panels) and performance (bottom panels) under saline conditions (filled symbols) and following administration of the highest dose of each drug that did not significantly disrupt performance accuracy (unfilled symbols). The 3.0 mg/kg dose of MDMA (left) increased acquisition errors prior to the delivery of the first reinforcer. By comparison with saline, more errors were also evident during the first few reinforcers until, by about the fifth trial, accuracy reached saline levels. These effects on acquisition at the 3.0 mg/kg MDMA dose occurred without any discernible effect on the performance trials. Neither MA nor MPD produced comparable increases in acquisition errors at the doses shown (0.56 mg/kg for MA and 3.0 mg/kg for MPD). The MA data in Fig. 3 are of interest given the non-significant trend toward a selective effect that was noted above at this dose, but it is apparent that, in contrast with the MDMA effects, errors after .56 MA were only slightly elevated and were fairly evenly distributed across trials. Although higher doses of MA and MPD than those presented here did interfere with acquisition accuracy, these were accompanied by disruptions of performance accuracy as well. Only MDMA disrupted acquisition at a dose that spared performance accuracy, and as Fig. 3 shows, this effect was primarily confined to the first few trials of acquisition.
Discussion
Consistent with the findings of Pitts et al. (2006), rats in the present study learned to respond to the reinforced target location with fairly high levels of accuracy by the tenth reinforcer within each session in the acquisition component while maintaining high accuracy levels in the performance component. Although relatively extensive training was required to reach stable performances (mean = 85.7 sessions), it should be noted that training duration was comparable to, and perhaps slightly more rapid than, that required in studies using multiple-component, RAP chain schedules with rats (Cohn and MacPhail, 1997; Winsauer et al., 1999). Thus, the touch-screen RAP procedure used here provides an automated and relatively efficient method for assessing drug effects on learning in rats.
MDMA, MA and MPD all impaired accuracy and speed in a dose-dependent fashion. However, the three drugs differed in the degree to which these impairments were selective to the acquisition component. MA and MPD were generally less selective than MDMA. That is, for MA and MPD, acquisition accuracy was impaired at doses that also impaired accuracy in the performance component. It should be noted that there was some tendency for MA effects to be selective at the 0.56 mg/kg dose, but these were not sufficiently robust to reach statistical significance. MPD also differed from MA in that decreases in accuracy produced by MPD only occurred at doses that also significantly decreased response speed. In contrast, MA impairment was not associated with decreased response speed (at least, until the highest 3.0 mg/kg dose). Thus, although MA impairments occurred in both performance and acquisition components, they were not simply due to impaired capacity to respond, but rather appeared to involve some generalized loss of stimulus control—both that associated with the well learned performance target location as well as that required for learning a new target location response in the acquisition component. This breakdown could involve some failure of control by the specific target stimuli, or the component stimuli, or both. It also should be noted that disruption of simple discriminative ability has been previously reported with high doses of amphetamines (Mayorga et al., 2000).
The non-selective effects of MA and MPD observed in the present study were somewhat surprising given previous findings showing that d-amphetamine and MPD disrupted acquisition of response chains or conditional discriminations at doses that failed to affect performance in pigeons and monkeys (Moerschbaecher et al., 1979; Thompson, 1976; Thompson and Moerschbaecher, 1979). Of course, doses intermediate to those chosen here might produce more selective effects, and more detailed exploration of the dose-effect functions would seem worthwhile. Alternatively, it is possible that rats are simply less sensitive to learning impairments produced by stimulant drugs than are pigeons or monkeys. Interestingly, Mayorga et al. (2000) found that both d-amphetamine and MPD interfered with acquisition of a response chain only at doses that also decreased overall responding in rats (i.e., they reported non-selective effects). Although they used an incremental repeated acquisition procedure that did not include a performance control condition, our results were consistent with theirs. However, Paule & McMillan (1982) found that d-amphetamine interfered with acquisition at doses that did not affect response rates using a similar incremental repeated acquisition procedure, suggesting that an account in terms of species-differences may be too simplistic. Rather, differences in procedures may be more likely to explain the differences between the present findings and the outcomes from previous RAP studies. The present study required learning a single nose-poke response to a new target location whereas the studies with pigeons and monkeys used more complex response chains or chains of conditional discriminations (Moerschbaecher et al., 1979; Thompson, 1976; Thompson and Moerschbaecher). Thus, the increased difficulty or complexity required by the task of learning a chain of responses, as opposed to learning a new target for a single response, may modulate the sensitivity of a RAP procedure to selective drug effects.
Consistent with previous RAP studies with MPD and amphetamines, no significant improvement of accuracy was observed at any dose of either MPD or MA in the present study. Because the baseline levels accuracy and speed in the acquisition component were generally well below those in the performance component, it would appear that the failure to detect improved accuracy can not simply be attributed to a low ceiling. Those studies that have detected learning enhancement by stimulants generally have used either a spatial learning task (Zhu, et al., 2007) or a discrimination-reversal procedure (Calhoun and Jones, 1974; Handley and Calhoun, 1978), although it should be noted that Paule & McMillan (1984) found enhancement at low d-amphetamine with the incremental repeated acquisition task. None of these studies used a RAP procedure, but RAP adaptations of spatial navigation tasks and reversal learning tasks are available (Galizio et al., 2006; Keith and Galizio, 1997) and it would be interesting to examine the effects of MA and MPD using these procedures.
Importantly, MDMA did produce reliably selective effects in the present study. That is, 3.0 mg/kg of MDMA impaired accuracy in the acquisition component while sparing the performance component. However, the selective effects of MDMA were complicated by the selective reductions in response speed that also occurred at this dose. Because no loss of response speed occurred in the performance component at the 3.0 mg/kg dose, the effect is clearly not a loss of response capacity or sensorimotor deficit. A possible account for this interesting effect may derive from consideration of the within-session analysis of Fig. 3. While MDMA increased errors in the acquisition component over the first few reinforcers of the session, the most striking feature was the very large number of errors that were emitted prior to the presentation of the first reinforcer. Thus, under MDMA, the acquisition component began with a series of unreinforced responses and these may be related to the reduced rate of responding. In any case, this observation suggests that MDMA may have produced perseverative behavior that interfered with the “win-stay/lose-shift” pattern that was associated with rapid acquisition under baseline conditions. Of more than passing interest, Harper and his colleagues (Harper et al., 2005; 2006) found that MDMA impaired delayed matching to sample performance in rats due to a proactive interference effect. They found that MDMA produced a perseverative tendency to respond to the lever chosen in the immediately preceding trial, an outcome consistent with the effects obtained in the present study. Harper et al. (2006) were able to attenuate these MDMA effects by lengthening the inter-trial interval (presumably reducing proactive interference) and it would be interesting to determine whether a similar outcome could be obtained with the RAP procedures studied here.
At first glance, the selective impairment of acquisition produced by MDMA in the present study may appear contrary to the results of Winsauer et al. (2004), who found only non-selective effects in rats. Closer analysis, however, suggests otherwise. Winsauer et al. studied only a single 10 mg/kg dose and this same dose produced non-selective impairments of both performance and acquisition in the present study as well. Only at the lower 3.0 mg/kg dose were selective effects apparent in the present study. The earlier finding of Thompson et al. (1987) of non-selective effects in monkeys appears more problematic. In that study, no increases in errors on a response chain in either the acquisition or performance component were observed at any dose that permitted responding. Again, although these different outcomes may involve species differences, another account would emphasize that the type of learning task is a critical determinant of drug effects in RAP procedures. Thompson et al. used the chain schedule that is most typical of repeated acquisitions designs, while only single response was required in the present study (and also in Harper et al., 2005; 2006). Another possible explanation of the differences between the present outcomes and those of more traditional RAP studies would emphasize the potential of pharmacological differences between spatial and non-spatial learning tasks. For example, a study from our laboratory found that morphine selectively affected acquisition in a spatial navigation task (Galizio et al., 2003), but morphine and other opiates have generally been associated with non-selective effects in RAP procedures involving response chains or other non-spatial learning tasks (c.f., Galizio et al., 2006). Although the procedures used in the present study do not involve spatial navigation, learning the target location on the two-dimensional screen certainly required spatial discrimination and perhaps can be viewed as presenting different requirements than more traditional RAP tasks.
Finally, the differences between the selective effects of MDMA and the relatively less selective effects of MA and MPD noted in the present study are of potential importance. Physiological, subjective, and behavioral effects of MDMA, amphetamines and methylphenidate are often quite similar (c.f., Harper, Wisnewski, Hunt and Schenk, 2005; Hegadoren, Baker, and Bourin, 1999; Liechti, Gamma and Vollenwieder, 2001), so the differences in the present assay are somewhat surprising. Common effects of these drugs are thought to be due to their common actions as dopamine agonists (Green, Mechan, Elliot, O'Shea & Colado, 2003; Perlow, Chiueh, Lake & Wyatt, 1980). The increase in 5-HT release produced by MDMA is an important neuropharmacological effect that differs from MA and MPD (Green et al., 2003), and thus, the more selective effects of MDMA on acquisition in the present study may implicate a serotonergic mechanism. Serotonin depletion is seen after chronic MDMA use, as is cognitive impairment (Morton, 2005). Previous studies have shown that selective 5-HT1A and 5-HT1B agonists can disrupt repeated acquisition (Winsauer et al., 1999; Winsauer & Moerschbaecher, 2000). Thus, further exploration of the serotonergic basis of the selective effects of MDMA on learning noted in the present study may be of value.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (DA12879). The procedures were approved by the UNCW Animal Care and Use Committee. The authors wish to thank Erica Blackwell, Stephanie Connor, Mitchell Ferguson, James Kurtessis, Joanna Lazzeri, Diane Lowder, and Shayna Nesbitt for their help with data collection and Rhiannon Thomas for the care and maintenance of the animals.
Funding: National Institute on Drug Abuse (DA12879).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Galizio, Department of Psychology, University of North Carolina Wilmington, 601 S. College Rd. Wilmington, NC 28403-5612, galizio@uncw.edu, Tel: 910-962-3813, Fax: 910-962-7010
P. McKinney, Department of Psychology, University of North Carolina Wilmington, 601 S. College Rd. Wilmington, NC 28403-5612
D. T. Cerutti, Department of Psychology, California State University-East Bay, Oakland CA
R. C. Pitts, Department of Psychology, University of North Carolina Wilmington, 601 S. College Rd. Wilmington, NC 28403-5612
References
- Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Cavallini R, Sala M. 3,4 Methylenedioxymethamphetamine (Ecstasy) impairs eight-arm radial maze performance and arm entry pattern in rats. Behav Neuroscience. 2002;116:298–304. doi: 10.1037//0735-7044.116.2.298. [DOI] [PubMed] [Google Scholar]
- Calhoun WH, Jones EA. Methamphetamine's effect on repeated acquisitions with serial discrimination reversals. Psychopharmacology. 1974;39:303–308. doi: 10.1007/BF00422969. [DOI] [PubMed] [Google Scholar]
- Chuhan YS, Taukulis HK. Impairment of single-trial memory formation by oral methylphenidate in the rat. Neurobiology of Learning and Memory. 2006;85:125–131. doi: 10.1016/j.nlm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Cohn J, MacPhail RC. Chlorpyrifos produces selective learning deficits in rats working under a schedule of repeated acquisition and performance. J Pharmacol Exp Ther. 1997;283:312–320. [PubMed] [Google Scholar]
- Galizio M, Keith JR, Mansfield W, Pitts RC. Repeated spatial acquisition: Effects of NMDA antagonists and morphine. Exp Clin Psychopharm. 2003;11:79–90. doi: 10.1037//1064-1297.11.1.79. [DOI] [PubMed] [Google Scholar]
- Galizio M, Miller L, Ferguson A, McKinney P, Pitts RC. Olfactory repeated discrimination reversal in rats: Effects of chlordiazepoxide, dizocilpine and morphine. Behav Neuro. 2006;120:1175–1179. doi: 10.1037/0735-7044.120.5.1175. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Handley GW, Calhoun WH. The effects of methylphenidate on repeated acquisition of serial discrimination reversals. Psychopharmacology. 1978;57:115–117. doi: 10.1007/BF00426967. [DOI] [PubMed] [Google Scholar]
- Harper DN, Hunt M, Schenk S. Attenuation of the disruptive effects of (+/-)3-4-methylenedioxymethamphetamine (MDMA) on delayed matching-to-sample performance in rats. Beh Neuro. 2006;120:201–205. doi: 10.1037/0735-7044.120.1.201. [DOI] [PubMed] [Google Scholar]
- Harper DN, Wisnewski R, Hunt M, Schenk S. 3,4-Methylenedioxymethamphetamine, d-amphetamine and cocaine impair delayed matching-to-sample performance by an increase in susceptibility to proactive interference. Beh Neuro. 2005;119:455–463. doi: 10.1037/0735-7044.119.2.455. [DOI] [PubMed] [Google Scholar]
- Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharm. 2008;33:1847–1855. doi: 10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegadoren KM, Baker GB, Bourin M. 3,4-methylenedioxy analogues of amphetamine: Defining the risks to humans. Neurosci and Biobehav Rev. 1999;23:539–553. doi: 10.1016/s0149-7634(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, Halkitis PN. Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychol Bull. 2008;134:301–310. doi: 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- Keller J, Strasburgert H, Cerutti DT, Sabel BA. Assessing spatial vision—automated measurement of the contrast-sensitivity function in the hooded rat. Journal of Neuroscience Methods. 2000;97:103–110. doi: 10.1016/s0165-0270(00)00173-4. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenwieder FX. Gender differences in the subjective effects of MDMA. Psychopharm. 2001;154:721–726. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Popke EJ, Fogle CM, Paule MG. Similar effects of amphetamine and methylphenidate on the performance of complex operant tasks in rats. Behav Brain Res. 2000;109:59–68. doi: 10.1016/s0166-4328(99)00165-5. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong Df, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Moerschbaecher JM, Boren JJ, Schrot J, Simoes-Fontes JC. Effects of cocaine and d-amphetamine on the repeated acquisition and performance of conditional discriminations. J Exp Anal Behav. 1979;31:127–140. doi: 10.1901/jeab.1979.31-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. Ecstasy: Pharmacology and neurotoxicity. Curr Opin Pharm. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Dohniwa M, Ibi D, Mizoguchi H, Kamei H, Nabeshima T, Yamada K. Repeated methamphetamine treatment impairs spatial working memory in rats: reversal by clozapine but not haloperidol. Psychopharm. 2007;194:21–32. doi: 10.1007/s00213-007-0820-1. [DOI] [PubMed] [Google Scholar]
- Paule MG, McMillan DE. Incremental repeated acquisition in the rat: Acute effects of drugs. Pharmacology, Biochemistry & Behavior. 1984;21:431–439. doi: 10.1016/s0091-3057(84)80106-9. [DOI] [PubMed] [Google Scholar]
- Perlow MJ, Chiueh CC, Lake R, Wyatt RJ. Increased dopamine and norepinephrine concentrations in primate CSF following amphetamine and phenylethylamine administration. Brain Res. 1980;186:469–473. doi: 10.1016/0006-8993(80)90993-2. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Buda DR, Keith JR, Cerutti DT, Galizio M. Chlordiazepoxide and dizocilpine, but not morphine, selectively impair acquisition under a novel repeated acquisition and performance task in rats. Psychopharm. 2006;18:135–143. doi: 10.1007/s00213-006-0538-5. [DOI] [PubMed] [Google Scholar]
- Thompson DM. Repeated acquisition of behavioral chains: effects of methylphenidate and imipramine. Pharmacol Biochem Behav. 1976;6:671–677. doi: 10.1016/0091-3057(76)90218-5. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Moerschbaecher JM. Drug effects on repeated acquisition. In: Thompson T, Dews PB, editors. Advances in behavioral pharmacology vol 2. Academic Press; New York: 1979a. pp. 229–259. [Google Scholar]
- Thompson DM, Moerschbaecher JM. An experimental analysis of the effects of d-amphetamine and cocaine on the acquisition and performance of response chains in monkeys. J Exp Anal Behav. 1979b;32:433–444. doi: 10.1901/jeab.1979.32-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Winsauer PJ, Mastropaolo J. Effects of phencyclidine, ketamine, and MDMA on complex operant behavior in monkeys. Pharmacol Biochem Behav. 1987;26:401–405. doi: 10.1016/0091-3057(87)90136-5. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM. Differential effects of 5-HT agonists and antagonists on the repeated acquisition of learning and performance of response sequences in monkeys. Beh Pharmacol. 2000;11:535–553. doi: 10.1097/00008877-200011000-00002. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Quinton MS, Porter JR, Corll CB, Moerschbaecher JM, Delatte MS, Leonard ST, Stroble SB. Effects of MDMA administration on scopolamine-induced disruptions of learning and performance in rats. Pharm Bio Behav. 2004;79:459–472. doi: 10.1016/j.pbb.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Rodriguez FH, Cha AE, Moerschbaecher JM. Full and partial 5-HT1A receptor agonists disrupt learning and performance in rats. J Pharmacol Exp Ther. 1999;288:335–347. [PubMed] [Google Scholar]
- Zhu N, Weedon J, Dow-Edwards DL. Oral methylphenidate improves spatial learning and memory in pre- and periadolescent rats. Behav Neuro. 2007;121:1272–1279. doi: 10.1037/0735-7044.121.6.1272. [DOI] [PubMed] [Google Scholar]