Abstract
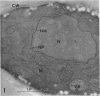
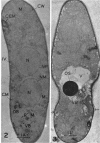
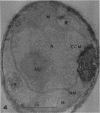
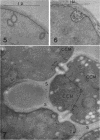
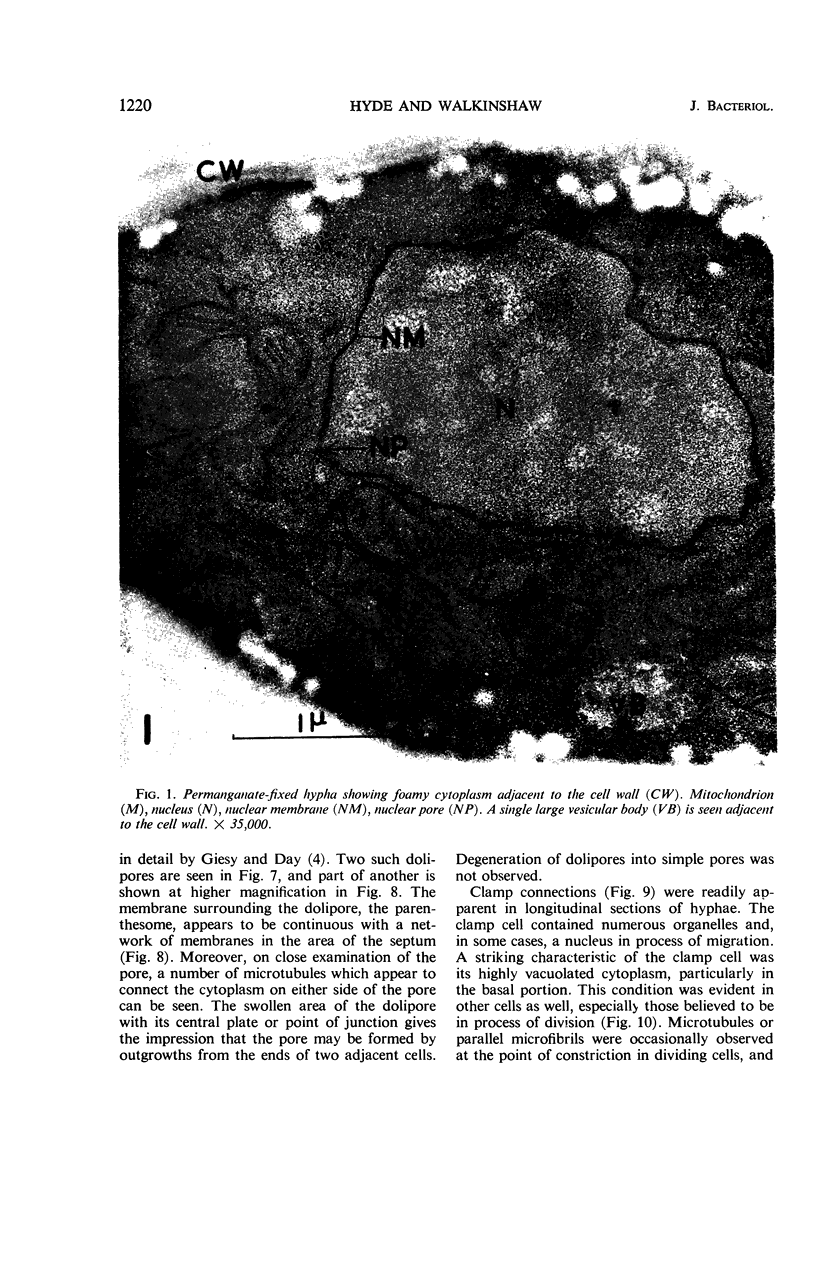
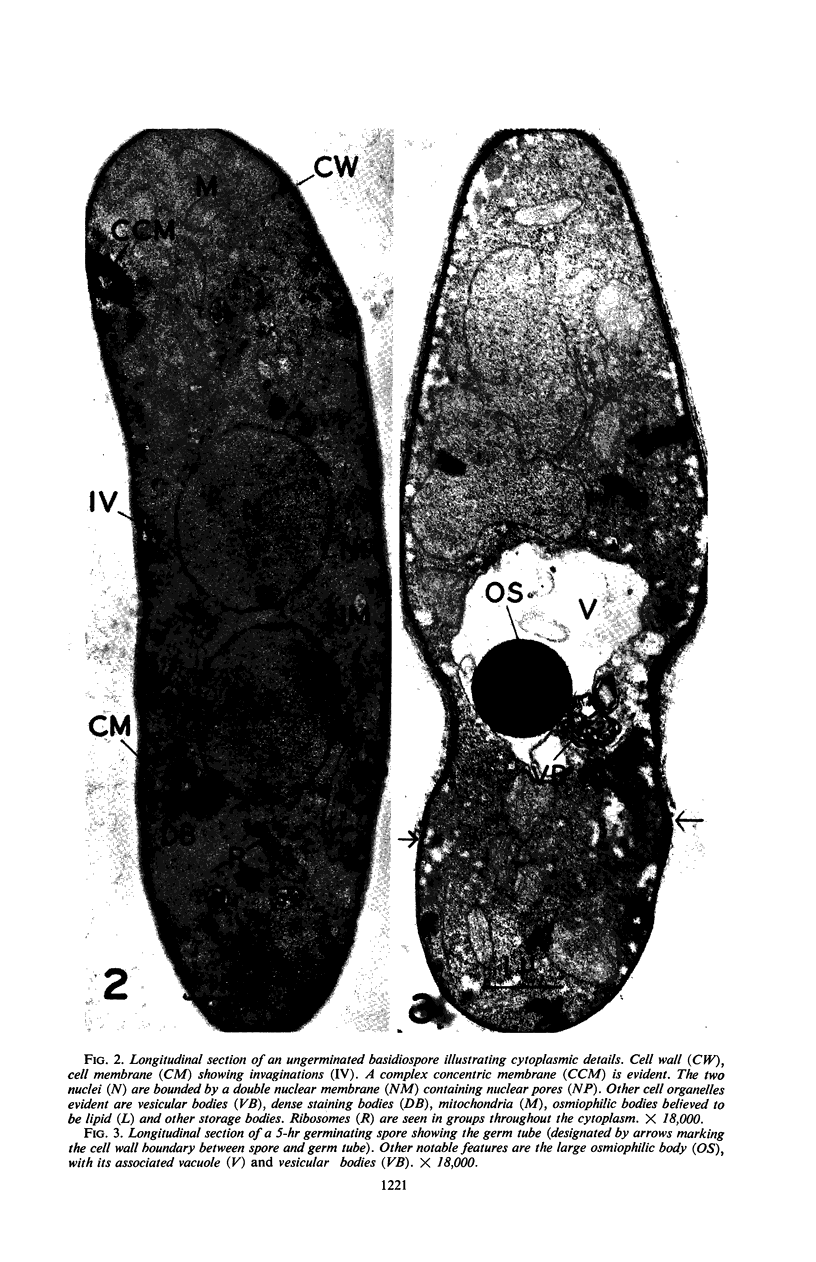
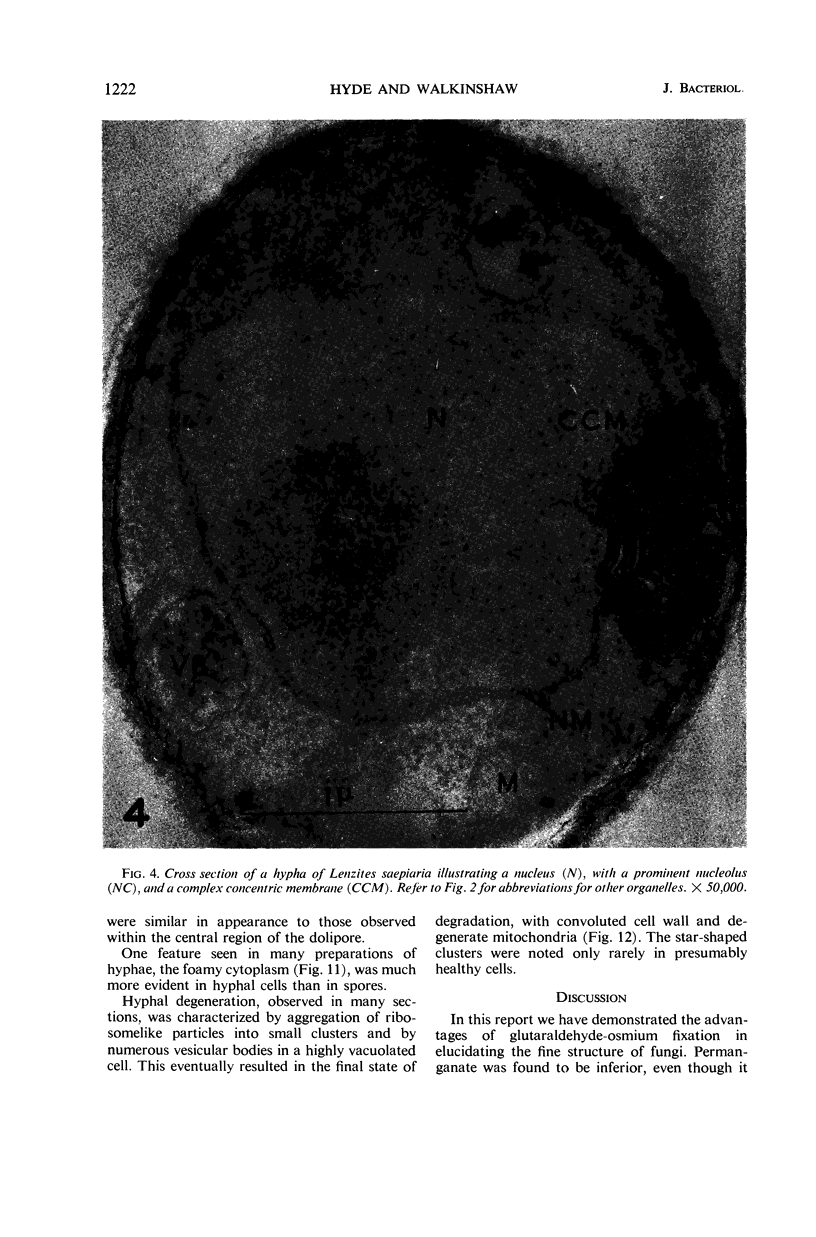
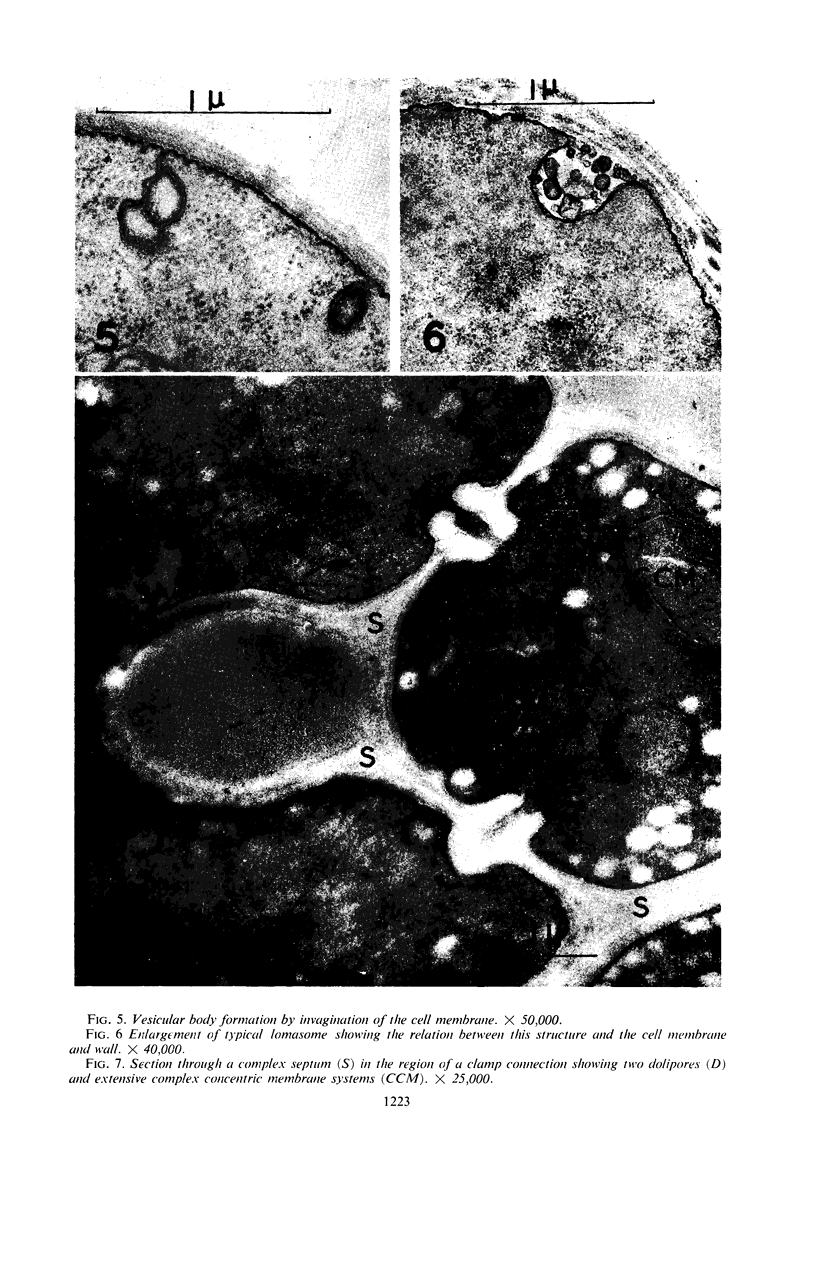
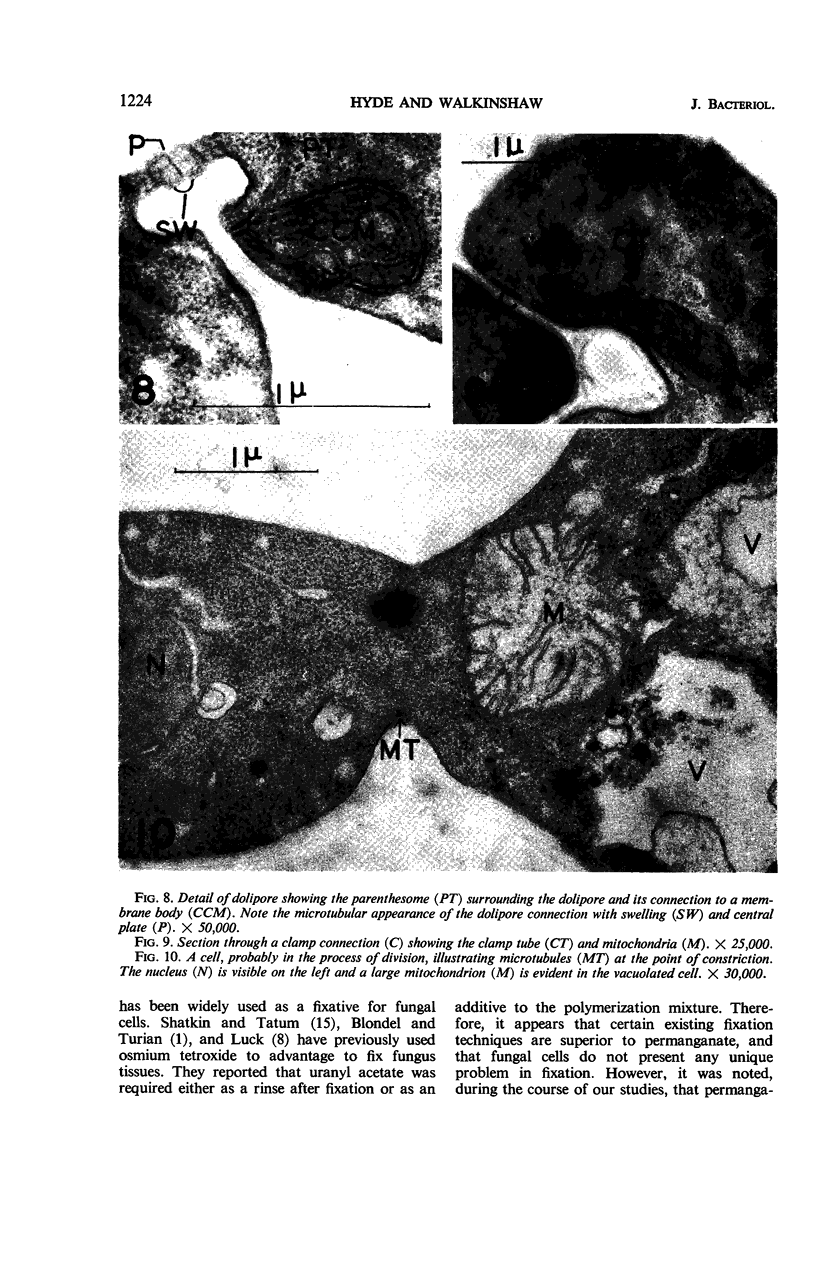
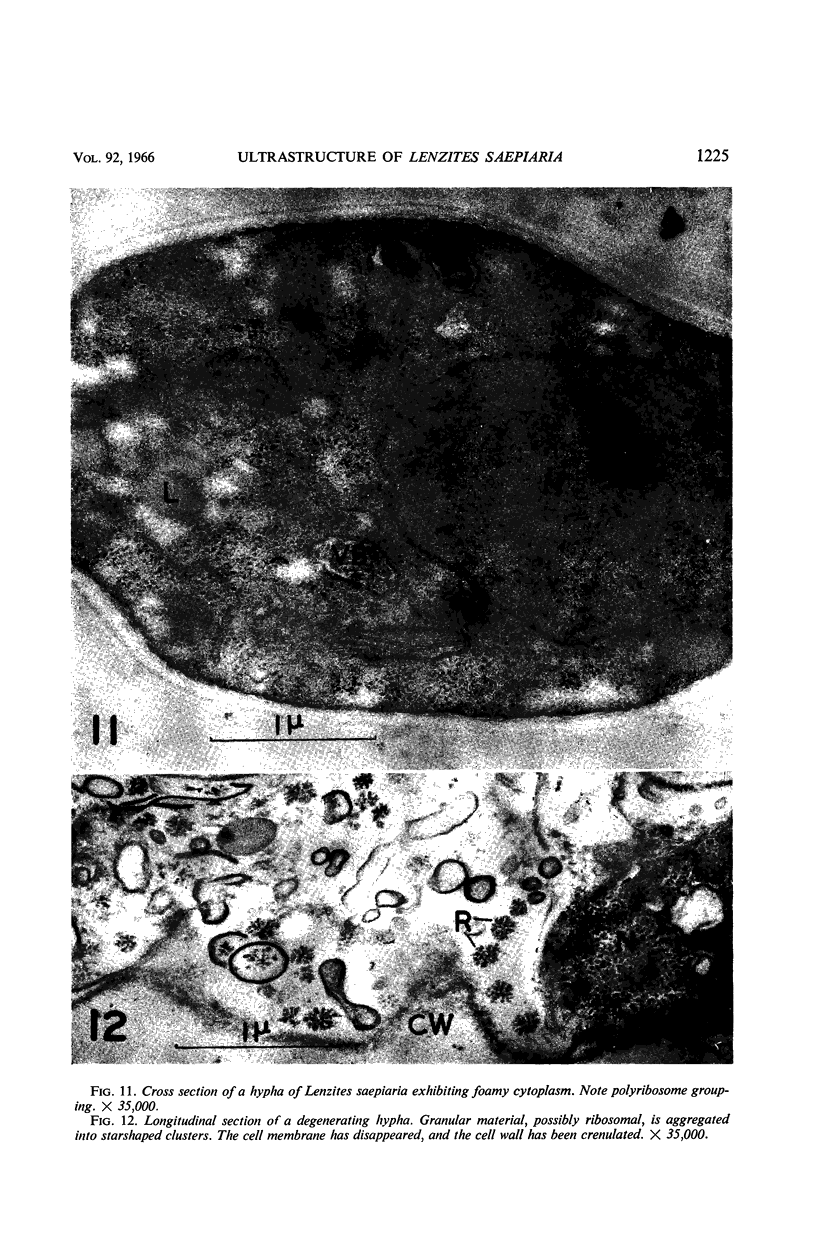
Hyde, James M. (University of Mississippi Medical School, Jackson), and Charles H. Walkinshaw. Ultrastructure of basidiospores and mycelium of Lenzites saepiaria. J. Bacteriol. 92:1218–1227. 1966.—Ungerminated and germinated basidiospores and 2-day-old mycelial cultures of Lenzites saepiaria were similar in their fine structure. Fixation with glutaraldehyde, followed by osmium tetroxide, was far superior to permanganate. Cell organelles were seen in cytoplasm of spores and hyphae, and clamp connections were abundant in hyphal elements. Numerous lomasomes, vesicular bodies, and complex concentric membranes occurred in the cytoplasm and were often associated with the cell membrane or the dolipore membrane (parenthesome) of the septum. Endoplasmic reticulum was not found, but numerous ribosomes were seen; polyribosome groupings were frequently noted. The nucleus was bounded by a double membrane which contained few pores. Germinating spores exhibited one or more large osmiophilic bodies in association with a vacuole and membranous elements. Other than possessing a thin wall, the emerging germ tube was similar in structure to the parent spore.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLONDEL B., TURIAN G. Relation between basophilia and fine structure of cytoplasm in the fungus Allomyces macrogynus Em. J Biophys Biochem Cytol. 1960 Feb;7:127–134. doi: 10.1083/jcb.7.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell W. W., Page R. M. The Use of Fragmented Mycelial Inoculum in the Culture of Fungi. J Bacteriol. 1947 Mar;53(3):360–361. [PMC free article] [PubMed] [Google Scholar]
- HAWKER L. E. FINE STRUCTURE OF FUNGI AS REVEALED BY ELECTRON MICROSCOPY. Biol Rev Camb Philos Soc. 1965 Feb;40:52–92. doi: 10.1111/j.1469-185x.1965.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Hohl H. R. Nature and Development of Membrane Systems in Food Vacuoles of Cellular Slime Molds Predatory upon Bacteria. J Bacteriol. 1965 Sep;90(3):755–765. doi: 10.1128/jb.90.3.755-765.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKWOOD W. R. A RELIABLE AND EASILY SECTIONED EPOXY EMBEDDING MEDIUM. Anat Rec. 1964 Oct;150:129–139. doi: 10.1002/ar.1091500204. [DOI] [PubMed] [Google Scholar]
- LUCK D. J. THE INFLUENCE OF PRECURSOR POOL SIZE ON MITOCHONDRIAL COMPOSITION IN NEUROSPORA CRASSA. J Cell Biol. 1965 Mar;24:445–460. doi: 10.1083/jcb.24.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol. 1956 Nov 25;2(6):799–802. doi: 10.1083/jcb.2.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHATKIN A. J., TATUM E. L. Electron microscopy of Neurospora crassa mycelia. J Biophys Biochem Cytol. 1959 Dec;6:423–426. doi: 10.1083/jcb.6.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOELZ H., NIEDERPRUEM D. J. FINE STRUCTURE OF BASIDIOSPORES OF SCHIZOPHYLLUM COMMUNE. J Bacteriol. 1964 Nov;88:1497–1502. doi: 10.1128/jb.88.5.1497-1502.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS B. AN ELECTRON MICROSCOPE AND BIOCHEMICAL STUDY OF NEUROSPORA CRASSA DURING DEVELOPMENT. J Gen Microbiol. 1965 Apr;39:85–94. doi: 10.1099/00221287-39-1-85. [DOI] [PubMed] [Google Scholar]