Abstract
Background
Ispinesib is a highly specific inhibitor of kinesin spindle protein (KSP, HsEg5), a mitotic kinesin required for separation of the spindle poles. Here we report the activity of ispinesib against the in vitro and in vivo panels of the Pediatric Preclinical Testing Program (PPTP).
Procedures
Ispinesib was tested against the PPTP in vitro panel cell lines at concentrations from 0.1 nM to 1 μM and against the in vivo tumor panel xenografts by intraperitoneal administration (5 or 10 mg/kg) every 4 days for 3 doses repeated at day 21.
Results
Ispinesib was highly potent against the PPTP’s in vitro cell lines with a median IC50 of 4.1 nM. Ispinesib (10 mg/kg) induced unexplained toxicity in mice bearing osteosarcoma xenografts and exceeded the MTD in 12 of 40 non-osteosarcoma xenografts. Ispinesib induced significant tumor growth delay in 88% (23/26) of evaluable xenografts. Using a time to event measure of efficacy, ispinesib had intermediate and high levels of activity against 4 (21%) and 5 (26%) of the 19 evaluable solid tumor xenografts, respectively. Ispinesib induced maintained complete responses (CR) in a rhabdoid tumor, a Wilms tumor and a Ewing sarcoma xenograft. Ispinesib (5 mg/kg) produced 2 complete and 2 partial responses among 6 evaluable xenografts in the ALL panel. The in vivo pattern of activity was distinctive from that previously reported for vincristine.
Conclusions
Ispinesib demonstrated broad in vivo antitumor activity, including maintained complete responses for several xenografts, although with high toxicity rates at the doses studied.
Keywords: Preclinical Testing, Developmental Therapeutics, Ispinesib
INTRODUCTION
Mitotic kinesins are members of the kinesin superfamily of motor proteins that play essential roles in mitotic spindle function, including spindle-pole organization, chromosome alignment and segregation, and the regulation of microtubule dynamics [1]. Kinesin spindle protein (KSP, HsEg5) is a mitotic kinesin that utilizes energy from adenosine triphosphate (ATP) hydrolysis to produce directed mechanical force along microtubules of the emerging mitotic spindle, thereby driving formation of a bipolar structure [2]. Thus, KSP is required for formation and maintenance of the bipolar spindle [3]. Highly specific small molecule inhibitors of KSP have been identified [1,4]. These small molecule inhibitors of KSP are not competitive with ATP for binding to KSP, but rather act as allosteric inhibitors by binding to the ADP-KSP complex and by inhibiting ADP release from KSP [5–8].
KSP inhibition produces a characteristic cellular phenotype with mitotically arrested cells showing a monopolar spindle with arrays of microtubules projecting from a pair of unseparated centrosomes surrounded by chromosomes. Following cell cycle arrest induced by KSP inhibition, some, but not all, cell lines undergo apoptosis [9]. KSP is highly expressed in proliferating cells during mitosis, but is absent in post-mitotic neurons [6], suggesting that targeting KSP offers the potential for lesser toxicity to normal tissues than the tubulin-binding antimitotic agents currently used for cancer treatment.
Small molecule inhibitors of KSP have entered clinical evaluation, including ispinesib (SB-715992) [10–14], SB-743921 [15], and MK-0741 [16]. Preclinical data for these inhibitors have shown high potency in vitro, and the ability to induce regressions in some preclinical models in vivo [1,4]. For example, ispinesib administered intraperitoneally (IP) on an intermittent schedule (every four days for three doses) was active against advanced human colon tumor xenografts, inducing complete regressions as well as tumor growth delay [17]. The related compound CK0106023 induced regressions against an ovarian cancer xenograft (SKOV3), and treated tumor cells showed circular mitotic figures, similar to the monopolar spindles seen in CK0106023-treated cells in culture [6]. Ispinesib was selected for testing by the PPTP because of the utility of conventional tubulin-binding antimitotic agents for many childhood cancers and because of the distinctive mechanism of action by which ispinesib blocks mitotic progression.
MATERIALS AND METHODS
In vitro testing
In vitro testing was performed using DIMSCAN, a semiautomatic fluorescence-based digital image microscopy system that quantifies viable (using fluorescein diacetate [FDA]) cell numbers in tissue culture multiwell plates [18]. Cells were incubated in the presence of ispinesib for 96 hours at concentrations from 0.1 nM to 1.0 μM and analyzed as previously described [19]. Vincristine was tested in a similar manner across the same concentration range.
In vivo tumor growth inhibition studies
CB17SC-M scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [20–22]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [23]. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [hCD45] cells [ALL xenografts] were determined as previously described [24]. Responses were determined using three activity measures as previously described [24]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SASR, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies. The Mann-Whitney test was used to test the difference of medians of in vitro parameters (e.g., EC50 and maximal effect) between the groups of lines with similar tumor types to the remaining lines of the panel. The relationship between in vitro parameters for ispinesib and vincristine were analyzed by linear regression analysis.
Drugs and Formulation
Ispinesib was provided to the Pediatric Preclinical Testing Program by Cytokinetics and GlaxoSmithKline through the Cancer Therapy Evaluation Program (NCI). Ispinesib was dissolved in a solution of Cremophor EL (2% v/v), dimethylacetamide (2% v/v), acidified water (pH 5), and administered intraperitoneally every 4 days for 3 doses, with the treatment course repeated at day 21. The dose of ispinesib was 10 mg/kg in the solid tumor models and 5 mg/kg in the ALL models and was based on toxicity testing in non-tumored animals. Ispinesib was provided to each consortium investigator in coded vials for blinded testing according the PPTP program standard operating procedures.
RESULTS
Ispinesib in vitro testing
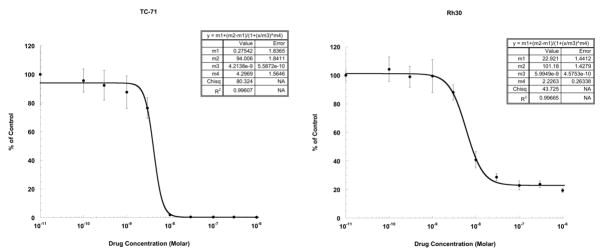
Ispinesib was active against most of the cell lines of the PPTP in vitro panel, with only a single rhabdomyosarcoma line (Rh18) having an IC50 greater than 1 μM. The median IC50 value for all of the cell lines in the panel was 4.1 nM (Table I). With the exception of a higher median IC50 for the rhabdomyosarcoma panel, reflecting in part the lack of response of Rh18, there was no relationship between histotype and IC50. T/C% values at the highest concentration tested (1 μM) were used as a measure of the maximal effect of ispinesib. Figure 1 illustrates the two primary patterns of response to ispinesib: one indicative of near complete cytotoxicity as typified by a Ewing cell line (TC-71) with a T/C% value at 1 μM of 0.3% and the other indicative of partial cytotoxicity or cytostasis as typified by a rhabdomyosarcoma cell line (Rh30) with a T/C% value at 1 μM of 22.8%. The four rhabdomyosarcoma lines evaluated had significantly higher T/C% values at 1 μM compared to the other lines in the panel (median of 21.1% versus 4.3%, p=0.013) (Table I). The ALL lines had T/C% values at 1 μM that were significantly lower than those of the remaining lines in the panel (0.1% versus 10.7%, p=0.003).
Table I.
Activity of Ispinesib against the PPTP in Vitro Panel
| Cell Line | Status | Histology | T/C% at1 μM | IC50(nM) |
|---|---|---|---|---|
| RD | Rhabdomyosarcoma | 11.4 | 5.8 | |
| Rh41 | Post-Therapy | Rhabdomyosarcoma | 22.8 | 11.1 |
| Rh18 | Diagnosis | Rhabdomyosarcoma | 71.2 | >1000 |
| Rh30 | Diagnosis | Rhabdomyosarcoma | 19.4 | 8.0 |
| BT-12 | Diagnosis | Rhabdoid | 6.2 | 2.1 |
| CHLA-266 | Diagnosis | Rhabdoid | 25.8 | 1.1 |
| TC-71 | Post-Therapy | Ewing sarcoma | 0.3 | 4.1 |
| CHLA-9 | Diagnosis | Ewing sarcoma | 0.9 | 2.8 |
| CHLA-10 | Post-Therapy | Ewing sarcoma | 2.2 | 3.6 |
| CHLA-258 | Post-Bone Marrow Transplant | Ewing sarcoma | 12.8 | 4.8 |
| SJ-GBM2 | Post-Therapy | Glioblastoma | 13.5 | 0.5 |
| NB-1643 | Diagnosis | Neuroblastoma | 9.9 | 3.0 |
| NB-EBc1 | Post-Therapy | Neuroblastoma | 6.5 | 11.4 |
| CHLA-90 | Post-Bone Marrow Transplant | Neuroblastoma | 11.6 | 3.6 |
| CHLA-136 | Post-Bone Marrow Transplant | Neuroblastoma | 14.9 | 7.0 |
| NALM-6 | Post-Therapy | ALL | 0.0 | 1.6 |
| COG-LL-317 | Post-Therapy | ALL | 0.1 | 2.5 |
| RS4; 11 | Post-Therapy | ALL | 0.2 | 0.6 |
| MOLT-4 | Post-Therapy | ALL | 0.1 | 7.9 |
| CCRF-CEM | ALL | 0.1 | 3.0 | |
| Kasumi-1 | Post-Bone Marrow Transplant | AML | 9.4 | 11.9 |
| Karpas-299 | Post-Therapy | ALCL | 4.3 | 8.9 |
| Ramos-RA1 | NHL | 0.0 | 8.0 | |
| Median | 6.5 | 4.1 | ||
| Minimum | 0.0 | 0.5 | ||
| Maximum | 71.2 | >1000 |
Figure 1. Ispinesib in vitro activity.
The figures illustrate typical cytotoxicity/growth inhibition dose-response curves for cell lines with T/C% values at 1 μM approaching 0% (TC-71) and for cell lines with T/C% values at 1 μM indicating a plateau agent effect of 10% or greater (Rh30). Error bars represent standard deviations for each concentration tested.
Because of previous reports suggesting that KSP inhibitors have in vitro activity profiles similar to those of antimitotic tubulin binders [25,26], we compared the in vitro activity of ispinesib to that of vincristine focusing on the T/C% values of each agent at the highest concentration tested (1μM) as a measure of the maximal effect of each agent (Supplemental Table 1). For both vincristine and ispinesib, Rh18 was the only cell line which had a T/C% value > 50% at the 1 μM concentration. There was a highly significant relationship between the T/C% values at 1 μM for ispinesib and vincristine (p<0.0001, r2 = 0.87), with the significant relationship being maintained even when Rh18 was omitted from the analysis (p=0.0003, r2 = 0.49). Both ispinesib and vincristine were highly active against the cell lines of the ALL panel, with T/C% values at 1 μM approaching zero for each of the cell lines, indicative of strong cytotoxic activity. For the rhabdomyosarcoma panel, however, T/C% values at 1 μM for both agents were > 10%, consistent with a cytostatic or at best a partial cytotoxic response.
Activity of ispinesib against the PPTP in vivo panel
Ispinesib was evaluated against 46 xenograft models using an every 4 day x 3 repeated at Day 21 schedule that was selected to model the weekly administration schedule evaluated in a pediatric phase 1 trial of ispinesib [27]. Of 1021 mice studied, 174 died during the study (17%), with 5 of 497 in the control arms (1.0%) and 169 of 524 in the ispinesib treatment arms (32%). Excessive toxicity was especially problematic for the osteosarcoma panel, with few animals surviving past day 7 of testing. The mice carrying osteosarcoma xenografts experienced early death even at a reduced dose of 5 mg/kg. For the non-osteosarcoma lines in the panel, toxicity was more manageable, although toxicity rates were still elevated [111/466 (24%)]. All six of the osteosarcoma xenografts, as well as 12 of 40 non-osteosarcoma xenografts, were excluded from analysis due to toxicity rates greater than 25 percent. A complete summary of results is provided in Supplemental Table II, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
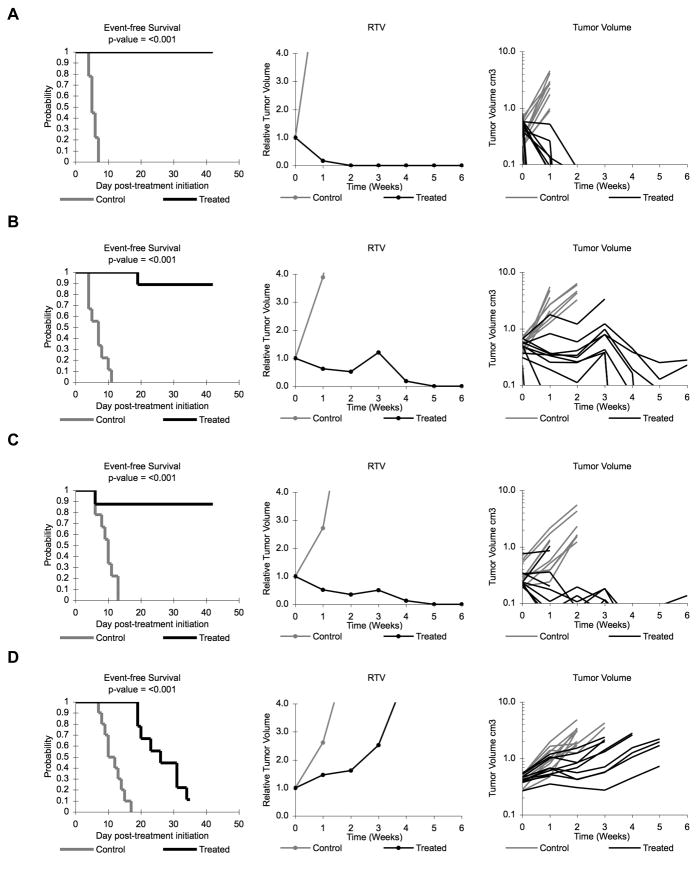
Antitumor effects were evaluated using the PPTP activity measures for time to event (EFS T/C), tumor growth delay (tumor volume T/C), and objective response. Ispinesib induced significant differences in EFS distributions compared to controls in 17/20 (85%) evaluable solid tumor models and 6/6 (100%) ALL models (Table II). Four solid tumor xenografts had objective responses and also met the criteria for high activity for the EFS T/C activity measure, including one xenograft each from the rhabdoid tumor, Wilms tumor, Ewing sarcoma, and glioblastoma panels. An additional 5 of 19 evaluable solid tumor xenografts met criteria for intermediate activity for the EFS T/C activity measure. Among the 6 evaluable ALL xenografts, all met criteria for either intermediate (n=4) or high (n=2) activity using the EFS T/C activity measure. Figure 2 Illustrates patterns of response to ispinesib for selected solid tumor models, and Figure 3 illustrates patterns of response of the ALL models.
Table II.
Activity for Ispinesib against the PPTP in Vivo Panel
| Xenograft Line | Histology | KM Estimate of Median Time to Event | P- value | EFS T/C | Median Final RTV | Tumor Volume T/C | T/C Activity | EFS Activity | Response Activity |
|---|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | > EP1 | <0.001 | > 4.0 | 3.8 | 0.33 | Int | Int | Int |
| KT-16 | Rhabdoid | > EP1 | <0.001 | > 4.2 | 0.0 | 0.35 | Int | High | High |
| KT-10 | Wilms | 17.5 | <0.001 | 1.7 | >4 | 0.37 | Int | Low | Int |
| KT-11 | Wilms | > EP1 | <0.001 | > 5.8 | 0.0 | 0.13 | High | High | High |
| KT-13 | Wilms | 16.9 | <0.001 | 2.4 | >4 | 0.46 | Low | Int | Int |
| SK-NEP-1 | Ewing | > EP1 | <0.001 | > 8.9 | 0.0 | 0.03 | High | High | High |
| EW8 | Ewing | 18.7 | 0.005 | 1.6 | >4 | 0.44 | Int | Low | Int |
| Rh30 | ALV RMS | 25.7 | <0.001 | 2.3 | >4 | 0.25 | Int | Int | Int |
| Rh30R | ALV RMS | 14.2 | 0.019 | 1.8 | >4 | 0.64 | Low | Low | Int |
| Rh41 | ALV RMS | 15.8 | <0.001 | 1.7 | >4 | 0.35 | Int | Low | Int |
| BT-28 | Medulloblastoma | 3.8 | 0.127 | 1.3 | >4 | 0.67 | Low | Low | Low |
| BT-46 | Medulloblastoma | 8.2 | 0.388 | 1.2 | >4 | 0.81 | Low | Low | Low |
| BT-41 | Ependymoma | > EP1 | 0.003 | > 1.3 | 1.1 | 0.52 | Low | NE2 | Int |
| GBM2 | Glioblastoma | 37.2 | <0.001 | 2.7 | >4 | 0.31 | Int | Int | Int |
| BT-39 | Glioblastoma | 31.7 | <0.001 | 4.0 | >4 | 0.53 | Low | Int | Int |
| D645 | Glioblastoma | 12.2 | <0.001 | 1.5 | >4 | 0.68 | Low | Low | Low |
| D456 | Glioblastoma | > EP1 | <0.001 | > 8.3 | 1.0 | 0.10 | High | High | High |
| NB-1771 | Neuroblastoma | 17.4 | <0.001 | 1.9 | >4 | 0.40 | Int | Low | Int |
| NB-1691 | Neuroblastoma | 4.8 | 0.163 | 1.1 | >4 | 0.79 | Low | Low | Low |
| CHLA-79 | Neuroblastoma | 19.2 | <0.001 | 1.8 | >4 | 0.32 | Int | Low | Int |
| ALL-2 | ALL B-precursor | > EP1 | <0.001 | > 3.2 | 0.1 | . | High | High | |
| ALL-3 | ALL B-precursor | > EP1 | <0.001 | > 8.4 | 9.4 | . | Int | High | |
| ALL-4 | ALL B-precursor | 17.9 | <0.001 | 4.4 | >25 | . | Int | Int | |
| ALL-8 | ALL T-cell | > EP1 | 0.041 | > 9.8 | 10.6 | . | Int | Int | |
| ALL-16 | ALL T-cell | > EP1 | <0.001 | >16.1 | 0.3 | . | High | High | |
| ALL-19 | ALL B-precursor | 42.5 | 0.011 | 6.8 | >25 | . | Int | High |
Greater than the Evaluation Period;
Not evaluable
Figure 2. Ispinesib activity against individual solid tumor xenografts.
Kaplan-Meier curves for EFS, median relative tumor volume graphs, and individual tumor volume graphs are shown for selected lines: (A) SK-NEP-1 (B) KT-11, (C) KT-16, and (D) Rh30. Controls (gray lines); Treated (black lines).
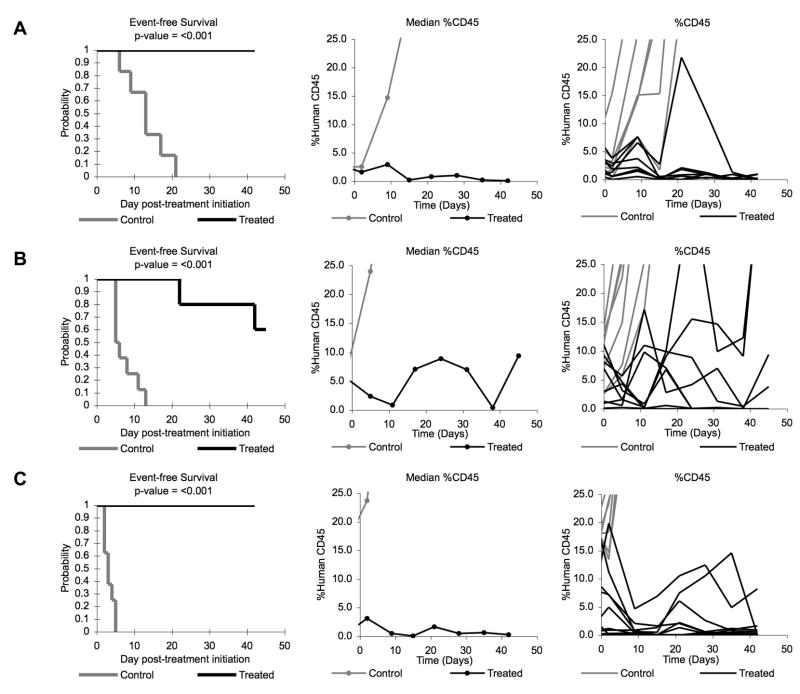
Figure 3. Ispinesib activity against individual ALL xenografts.
Kaplan-Meier curves for EFS, median %hCD45 graphs, and individual %hCD45 graphs are shown for selected lines: (A) ALL-2, (B) ALL-3, and (C) ALL-16. Controls (gray lines); Treated (black lines).
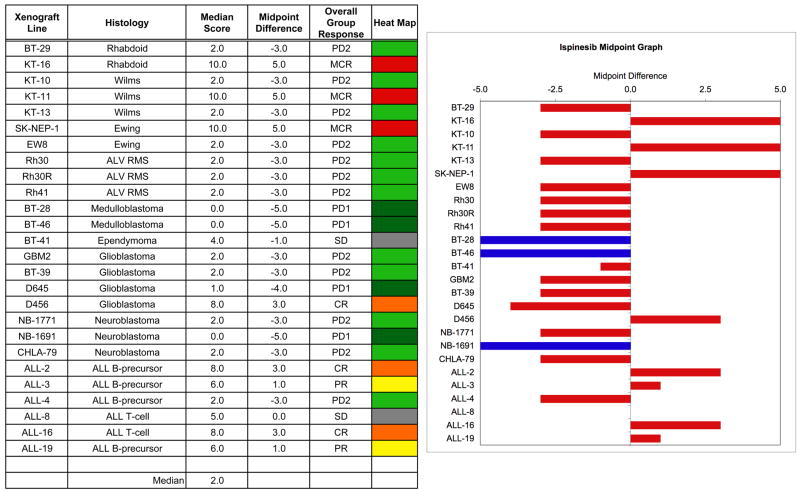
The in vivo testing results for the objective response measure of activity are presented in Figure 4 in a ‘heat-map’ format as well as a ‘COMPARE’-like format, based on the scoring criteria described in the Material and Methods and the Supplemental Response Definitions section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of 5 (stable disease). Objective responses [either complete response (CR) or maintained CR)] were seen in 4 of 20 solid tumor models. Objective responses were observed in 4 of 6 ALL models, with two PRs (ALL-3 and ALL-19) and two CRs (ALL-2 and ALL-16).
Figure 4. Ispinesib in vivo objective response activity.
Left: The colored ‘heat map’ depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of ≥ 2 but < 6, and low activity by a score of < 2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive and that achieve regression as a best response. Bars to the left are tumor models that are less sensitive and that achieve tumor progression with growth delay or no treatment effect as their best response. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
To evaluate ispinesib’s activity profile at lower, better tolerated doses, three responsive lines (KT-11, SK-NEP-1, and Rh28) were evaluated at 4, 5, or 7.5 mg/kg. The former two xenografts achieved maintained CRs at 10 mg/kg, while Rh28 achieved PR at 10 mg/kg (but with excessive toxicity). There were no deaths among animals treated at these lower doses. SK-NEP-1 responded similarly at the lower doses with maintained CR responses at 4 and 7.5 mg/kg. The other two lines exhibited progressive disease at the reduced doses. Complete results from the dose-response testing are provided in Supplemental Table III.
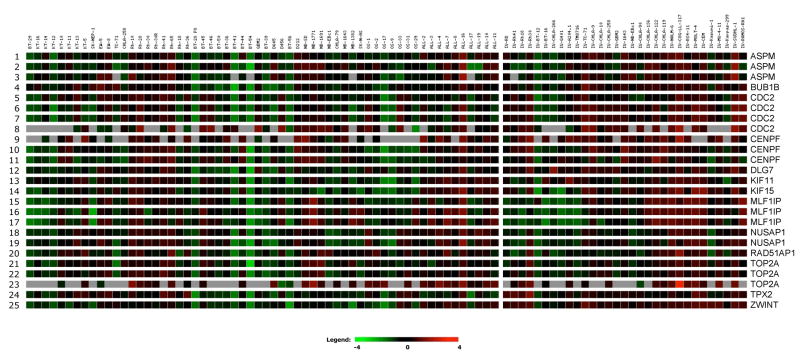
KSP (KIF11) gene expression for the PPTP cell lines and xenografts is shown in Figure 5. KSP was expressed at significantly higher levels in the ALL xenografts and cell lines compared to the solid tumor xenografts (Supplemental Table IV). Among the solid tumor panels, KSP expression was highest for the rhabdomyosarcoma xenografts and lowest for the osteosarcoma xenografts. There was no correlation between the objective response score and KSP expression levels (r2 = 0.02). KSP expression was highly correlated with the expression of other genes whose expression is associated with mitosis and the mitotic checkpoint machinery (e.g., CDC2, BUBR1, and CENPF) [28].
Figure 5. Gene expression for KSP (KIF11) and genes with correlated expression patterns.
Relative expression of KSP (KIF11) based on Affymetrix U133 Plus 2.0 expression profiles for PPTP xenografts (left panel) and cell lines (right panel), as visualized using GeneSifter software (VizX Labs, Seattle, WA). Gray indicates an absent call from Affymetrix quality control. The expression profiles for genes whose expression is highly correlated with that of KSP are also shown.
DISCUSSION
Ispinesib is a potent, highly specific inhibitor of KSP. The in vitro activity profile of ispinesib was characterized by nanomolar range IC50 values for almost all of the PPTP cell lines. The maximal effect of ispinesib, as measured by its T/C% value at the highest concentration tested (1 μM), ranged from near complete cytotoxicity (T/C% values approaching 0% at 1 μM) for the ALL cell lines and most of the Ewing sarcoma cell lines to partial cytotoxic or cytostatic activity (T/C% values > 10% at 1 μM) for the rhabdomyosarcoma cell lines. For small molecule KSP inhibitors, the determinants of cellular response (cell cycle arrest versus apoptosis induction) are not yet well defined [9]. The activity pattern for ispinesib against the PPTP’s in vitro panel is similar to that observed for the antimitotic agent vincristine. This observation is consistent with NCI-60 cell line panel testing results, for which similar activity profiles for antitubulin agents such as vincristine and the KSP inhibitor S-trityl-L-cysteine (STLC) were also observed [25,26].
In vivo activity for ispinesib was noted across a diverse range of histotypes, with CR or maintained CRs observed in single xenografts from the rhabdoid tumor, Ewing sarcoma, glioblastoma, and Wilms tumor panels. While ispinesib’s in vitro activity profile is similar to that of vincristine, its in vivo activity profile appears to be distinctive. Overall for the PPTP solid tumor panels, vincristine demonstrated high activity using the objective response activity measure in 6 of 24 xenografts [24], compared to high activity for ispinesib in 4 of 20 evaluable xenografts. However, two lines with maintained CRs to ispinesib (KT-16 and SK-NEP-1) had progressive disease (PD2) in response to vincristine. Conversely, KT-13 (Wilms) and BT-39 (GBM), which had maintained CRs to vincristine, had progressive disease (PD2) following treatment with ispinesib. The six ALL xenografts were generally responsive to both vincristine and ispinesib, although the response categories were higher for vincristine (4 maintained CRs, 2CRs, and 1PR) than for ispinesib (2CRs, 2PRs, 1SD, and 1PD2) [24].
Ispinesib produced a high level of toxicity in tumor-bearing mice at the 10 mg/kg dose. Lower doses (4 – 7.5 mg/kg) were better tolerated, but activity was substantially reduced for two of three responsive solid tumor xenografts for which dose-response testing was performed. For the ALL panel, toxicity at the 5 mg/kg dose precluded analysis of results in two of eight xenograft models. Preliminary testing at a dose of 2.5 mg/kg in the ALL-panel (data not shown) point to reduced efficacy, with 6 of 7 xenografts achieving a lower response score than at the 5 mg/kg dose and with only one objective response at the lower dose as compared to 4 for the higher dose. These results suggest a relatively narrow therapeutic range, which is typical of many cytotoxic agents. Ispinesib-associated toxicity was especially severe in the osteosarcoma panel, an observation for which there is no obvious explanation. There is to our knowledge no report of a similar “tumor specific” toxicity profile, and thus the clinical significance of this observation is not known.
Ispinesib has completed its phase 1 evaluation in children with recurrent solid tumors using a weekly for 3 weeks repeated every 28 day schedule [27]. In adults, ispinesib has been evaluated using a variety of schedules [10–12], with the primary focus of clinical development being the every three week schedule. Objective responses were not observed for ispinesib in phase II trials using the every three week schedule for head and neck squamous cell carcinoma [30], colorectal cancer [13], hepatocellular carcinoma [31], and melanoma [32]. For a cohort of patients with metastatic breast cancer previously treated with both an anthracycline and a taxane, partial responses were observed in 4 of 45 evaluable patients using the every 21 day schedule (K. Wood, personal communication), and an every two week schedule is under evaluation in chemotherapy-naive breast cancer patients [14].
The PPTP in vivo testing results suggest that ispinesib may be an active agent for several pediatric cancers, although the biological characteristics associated with responsiveness are not apparent and there appears to be a relatively narrow therapeutic range for responsive pediatric models. Further clinical evaluations will be required to determine whether the potential therapeutic benefits associated with a novel antimitotic agent like ispinesib that causes minimal toxicity to non-proliferating cells can be realized in the clinical setting.
Supplementary Material
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used ispinesib supplied by Cytokinetics and GlaxoSmithKline. In addition to the authors, represents work contributed by the following: Sherry Ansher, Joshua Courtright, Edward Favours, Henry S. Friedman, Nino Keshelava, Tiebin Liu, Debbie Payne-Turner, Charles Stopford, Mayamin Tajbakhsh, Chandra Tucker, Jianrong Wu, Joseph Zeidner, Wendong Zhang, Leticia Campbell, Maya Willie, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children’s Hospital.
References
- 1.Jackson JR, Patrick DR, Dar MM, et al. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7(2):107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 2.Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288(5463):88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 3.Blangy A, Lane HA, d’Herin P, et al. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83(7):1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 4.Jiang C, You Q, Li Z, et al. Kinesin spindle protein Inhibitors as anticancer agents. Expert Opinion on Therapeutic Patents. 2006;16(11):1517–1532. [Google Scholar]
- 5.Yan Y, Sardana V, Xu B, et al. Inhibition of a mitotic motor protein: where, how, and conformational consequences. J Mol Biol. 2004;335(2):547–554. doi: 10.1016/j.jmb.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 6.Sakowicz R, Finer JT, Beraud C, et al. Antitumor activity of a kinesin inhibitor. Cancer Res. 2004;64(9):3276–3280. doi: 10.1158/0008-5472.can-03-3839. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, Carson JD, Dhanak D, et al. Mechanism of inhibition of human KSP by monastrol: insights from kinetic analysis and the effect of ionic strength on KSP inhibition. Biochemistry. 2004;43(48):15258–15266. doi: 10.1021/bi048282t. [DOI] [PubMed] [Google Scholar]
- 8.Skoufias DA, DeBonis S, Saoudi Y, et al. S-trityl-L-cysteine is a reversible, tight binding inhibitor of the human kinesin Eg5 that specifically blocks mitotic progression. J Biol Chem. 2006;281(26):17559–17569. doi: 10.1074/jbc.M511735200. [DOI] [PubMed] [Google Scholar]
- 9.Vijapurkar U, Wang W, Herbst R. Potentiation of kinesin spindle protein inhibitor-induced cell death by modulation of mitochondrial and death receptor apoptotic pathways. Cancer Res. 2007;67(1):237–245. doi: 10.1158/0008-5472.CAN-06-2406. [DOI] [PubMed] [Google Scholar]
- 10.Burris HA, LoRusso P, Jones S, et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV days 1, 8, 15 q 28 days. J Clin Oncol. 2004;22(14S) Abstr #2004. [Google Scholar]
- 11.Chu QS, Holen KD, Rowinsky EK, et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV Q 21 days. J Clin Oncol. 2004;22(14S) Abstr #2078. [Google Scholar]
- 12.Heath EI, Alousi A, Eder JP, et al. A phase I dose escalation trial of ispinesib (SB-715992) administered days 1–3 of a 21-day cycle in patients with advanced solid tumors. J Clin Oncol. 2006;24(18S) Abstr #2026. [Google Scholar]
- 13.El-Khoueiry AB, Iqbal S, Singh DA, et al. A randomized phase II non-comparative study of Ispinesib given weekly or every three weeks in metastatic colorectal cancer. A California Cancer Consortium Study (CCC-P) J Clin Oncol. 2006;24(18S) Abstr #3595. [Google Scholar]
- 14.Philco M, Falcon S, Gomez H, et al. A phase I-II open-label trial of ispinesib on an alternate dosing schedule in chemotherapy-naive patients with locally advanced or metastatic breast cancer (MBC) J Clin Oncol. 2008 May 20;26(suppl) Abstr #1143. [Google Scholar]
- 15.Holen KD, Belani CP, Wilding G, et al. Phase I study to determine tolerability and pharmacokinetics (PK) of SB-743921, a novel kinesin spindle protein (KSP) inhibitor. J Clin Oncol. 2006;24(18S) Abstr #2000. [Google Scholar]
- 16.Stein MN, Rubin EH, Scott PD, et al. Phase I clinical and pharmacokinetic (PK) trial of the kinesin spindle protein (KSP) inhibitor MK-0731 in cancer patients. J Clin Oncol. 2006;24(18S) Abstr #2001. [Google Scholar]
- 17.Johnson RK, McCabe FL, Caulder E, et al. SB-715992, a potent and selective inhibitor of the mitotic kinesin KSP, demonstrates broad-spectrum activity in advanced murine tumors and human tumor xenografts. Proc Annu Meet Am Assoc Cancer Res. 2002;43:269. [Google Scholar]
- 18.Keshelava N, Frgala T, Krejsa J, et al. DIMSCAN: a microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy. Methods Mol Med. 2005;110:139–153. doi: 10.1385/1-59259-869-2:139. [DOI] [PubMed] [Google Scholar]
- 19.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(1):37–45. doi: 10.1002/pbc.21214. [DOI] [PubMed] [Google Scholar]
- 20.Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988;48(15):4189–4195. [PubMed] [Google Scholar]
- 21.Graham C, Tucker C, Creech J, et al. Evaluation of the antitumor efficacy, pharmacokinetics, and pharmacodynamics of the histone deacetylase inhibitor depsipeptide in childhood cancer models in vivo. Clin Cancer Res. 2006;12(1):223–234. doi: 10.1158/1078-0432.CCR-05-1225. [DOI] [PubMed] [Google Scholar]
- 22.Peterson JK, Tucker C, Favours E, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11(19 Pt 1):6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 23.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 24.Houghton PJ, Morton CL, Tucker C, et al. The Pediatric Preclinical Testing Program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 25.Paull KD, Lin CM, Malspeis L, et al. Identification of novel antimitotic agents acting at the tubulin level by computer-assisted evaluation of differential cytotoxicity data. Cancer Res. 1992;52(14):3892–3900. [PubMed] [Google Scholar]
- 26.DeBonis S, Skoufias DA, Lebeau L, et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol Cancer Ther. 2004;3(9):1079–1090. [PubMed] [Google Scholar]
- 27.Souid A, Dubowy RL, Triplett DG, et al. Pediatric phase I trial and pharmacokinetic (PK) study of ispinesib (SB715992): A Children’s Oncology Group phase I consortium study. J Clin Oncol. 2008 May 20;26(suppl) Abstr #10014. [Google Scholar]
- 28.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7(5):637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Neale G, Su X, Morton CL, et al. Molecular characterization of the Pediatric Preclinical Testing Panel. 2008 doi: 10.1158/1078-0432.CCR-07-5090. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang PA, Siu LL, Chen EX, et al. Phase II study of ispinesib in recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2008;26(3):257–264. doi: 10.1007/s10637-007-9098-8. [DOI] [PubMed] [Google Scholar]
- 31.Knox JJ, Gill S, Synold TW, et al. A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168) Invest New Drugs. 2008;26(3):265–272. doi: 10.1007/s10637-007-9103-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee CW, Belanger K, Rao SC, et al. A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2008;26(3):249–255. doi: 10.1007/s10637-007-9097-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.