Abstract
Synthetic biology encompasses the design of new biological parts and systems as well as the modulation of existing biological networks to generate novel functions. In recent years, increasing emphasis has been placed on the engineering of population-level behaviors using cell-cell communication. From the engineering perspective, cell-cell communication serves as a versatile regulatory module that enables coordination among cells in and between populations and facilitates the generation of reliable dynamics. In addition to exploring biological “design principles” via the construction of increasingly complex dynamics, communication-based synthetic systems can be used as well-defined model systems to study ecological and social interactions such as competition, cooperation and predation. Here we discuss the dynamic properties of cell-cell communication modules, how they can be engineered for synthetic circuit design, and applications of these systems.
1. Introduction
Synthetic biology aims to generate well-defined controllable functions in living organisms. The design of such functions involves using well-characterized biological parts from natural systems, manipulating existing cellular networks, or creation of new parts altogether [1,2], to form new genetic networks called “synthetic circuits”. This past decade has seen an explosion in the design of such synthetic circuits with increasing complexity and a rapid expansion of engineered functions [3–5]. The ability to generate desired functionality using synthetic circuits is useful in a wide variety of applications from therapeutics to green chemistry [5,6]. In addition, the use of existing biological parts in synthetic circuits provides vital insights into the roles of these parts in their natural context.
Cell-cell communication leading to multicellular behavior has attracted great attention for use in synthetic circuits. In nature, communication is critical for the physiological functions of diverse organisms. In the nervous system, activity-dependent ATP release by nervous system cells acts as an extracellular signal detected by purinergic membrane receptors that modulate intracellular calcium and cyclic AMP [7]. In this case, cell-cell communication links together a wide variety of cells essential to the functioning of the nervous system within a complex organism. During development in Drosophila, intercellular signaling combines with spatial signal gradient sensing for the formation of vein structure on the wing [8]. Here, a well-defined, multicellular pattern emerges from the controlled action of individual cells. In bacteria, populations monitor their own density and achieve coordinated expression of target genes in a phenomenon known as quorum sensing (QS) [9]. The coordination that results from QS-dependent communication is postulated to benefit the population as a whole [10]. In each case, cell-cell communication is essential to the formation of a well controlled, coordinated, multicellular system. These properties make communication an important feature for synthetic circuits.
Natural communication modules, especially those from bacteria [11], have been exploited to program synthetic population behavior [3,12]. Here, communication is typically via diffusible chemicals that are synthesized and detected by individual cells where they alter downstream gene expression. Other cellular modules, such as metabolic networks, have also been re-engineered to realize communication [13]. To encompass all these efforts, we define ‘communication’ simply as any interaction between cells where the sender, the carrier and the receiver of specific information can be identified [14,15]. Here we discuss the basic properties of cell-cell communication, the design of communication-based multicellular systems and their applications.
2. Cell-cell communication and its properties
The natural QS systems in Gram-negative bacteria often use acyl homoserine lactones (AHLs) as communication signals [16,17]. These AHLs are typically synthesized by LuxI-type enzymes from fatty acids, where LuxI is the canonical AHL synthase from Gram-negative bacterium Vibrio fischeri. Gram-positive bacteria often use small peptides as the QS signals [18,19]. In all QS systems, signals are produced intracellularly and transported to the extracellular environment. The smaller AHLs diffuse freely across bacterial cell membranes [20] while peptides and large AHLs appear to be actively transported by pumps [19,21,22]. These signals are detected by different strategies; AHL signals often lead to activation of cytoplasmic regulator proteins such as LuxR in V. fischeri [17,20], which then activates target gene expression. Peptide signals and also some AHLs, are typically sensed by membrane-associated receptors to initiate a phosphorylation cascade that leads to target gene expression [18,19]. The list of target genes under QS control is diverse, such as bioluminescence in Vibrio harveyi [23], competence regulation in Streptococcus pneumoniae [24], exoenzyme secretion in P. aeruginosa and other plant pathogens [25,26], conjugation in Agrobacterium tumefaciencs [27] and virulence in Staphylococcus aureus [28].
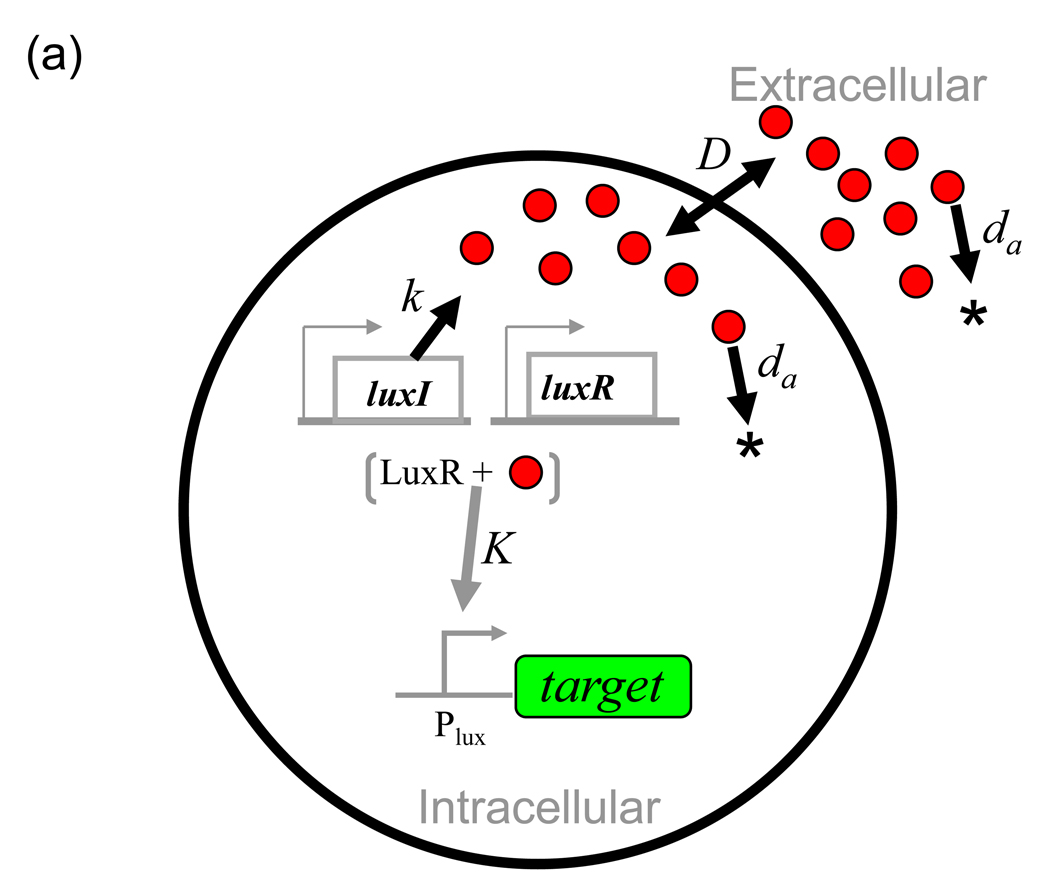
To date, most communication-based synthetic circuits have exploited bacterial QS especially those from Gram-negative bacteria. These systems are tremendously diverse in terms of their sensory components, the biochemical and transport properties of signaling molecules, and the genes and functions that are controlled in a density-dependent manner. We illustrate their general functioning with a minimal motif comprised of signal synthesis, secretion, degradation and detection elements (Fig. 1a), and use the lux system of V. fischeri as a canonical example. At low cell density, the AHL concentration is low both inside and outside of the cells. As cell density increases, the local AHL concentration increases. Within the cells, the cytoplasmic transcription factor LuxR recognizes AHL and activates gene expression of the well-characterized luxI promoter (PluxI) [29]. Therefore, the expression of PluxI is correlated to local population density through the production and detection of the AHL signal molecule.
Fig. 1. Cell-cell communication and its properties.
Single black arrows indicate reactions, double headed arrows indicate diffusion, grey arrows indicate activation and grey blunt arrows indicate inhibition.
(a) A minimal QS module.
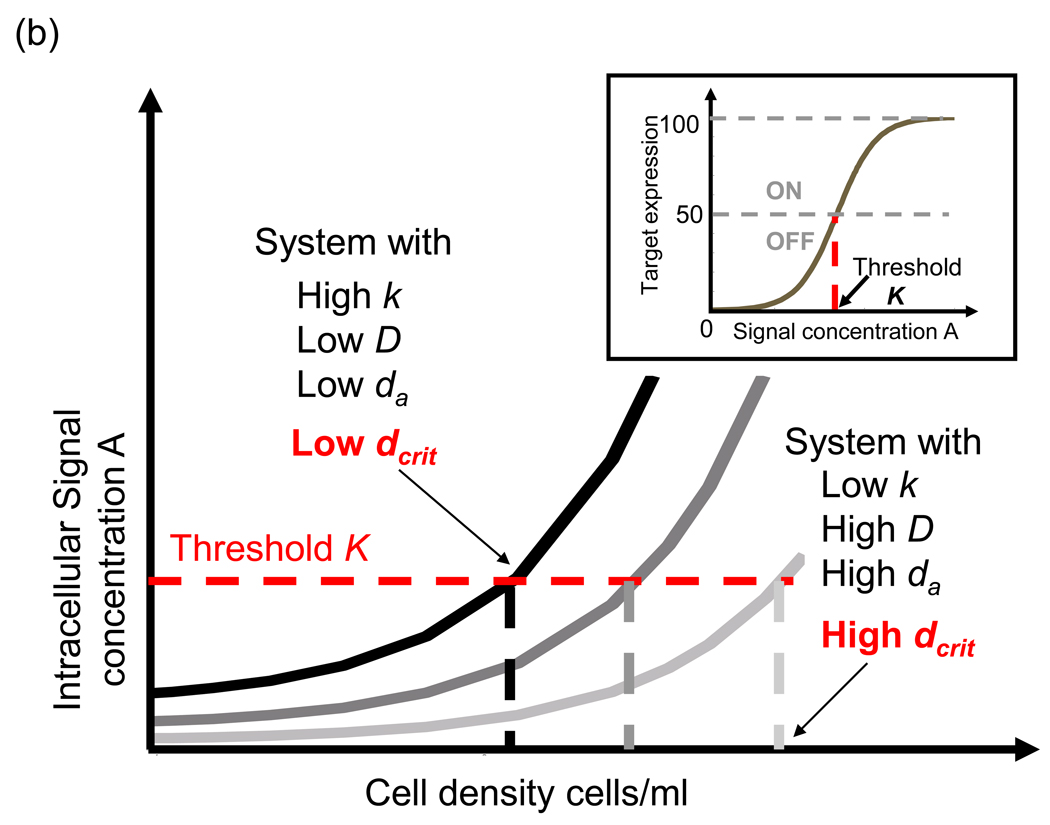
(b) Target expression versus cell density. Each curve represents the action of cell-cell communication as in (a) but with different parameters. K refers to the threshold signal concentration leading to 50% target expression (inset). The effect of parameters is indicated. Vertical stippled lines for each case marks the critical density at which signal concentration exceeds K. Note that increasing k, decreasing D and da lower the critical density (lower dcrit) for target expression. Lowering K (horizontal stippled line) decreases dcrit too.
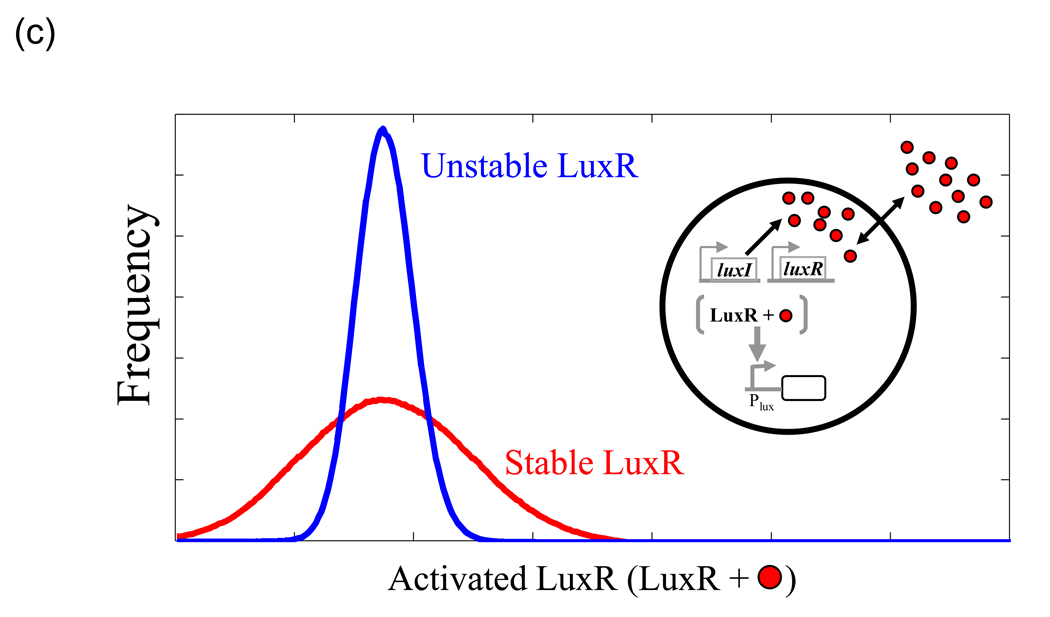
(c) Noise reduction by quorum sensing. Histogram of activated LuxR for unstable (blue) and stable (red) LuxR. Y axis shows the frequency with which corresponding number of activated LuxR molecules on the×axis is observed over time. Each histogram is generated from a time course simulation of the minimal QS system (inset). Parameters are chosen so that the mean number of activated LuxR is the same in either case. Unstable LuxR reduces noise, resulting in a tighter control on the number of activated LuxR molecules than in the case without diffusion. See Tanouchi et al. [34] for details.
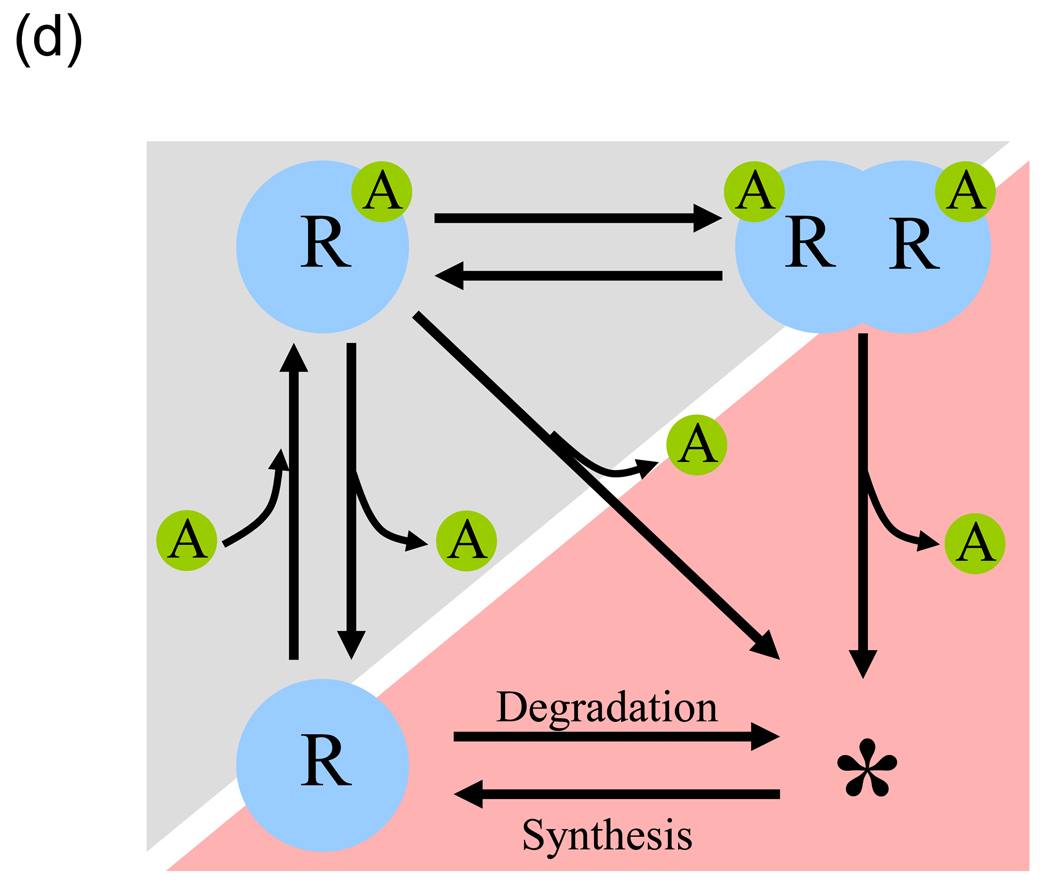
(d) Kinetic proofreading in QS signal recognition. Sequential reactions involving signal (A) binding to an R-protein (R) and dimerization of R-A complex constitute a mechanism analogous to the canonical Hopfield-Ninio model of kinetic proofreading (gray shade). Stabilization of R by A provides another layer of kinetic proofreading especially when R is unstable (red shade). Signal A could be cognate or non-cognate, the difference lying in the reaction rates of the steps shown. Redrawn from ref. [36].
We define a dcrit as the critical cell density at which a threshold concentration (K) of signal is reached (Fig. 1b), where K is commonly defined as the signal concentration at which target gene expression is half maximal (Fig. 1b inset). This dcrit acts as a simple measure of the module’s characteristics and can be derived in terms of the basic QS parameters: signal synthesis, secretion, degradation and detection [30]. Increasing signal synthesis while decreasing signal diffusion, degradation and threshold, decrease dcrit. That is, doing any of this will lower the density at which the population activates (Fig. 1b). We can also use an alternative perspective of a single cell and define a metric ‘sensing potential’ (v) that, instead of a critical density, considers the microenvironment size Ve that results in the activation of an individual confined cell [30]. Mathematically, where Vc is the cell volume and Ve the critical microenvironment size for activation. v is equivalent to dcrit as density can be interpreted in terms of average volume available to each cell giving v = 1/(dcritVc). However, v allows a consistent connection between an individual QS cell’s activation and its population-level phenotype and is, in that sense, more general.
The metric and equations show that if signal synthesis is too high compared to its rate of diffusion, then signal accumulation within the cells can result in the target gene expression being ON (above half-maximal) regardless of cell density [30]. When this ‘self-activation’ occurs, the role of communication-based activation is reduced (in terms of increase in fold target induction from low to high cell density).
Other variations in QS modules, such as when the extracellular signal is sensed by cell surface receptors [9,19], when there is positive feedback on signal synthesis [31], or when signal molecules are actively transported by pumps rather than by passive diffusion [22], can similarly be accounted for and in each case, the relationships between dcrit and the system parameters can be derived [30].
A salient characteristic of many QS modules is the stabilization of ‘LuxR’ homologs (R-protein) upon AHL binding [32,33]. As an example, the R-protein TraR from A. tumefaciens is extremely unstable with a half-life of 2–3 min and binding its cognate signal increases its half-life by 25-fold [32]. This instability appears to be wasteful as cells would have to synthesize it with a higher rate to sustain R-protein levels. Recent theoretical studies indicate at least two potential roles for R-protein instability. First, an unstable R-protein could reduce variability in QS circuit behavior [34] (Fig. 1c). Gene expression is intrinsically noisy, primarily due to small numbers of interacting molecules within cells and fluctuations in environmental conditions [35]. The fast turnover of R-proteins together with AHL diffusion across cell membrane is predicted to reduce variability in QS-mediated target gene expression, via a mechanism termed “diffusional dissipation” [34].
A second potential role for R-protein instability is signal discrimination [36]. In nature, diverse AHL-dependent QS systems exist within and among different species of bacteria. While R-proteins generally have a strong preference for their native AHL signal, at high concentrations they can bind non-cognate AHLs and induce gene expression, a phenomenon typically termed “crosstalk”. This implies that bacteria would need some mechanism to differentiate between their cognate and other non-cognate AHL signals to minimize crosstalk. One way to do this is to have asymmetry between the binding reactions of the two to the R-protein. An asymmetry could also exist in the subsequent dimerization of their R-protein-AHL complex [37,38]. This sequence of reactions constitutes a mechanism analogous to the canonical Hopfield-Ninio model of kinetic proofreading [39,40] (Fig. 1d). Smith et al. [36] proposed that asymmetry in another step, stabilization of the R-protein by signal binding, adds an additional layer to the kinetic proofreading. That is, given the asymmetry in binding reactions, signal crosstalk can be further reduced when the cognate signal stabilizes R-protein to a higher degree than the non-cognate signal. Importantly, the effect of this asymmetry in R-protein stabilization is amplified if the R-protein is unstable. Although these features of R-protein dynamics remain to be tested experimentally, these theoretical studies [34,36] have shed light on possible consequences of R-protein instability.
By themselves, the coordination, noise reduction and signal discrimination properties of communication could be useful in synthetic circuits. Koseska et al. [41] modeled theoretical populations where cellular decision making is governed by multi-stable genetic switches. These switches are particularly susceptible to noise induced fluctuations, causing random jumps between alternative cellular states. The study shows that cell-cell communication in a population allows reliable synchronization of decision making among individuals, even in the presence of noise. Similarly, the synthetic gene circuit from Elowitz and Leibler [42] (the ‘repressilator’) effectively generates oscillations but these are highly variable and show rapid loss of synchrony between cells. Computational studies indicate that cell-cell communication could synchronize the oscillations and make them more robust to perturbations [43,44]. This communication induced oscillation synchrony remains to be demonstrated experimentally. However, robust oscillations using cell-cell communication circuits have been demonstrated in a microchemostat [45] using a version of the population controller (described below) [46].
3. Engineering the communication toolkit
Several well characterized natural communication modules [12] are now widely used in synthetic circuit design. However, a large pool of natural communication modules still remain to be explored, such as the oligopeptide-based QS systems in Gram-positive bacteria [19]. The autoinducer-2 (AI-2) QS signal [47,48] can act as an intra-species [49,50] or inter-species signal [51] and has only recently been incorporated into synthetic circuits [52].
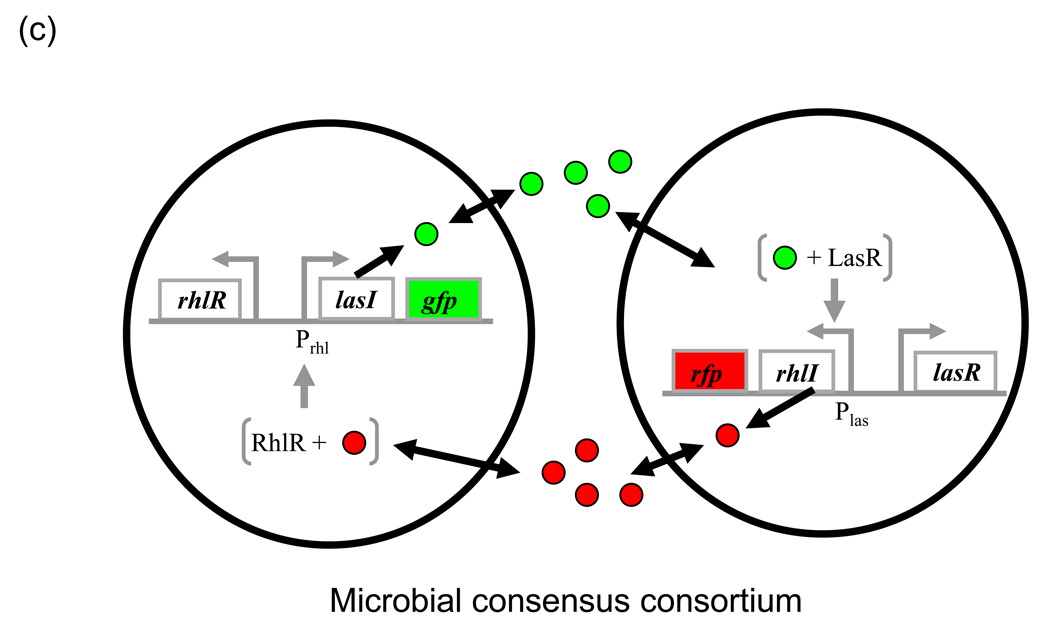
Engineering these circuits involves the ad hoc choice of different QS elements and their optimization, depending on considerations such as “cross-talk”, and critical activation density (dcrit). As an example, consider the design of a microbial consensus consortium (MCC) by Brenner et al. [53] (Fig. 3c). Here, the lasRI and rhlRI systems from P. aeruginosa were used for bidirectional communication between two populations. These two systems did show low-level crosstalk but the authors used detailed circuit modeling to arrive at an architecture that maximized the consensus population response while minimizing any response due to crosstalk. Another strategy is to specifically engineer the R-protein for desired properties. Collins et al. [33] used a dual selection strategy to engineer LuxR mutants that respond to different AHLs with varying specificity. LuxR variants with increased affinity for a broad range of AHLs were also generated [54]. Such strategies provide a method to engineer parts that possess or lack a specific response to a signal. One of the engineered LuxR mutants was instrumental in the development of a ‘band-pass’ biological circuit [55]. Modulating the R-protein degradation rate without compromising binding characteristics could also improve signal discrimination [36].
Fig. 3. Synthetic systems with communication between populations.
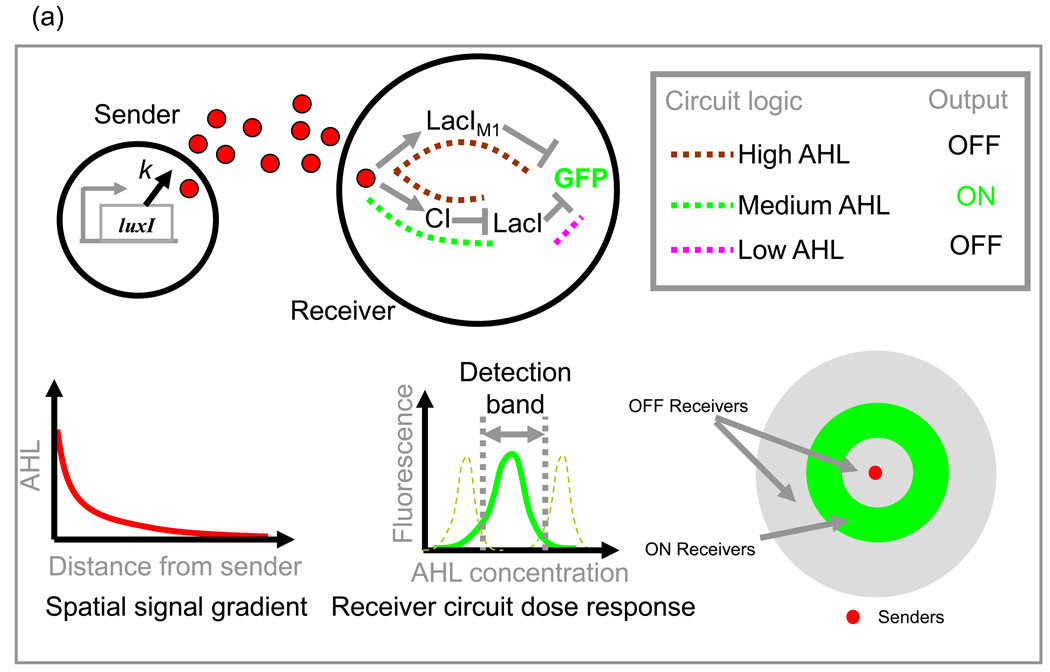
(a) Pattern formation using signal senders and receivers (top left) with spatial signal gradient around senders (Bottom left). Receivers (top center) are programmed to express fluorescence (green curve) in a narrow range of signal concentrations (bottom center). The positioning of the detection band can be changed (dotted green) by manipulating the receiver circuit elements. Active parts of receiver circuit logic at different AHL concentrations are shown (Top right). In a circular domain with senders in the middle and receivers everywhere on the surface of the circle, a ring like pattern will emerge. Redrawn from ref. [55]
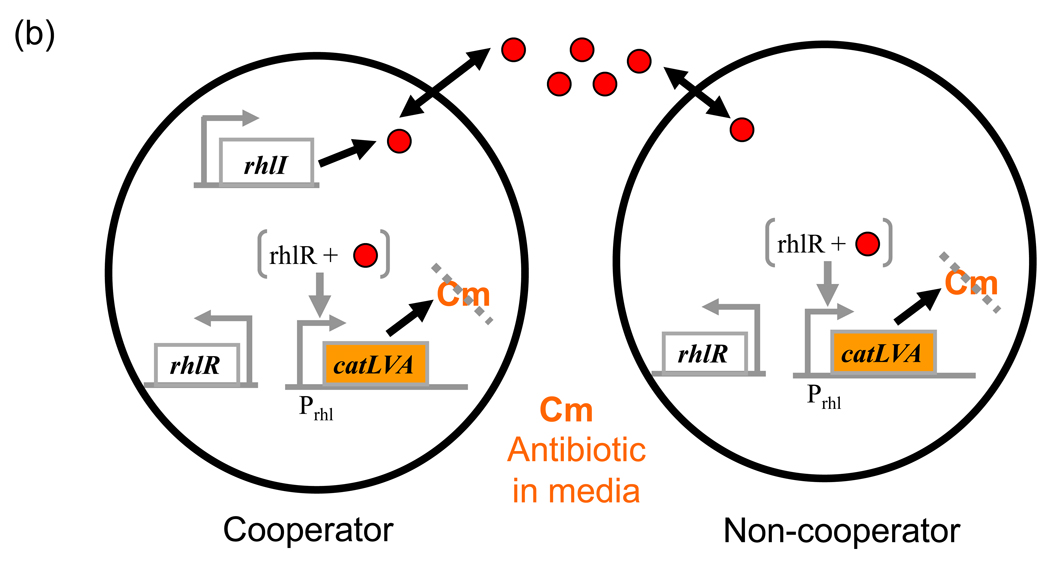
(b) Synthetic cooperative system. Cooperators constitutively synthesize the AHL signal C4HSL via RhlI. Non-cooperators do not express RhlI. Both populations constitutively express RhlR which, when signal-bound, induces the expression of a chloramphenicol resistance gene (catLVA). Redrawn from ref. [76]
(c) Microbial consensus consortium (MCC). LasI and RhlI catalyze the synthesis of AHLs, 3OC12HSL (red circles) and C4HSL (green circles), which activate LasR and RhlR, respectively. The activated regulators then activate their target reporters, RFP and GFP respectively. Both populations need to be at sufficient density (not equal) for concurrent expression of both reporters. Redrawn from ref. [53]
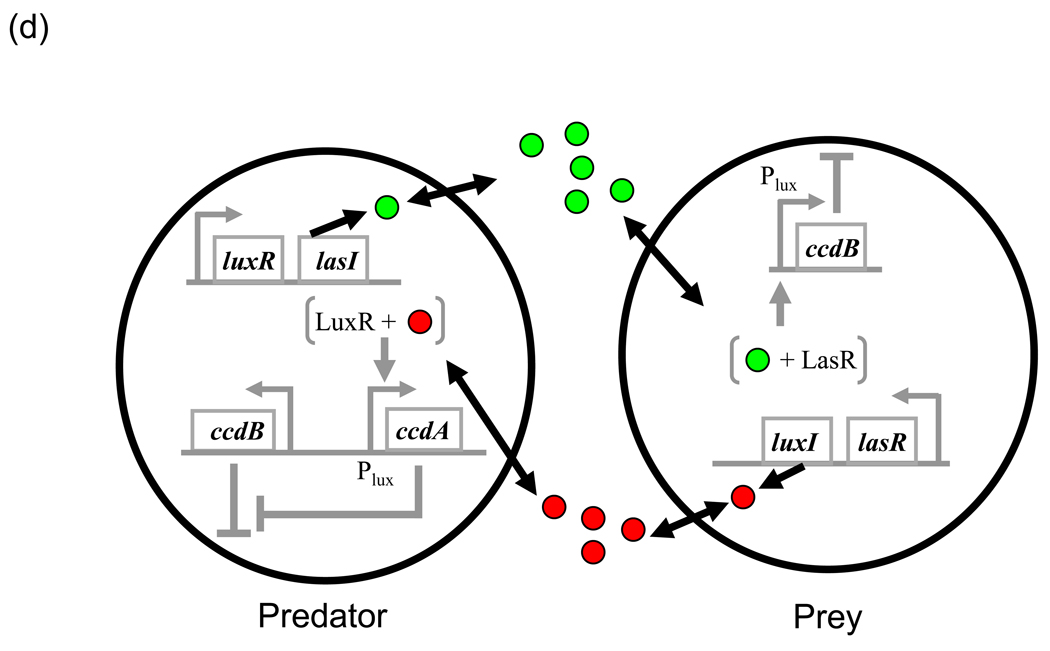
(d) Synthetic predator-prey system. Predator and prey synthesize 3OC12HSL (green circles) and 3OC6HSL (red circles) via LasI and LuxI, respectively. Toxin, CcdB, is produced constitutively in the predator but is under PluxI control in the prey. In the predator, antidote CcdA is under PluxI control. 3OC12HSL-bound LasR does induce expression from PluxI. The predator requires sufficient number of prey cells to sustain enough expression of CcdA to survive. On the other hand, the prey dies due to CcdB expression when the density of predator is high. Redrawn from ref. [80]
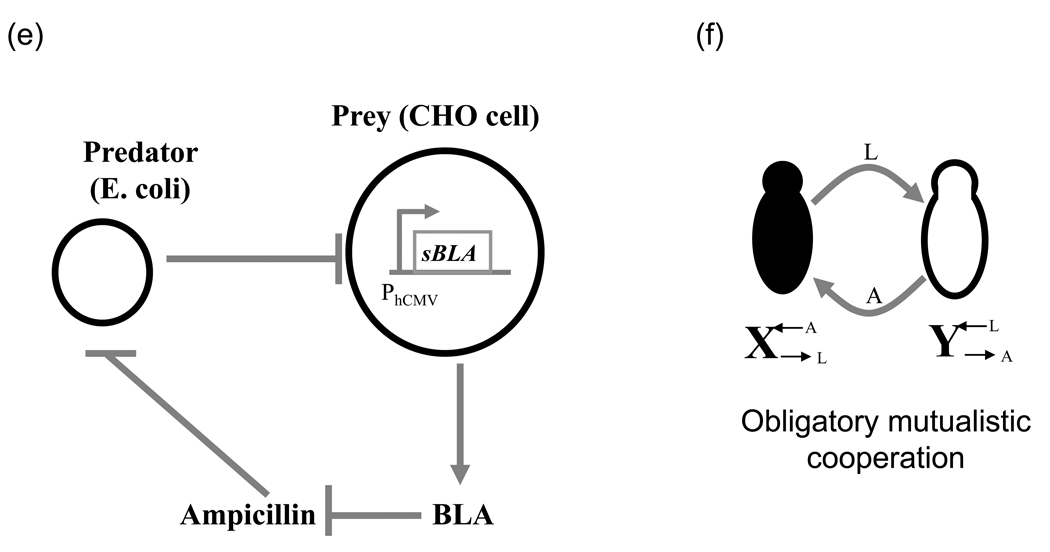
(e) Another design of synthetic predator-prey system. The system consists of wild-type E. coli cells (predator) and Chinese hamster ovary cells (prey) in a medium containing ampicillin. Rapid growth of the predator limits the prey’s growth by depleting nutrients. Prey constitutively expresses BLA that degrades ampicillin. Redrawn from ref. [82]
(e) A synthetic obligatory cooperation circuit with two mutually dependent yeast populations. indicates yeast cell that overproduces lysine but requires external adenine. overproduces adenine but requires external lysine supply. Redrawn from ref. [83]
Analysis of the minimal QS motif can guide the modulation of dcrit, which can be decreased by increasing signal production, decreasing signal degradation, transport or the threshold for activation (Fig. 1b). Signal production can be modulated by controlling the induction of its synthase gene. Signal degradation can be modulated by controlling the pH of the culture medium [46,56] while the threshold can be changed by using different R-protein variants [33,54,57] or by changing the signal inducible promoter (PluxI) region. The dynamic properties of the individual elements themselves, such as the signal synthesis rate from a synthase, can also be systematically engineered. Kambam et al. [58,59] have used directed evolution to generate LuxI and RhlI mutants that display increased signal production rate compared to wild-type AHL synthases.
Existing cellular modules can also be co-opted to realize communication. Bulter et al. [13] reengineered carbon metabolism in Escherichia coli such that acetate, a commonly secreted metabolite, acts as a density dependent signal and parts of the nitrogen starvation regulon act as detectors to achieve cell-cell communication. Chen and Weiss [60] created a QS circuit in yeast by using a signal (IP, a cytokinin) and receptor (AtCRE1) from Arabidopsis thaliana (Fig. 2d), demonstrating that communication circuits can also be generated by mixing and matching components from different natural systems.
Fig. 2. Synthetic systems with communication within a population.
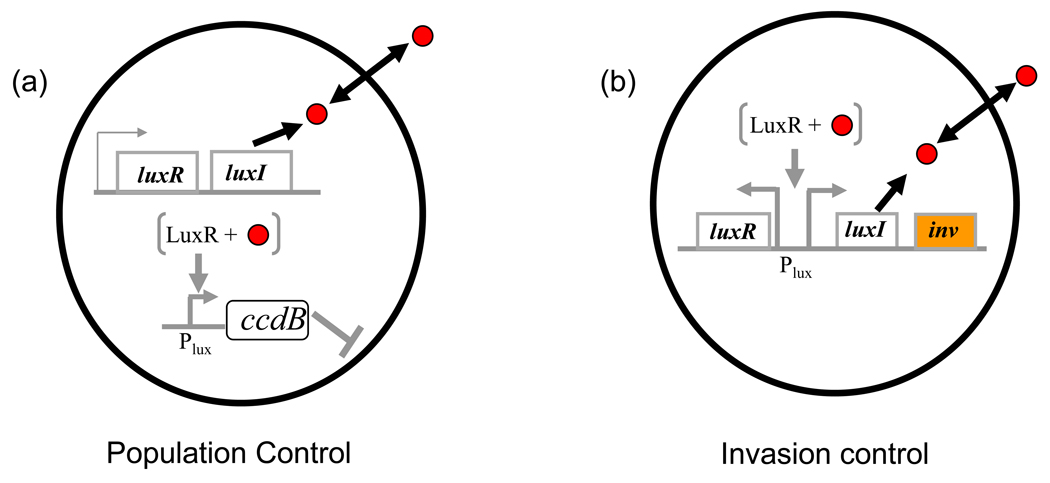
(a) A population controller. The circuit uses the schematic in Fig.1a but here the signal-bound LuxR drives expression of a toxin CcdB. Redrawn from ref. [46]
(b) Density dependent invasion circuit. The circuit architecture differs slightly from (a) with the invasin gene (inv) expression controlled by communication. Redrawn from ref. [65]
4. Communication systems and their applications
Natural QS systems are typically much more complex than illustrated in Fig. 1a and much effort has been put into dissecting their dynamics [61]. These studies reveal the substantial complexity in commonly used QS modules. An alternative, therefore, is to examine QS dynamics in well-defined synthetic gene circuits with QS components. Haseltine and Arnold [62] examined the luxRI system regulation characteristics by rearranging the individual components of the system. They observed that target function activation could be either graded (linearly increasing with cell density), threshold (all or none above or below a critical cell density) or bistable (have a different threshold depending on initial conditions), depending on the arrangement.
In single populations, by using different target functions under QS control, interesting circuits have been illustrated [63,64]. A population controller [46] uses the luxRI system to achieve autonomous control of E. coli density. Here, a toxic protein, CcdB, is placed under the control of PluxI, conferring a density-dependent increase in CcdB expression (Fig. 2a). The result is an increase in growth inhibition as cell density increases, leading to a controllable steady state cell density. Analogous to dcrit (Fig. 1c), this steady state can be modulated by changing the communication module parameters such as signal degradation rate [46]. The invasin circuit [65] is based on the observation that many bacterial species, including E. coli, have been observed to localize to tumors at high densities following intravenous injection [66]. Anderson et al. [65] surmised that this localization could act as a feature to identify tumors and invade them with engineered E. coli. Their design uses the luxRI system to control the invasin gene that enables E. coli to invade mammalian cells (Fig. 2b). In vitro studies showed that invasion of cancer-derived cells by E. coli carrying the circuit took place only when the inoculated E. coli were at high density. While the study does not directly contrast the cell-density based tumor invasion with other localization cues such as hypoxia, it demonstrates that autonomous communication based regulation could be useful in therapeutic applications.
A single cell-cell communication system can be used to design multiple communicating species by placing the signal synthesis and response elements into different cells, generating a sender and receiver respectively. This strategy was used in the design of a biological ‘pulse’ generator [67] and a ‘band-pass’ filter [55] in E. coli. In the latter study, sender cells express luxI and synthesize the diffusible AHL signal while receiver cells contain a circuit with the response elements luxR, promoter PluxI, and repressors CI, LacI and LacIM1 (a LacI mutant) (Fig. 3a). The receiver circuit logic allows for GFP expression only when the AHL concentration is within a narrow range. On a solid surface, the diffusing signals form a spatial concentration gradient around sender cells. The distance of receivers from senders determines the signal concentration they encounter and hence also their response. The result is fascinating spatial patterns depending on the arrangement of senders and receivers. The authors combined experimental observations with simulations of pattern formation to study the roles of circuit parameters. The decay rate of LacI emerged as the major determinant of the time to pattern formation as well as any shift of the pattern itself. Signal production and sensing can be combined with other synthetic genetic parts in new and interesting ways as demonstrated recently by the construction of a biological ‘edge detector’ circuit [68]. Here, signal production in the circuit was tied to a light sensor and a genetic ‘inverter’ circuit to generate a variant of an AND gate. This logic programmed the cells to produce a pigment (through LacZ expression) only at the edge of light and dark regions. Similar studies on artificial pattern formation systems could aid investigations [69,70] into natural pattern formation questions, such as the surprising robustness [71] in morphogen gradient-based patterning observed in Drosophila embryos despite large variations in the gradient profiles in individual embryos. Similarly, the patterns formed by swarming microbes [72,73] could be investigated by using circuits that tie the motility of cells with communication [74,75]. Overall, we see that signal production and its detection - as separately controlled parts-combined with the diffusion properties of the signal and other genetic parts can yield numerous applications.
The strategy of separating signal synthesis and signal detection was also used by Chuang et al. [76] to create a heterogeneous population of cooperating and non-cooperating cells (Fig. 3b). In cooperators, the rhlRI system controls the induction of an antibiotic resistance gene, the corresponding antibiotic of which is present in the media. Non-cooperators carry the same circuit except for the rhlI gene. In the combined population, the AHL produced by the cooperators acts as public goods for non-cooperators to survive as well. The authors considered a scenario where the overall population consists of subpopulations of different sizes with varying ratios of cooperators to non- cooperators. Within any subpopulation, non-cooperators benefit from public goods without paying any cost of its synthesis and thus grow faster than cooperators. Despite this, in some cases, the overall proportion of producers in the overall population can actually increase with time; a paradoxical statistical effect known as Simpson’s paradox. A large variance between the initial subpopulation compositions is required for this effect to happen. The study shows that such large variance can be simply generated by large dilutions of the subpopulations resulting in stochastic fluctuations in compositions among them. Overall, the study provides a simple scenario where a trait beneficial to the population can emerge despite it not being favorable at the individual level. This design also offers the ability to tune various aspects of cooperation, such as changing cost and benefit by replacing the antibiotic resistance with another costly but beneficial system, and could be used to study other aspects such as the conditions for cooperation and its stability [77]. In self-destructive cooperation, cooperators die to benefit the remaining members of their population [78]. To address this, the same circuit could be modified to force the cooperators to commit suicide [46].
Multiple communicating populations can also be created by using two or more communication systems. In the two-population MCC system [53] (Fig. 3c), each population expresses its reporter only when the other is present at sufficiently high density, resulting is a population-level AND gate. The system presents the possibility of dividing a complex task among different microbial populations (division of labor) which coordinate in its execution [79]. Balagadde et al. [80] used the luxRI and lasRI systems in combination with a toxin and an anti-toxin to generate predator and prey populations (Fig. 3d). Depending on the operating conditions, the predator-prey populations display diverse ecological consequences such as species extinction, coexistence, as well as oscillations that can be predicted by mathematical modeling. By modulating growth rate, death rate and the strength of cell–cell communication, their effects on the interaction consequences can be studied. The same circuits have also been used to explore the maintenance of biodiversity in chemically-mediated ecosystems (H. Song et al., unpublished). Synthetic communication circuits can thus be convenient tools to study biological questions [81] such as various aspects of cooperation (as seen in the previous paragraph) as well as species interactions.
Lastly, two or more populations can also be linked via an essential chemical (instead of an explicit signal). Weber et al. [82] used this approach to engineer inter- and intra-kingdom interactions with a variety of behaviors: predator-prey, commensalism, amensalism, parasitism and cooperation. In the predator-prey case, wild-type E. coli (predator) and Chinese hamster ovary (CHO) cells (prey) that constitutively express the ampicillin-degrading enzyme β-lactamase were used (Fig. 3e). Growth of the CHO cells is inhibited by rapid growth of E. coli in the same environment. In turn, E. coli cells, which are sensitive to ampicillin, require sufficient number of CHO cells to sustain their growth in a medium with ampicillin. Shou et al. [83] similarly co-opted an essential chemical requirement to engineer cooperation between two yeast populations (Fig. 3f). Here, one strain requires the external supply of adenine and the other lysine. The adenine-requiring strain overproduces lysine and the lysine-requiring strain overproduces adenine so each can supply the essential metabolite for the other. The study points out that factors such as starvation tolerance, nutrient release timing and the initial population ratios of the two strains can restrict cooperation. Importantly, it also observes that the mutualistic and obligatory population is stable to sudden population reductions. The approach of linking individual cells via a diffusible chemical was also recently used to generate tunable band-pass circuits causing spatial patterns [84,85]. Here, the ‘band’ was defined by a zone of appropriate antibiotic concentration in which cells could grow while the position and width of the band could be externally tuned using ampicillin and tetracycline. Thus, just as was seen with communication based circuits earlier, these circuits that are linked via an essential chemical rather than an explicit signal, can similarly be used to study various biological questions.
Conclusions and Prospects
An impressive number of multicellular systems have been built in the past few years using natural or synthetic communication modules. However, a large number of natural communication systems, particularly non-bacterial ones, remain to be explored and adopted into the synthetic biology framework. We have listed several of their applications, stretching from therapeutics to replicating social behavior. Many other uses will emerge from tying communication to the power of synthetic biology and metabolic engineering. Autonomous density sensing can be coupled with motility control to realize bio-computation in the spatial domain [86]. Tasks that involve many reaction steps such as breaking down a complex substance in bioremediation [87] could be divided into several interacting populations that perform it under a division of labor [5,15]. Different microbes possess their own unique abilities and it may be easier to make them communicate and coordinate on a task rather than force the required gene sets from each into a single organism. The autonomous population control possible by communication (Fig. 3) can be combined with metabolic engineering to optimize the growth of circuit carrying microbes in bioreactors [5,88]. Engineering multicellular behavior thus displays a compelling range of applications.
Acknowledgments
L.Y acknowledges generous research support by the National Institutes of Health, the National Science Foundation, the DuPont Company, and the Packard Foundation. Y. T. acknowledges the James McElhaney fellowship from the Department of Biomedical Engineering at Duke University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Voigt CA. Genetic parts to program bacteria. Curr Opin Biotechnol. 2006;17:548–557. doi: 10.1016/j.copbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Lucks JB, Qi L, Whitaker WR, Arkin AP. Toward scalable parts families for predictable design of biological circuits. Curr Opin Microbiol. 2008;11:567–573. doi: 10.1016/j.mib.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Chin JW. Programming and engineering biological networks. Curr Opin Struct Biol. 2006;16:551–556. doi: 10.1016/j.sbi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Haseltine EL, Arnold FH. Synthetic gene circuits: design with directed evolution. Annu Rev Biophys Biomol Struct. 2007;36:1–19. doi: 10.1146/annurev.biophys.36.040306.132600. [DOI] [PubMed] [Google Scholar]
- 5.Marguet P, Balagadde F, Tan C, You L. Biology by design: reduction and synthesis of cellular components and behaviour. J R Soc Interface. 2007;4:607–623. doi: 10.1098/rsif.2006.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprinzak D, Elowitz MB. Reconstruction of genetic circuits. Nature. 2005;438:443–448. doi: 10.1038/nature04335. [DOI] [PubMed] [Google Scholar]
- 7.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- 9.Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 11.Fuqua C, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 12.Hooshangi S, Bentley WE. From unicellular properties to multicellular behavior: bacteria quorum sensing circuitry and applications. Curr Opin Biotechnol. 2008;19:550–555. doi: 10.1016/j.copbio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Bulter T, Lee SG, Wong WW, Fung E, Connor MR, Liao JC. Design of artificial cell-cell communication using gene and metabolic networks. Proc Natl Acad Sci U S A. 2004;101:2299–2304. doi: 10.1073/pnas.0306484101.An example of how metabolic pathways and metabolites may be co-opted to serve as a communication system. The acetate production pathway was rewired along with the nitrogen starvation regulon to build a QS system.
- 14.Brenner K, You L, Arnold FH. Response to Goldman and Brown: Making sense of microbial consortia using ecology and evolution. Trends Biotechnol. 2009;27:4. doi: 10.1016/j.tibtech.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Brenner K, You L, Arnold FH. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008;26:483–489. doi: 10.1016/j.tibtech.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Williams P, Winzer K, Chan WC, Camara M. Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazdunski AM, Ventre I, Sturgis JN. Regulatory circuits and communication in Gram-negative bacteria. Nat Rev Microbiol. 2004;2:581–592. doi: 10.1038/nrmicro924. [DOI] [PubMed] [Google Scholar]
- 18.Kleerebezem M, Quadri LE, Kuipers OP, de Vos WM. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Grampositive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 19.Sturme MH, Kleerebezem M, Nakayama J, Akkermans AD, Vaugha EE, de Vos WM. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek. 2002;81:233–243. doi: 10.1023/a:1020522919555. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan HB, Greenberg EP. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyon GJ, Novick RP. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides. 2004;25:1389–1403. doi: 10.1016/j.peptides.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomasz A. Control of the competent state in Pneumococcus by a hormone-like cell product: an example for a new type of regulatory mechanism in bacteria. Nature. 1965;208:155–159. doi: 10.1038/208155a0. [DOI] [PubMed] [Google Scholar]
- 25.Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6:56–60. doi: 10.1016/s1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 26.Von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003;41:455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- 27.Fuqua WC, Winans SC. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winzer K, Williams P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int J Med Microbiol. 2001;291:131–143. doi: 10.1078/1438-4221-00110. [DOI] [PubMed] [Google Scholar]
- 29.Egland KA, Greenberg EP. Quorum sensing in Vibrio fischeri: elements of the luxl promoter. Mol Microbiol. 1999;31:1197–1204. doi: 10.1046/j.1365-2958.1999.01261.x. [DOI] [PubMed] [Google Scholar]
- 30.Pai A, You L. Optimal tuning of bacterial sensing potential. Mol Syst Biol. 2009;5:286. doi: 10.1038/msb.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engebrecht J, Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 1987;15:10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Winans SC. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci U S A. 2001;98:1507–1512. doi: 10.1073/pnas.98.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins CH, Leadbetter JR, Arnold FH. Dual selection enhances the signaling specificity of a variant of the quorum-sensing transcriptional activator LuxR. Nat Biotechnol. 2006;24:708–712. doi: 10.1038/nbt1209.Describes the use of a positive-negative selection strategy to create a LuxR variant that is sensitive to AHLs other than it’s native one. Together with ref. [54] this demonstrates the engineering of regulator proteins for desired response characteristics.
- 34.Tanouchi Y, Tu D, Kim J, You L. Noise reduction by diffusional dissipation in a minimal quorum sensing motif. PLoS Comput Biol. 2008;4:e1000167. doi: 10.1371/journal.pcbi.1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaern M, Elston TC, Blake WJ, Collins JJ. Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet. 2005;6:451–464. doi: 10.1038/nrg1615. [DOI] [PubMed] [Google Scholar]
- 36.Smith C, Song H, You L. Signal discrimination by differential regulation of protein stability in quorum sensing. J Mol Biol. 2008;382:1290–1297. doi: 10.1016/j.jmb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi SH. Genetic evidence for multimerization of LuxR, the transcriptional activator of Vibrio fischeri luminescence. Molecular marine biology and biotechnology. 1992;1:408. [Google Scholar]
- 38.Qin Y, Luo ZQ, Smyth AJ, Gao P, Beck von Bodman S, Farrand SK. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. Embo J. 2000;19:5212–5221. doi: 10.1093/emboj/19.19.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopfield JJ. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974;71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57:587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- 41.Koseska A, Zaikin A, Kurths J, Garcia-Ojalvo J. Timing cellular decision making under noise via cell-cell communication. PLoS ONE. 2009;4:e4872. doi: 10.1371/journal.pone.0004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Ojalvo J, Elowitz MB, Strogatz SH. Modeling a synthetic multicellular clock: repressilators coupled by quorum sensing. Proc Natl Acad Sci USA. 2004;101:10955–10960. doi: 10.1073/pnas.0307095101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMillen D, Kopell N, Hasty J, Collins JJ. Synchronizing genetic relaxation oscillators by intercell signaling. Proc Natl Acad Sci USA. 2002;99:679–684. doi: 10.1073/pnas.022642299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balagaddé FK, You L, Hansen CL, Arnold FH, Quake SR. Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science. 2005;309:137–140. doi: 10.1126/science.1109173. [DOI] [PubMed] [Google Scholar]
- 46.You L, Cox RS, 3rd, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing. Nature. 2004;428:868–871. doi: 10.1038/nature02491.One of the earliest synthetic circuits to use cell-cell communication to control cellular action. The expression of toxin ccdB under luxRI QS control provided for density-dependent programming of cell death.
- 47.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 48.Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 49.Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 51.Federle MJ, Bassler BL. Interspecies communication in bacteria. J Clin Invest. 2003;112:1291–1299. doi: 10.1172/JCI20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandes R, Bentley WE. AI-2 biosynthesis module in a magnetic nanofactory alters bacterial response via localized synthesis and delivery. Biotechnol Bioeng. 2009;102:390–399. doi: 10.1002/bit.22078. [DOI] [PubMed] [Google Scholar]
- 53.Brenner K, Karig DK, Weiss R, Arnold FH. Engineered bidirectional communication mediates a consensus in a microbial biofilm consortium. Proc Natl Acad Sci U S A. 2007;104:17300–17304. doi: 10.1073/pnas.0704256104.Uses two QS systems from P. aeruginosa to create two populations that give a consensus response only when both are present and at sufficient density. The design strategy involved modeling of circuit dynamics and using this information to redesign circuit architecture to minimize the effect of crosstalk between the QS systems.
- 54.Collins CH, Arnold FH, Leadbetter JR. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol Microbiol. 2005;55:712–723. doi: 10.1111/j.1365-2958.2004.04437.x. [DOI] [PubMed] [Google Scholar]
- 55.Basu S, Gerchman Y, Collins CH, Arnold FH, Weiss R. A synthetic multicellular system for programmed pattern formation. Nature. 2005;434:1130–1134. doi: 10.1038/nature03461.A band-pass biological filter was designed for receiver cells to respond to a narrow range of signal concentrations. Spatially separating signal sender and receiver cells results in fascinating two dimensional patterns on an agar surface. Pattern formation under this circuit was mathematically modeled and provides insights into parameters that affect pattern formation characteristics.
- 56.Wang YJ, Huang JJ, Leadbetter JR. Acyl-HSL signal decay: intrinsic to bacterial cell-cell communications. Adv Appl Microbiol. 2007;61:27–58. doi: 10.1016/S0065-2164(06)61002-2. [DOI] [PubMed] [Google Scholar]
- 57.Sayut DJ, Niu Y, Sun L. Construction and engineering of positive feedback loops. ACS Chem Biol. 2006;1:692–696. doi: 10.1021/cb6004245. [DOI] [PubMed] [Google Scholar]
- 58.Kambam PK, Eriksen DT, Lajoie J, Sayut DJ, Sun L. Altering the substrate specificity of RhlI by directed evolution. Chembiochem. 2009;10:553–558. doi: 10.1002/cbic.200800636. [DOI] [PubMed] [Google Scholar]
- 59.Kambam PK, Sayut DJ, Niu Y, Eriksen DT, Sun L. Directed evolution of LuxI for enhanced OHHL production. Biotechnol Bioeng. 2008;101:263–272. doi: 10.1002/bit.21901. [DOI] [PubMed] [Google Scholar]
- 60.Chen MT, Weiss R. Artificial cell-cell communication in yeast Saccharomyces cerevisiae using signaling elements from Arabidopsis thaliana. Nat Biotechnol. 2005;23:1551–1555. doi: 10.1038/nbt1162.Demonstrates the use of mixed signaling components from yeast and Arabidopsis thaliana to generate a QS system in yeast.
- 61.Williams JW, Cui X, Levchenko A, Stevens AM. Robust and sensitive control of a quorum-sensing circuit by two interlocked feedback loops. Mol Syst Biol. 2008;4:234. doi: 10.1038/msb.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haseltine EL, Arnold FH. Implications of rewiring bacterial quorum sensing. Appl Environ Microbiol. 2008;74:437–445. doi: 10.1128/AEM.01688-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss R, Knight TF., Jr . DNA Computing, 6th International Workshop on DNA-Based Computers, DNA 2000. Leiden, The Netherlands: Springer, Berlin; 2001. Engineered communications for microbial robotics. [Google Scholar]
- 64.Kobayashi H, Kaern M, Araki M, Chung K, Gardner TS, Cantor CR, Collins JJ. Programmable cells: interfacing natural and engineered gene networks. Proc Natl Acad Sci U S A. 2004;101:8414–8419. doi: 10.1073/pnas.0402940101.Modularity of the toggle switch as a synthetic circuit part was demonstrated by interfacing it with natural cellular networks to generate a programmed response. The switch combined with a cell’s SOS response pathway or with a (transgenic) QS circuit produced predictable and controllable gene expression.
- 65.Anderson JC, Clarke EJ, Arkin AP, Voigt CA. Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol. 2006;355:619–627. doi: 10.1016/j.jmb.2005.10.076.Environmental cues of tumors such as their ability to localize injected bacteria or their hypoxic environment were used to design E. coli with synthetic circuits programmed to invade cells based on these cues.
- 66.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, Goebel W, Szalay AA. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 67.Basu S, Mehreja R, Thiberge S, Chen MT, Weiss R. Spatiotemporal control of gene expression with pulse-generating networks. Proc Natl Acad Sci U S A. 2004;101:6355–6360. doi: 10.1073/pnas.0307571101.Receiver cells were designed that display a transient ‘pulse-like’ response to long-lasting AHL signals from signal cells placed at a distance. The authors also modulate the pulse duration and intensity. This work was extended to create the ‘band-pass’ circuit in ref [44].
- 68.Tabor JJ, Salis HM, Simpson ZB, Chevalier AA, Levskaya A, Marcotte EM, Voigt CA, Ellington AD. A synthetic genetic edge detection program. Cell. 2009;137:1272–1281. doi: 10.1016/j.cell.2009.04.048.An earlier synthetic 'dark sensor' circuit was used to control signal production and was combined cleverly with genetic logic gates and signal detection such that cells imprint the edge between light and dark regions on a solid surface.
- 69.Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature. 2005;434:1134–1138. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Isalan M, Lemerle C, Serrano L. Engineering gene networks to emulate Drosophila embryonic pattern formation. PLoS Biol. 2005;3:e64. doi: 10.1371/journal.pbio.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Houchmandzadeh B, Wieschaus E, Leibler S. Establishment of developmental precision and proportions in the early Drosophila embryo. Nature. 2002;415:798–802. doi: 10.1038/415798a. [DOI] [PubMed] [Google Scholar]
- 72.Brenner MP, Levitov LS, Budrene EO. Physical mechanisms for chemotactic pattern formation by bacteria. Biophys J. 1998;74:1677–1693. doi: 10.1016/S0006-3495(98)77880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Budrene EO, Berg HC. Dynamics of formation of symmetrical patterns by chemotactic bacteria. Nature. 1995;376:49–53. doi: 10.1038/376049a0. [DOI] [PubMed] [Google Scholar]
- 74.Weiss LE, Badalamenti JP, Weaver LJ, Tascone AR, Weiss PS, Richard TL, Cirino PC. Engineering motility as a phenotypic response to LuxI/R-dependent quorum sensing in Escherichia coli. Biotechnol Bioeng. 2008;100:1251–1255. doi: 10.1002/bit.21862. [DOI] [PubMed] [Google Scholar]
- 75.Topp S, Gallivan JP. Guiding bacteria with small molecules and RNA. J Am Chem Soc. 2007;129:6807–6811. doi: 10.1021/ja0692480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chuang JS, Rivoire O, Leibler S. Simpson's paradox in a synthetic microbial system. Science. 2009;323:272–275. doi: 10.1126/science.1166739.The authors rewire AHL signaling to cast the signal in the role of ‘public goods’. The result is a heterogeneous population of cooperators and non-cooperators using a single communication system.
- 77.Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314:1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ackermann M, Stecher B, Freed NE, Songhet P, Hardt WD, Doebeli M. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. doi: 10.1038/nature07067. [DOI] [PubMed] [Google Scholar]
- 79.Wong JV, Song H, You L. A whole more than the sum of its synthetic parts. ACS Chem Biol. 2008;3:27–29. doi: 10.1021/cb700256w. [DOI] [PubMed] [Google Scholar]
- 80.Balagadde FK, Song H, Ozaki J, Collins CH, Barnet M, Arnold FH, Quake SR, You L. A synthetic Escherichia coli predator-prey ecosystem. Mol Syst Biol. 2008;4:187. doi: 10.1038/msb.2008.24.Two QS systems in combination with a toxin anti-toxin system were used to design circuits that realize predator-prey behavior in an artificial ecosystem. Long-term monitoring of predator-prey dynamics was carried out in a microchemostat.
- 81.Tanouchi Y, Pai A, You LC. Decoding biological principles using gene circuits. Molecular Biosystems. 2009;5:695–703. doi: 10.1039/b901584c. [DOI] [PubMed] [Google Scholar]
- 82.Weber W, Daoud-El Baba M, Fussenegger M. Synthetic ecosystems based on airborne inter- and intrakingdom communication. Proc Natl Acad Sci U S A. 2007;104:10435–10440. doi: 10.1073/pnas.0701382104.This work describes the creation of a large number of communication circuits using non-AHL based signals to achieve communication with and between bacteria, yeast, mammalian cells and plants.
- 83.Shou W, Ram S, Vilar JM. Synthetic cooperation in engineered yeast populations. Proc Natl Acad Sci U S A. 2007;104:1877–1882. doi: 10.1073/pnas.0610575104.Two yeast strains were engineered to be display mutualistic, obligatory cooperation by relying on each other for an essential metabolite.
- 84.Sohka T, Heins RA, Ostermeier M. Morphogen-defined patterning of Escherichia coli enabled by an externally tunable band-pass filter. J Biol Eng. 2009;3:10. doi: 10.1186/1754-1611-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sohka T, Heins RA, Phelan RM, Greisler JM, Townsend CA, Ostermeier M. An externally tunable bacterial band-pass filter. Proc Natl Acad Sci U S A. 2009;106:10135–10140. doi: 10.1073/pnas.0901246106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan C, Song H, Niemi J, You L. A synthetic biology challenge: making cells compute. Mol Biosyst. 2007;3:343–353. doi: 10.1039/b618473c. [DOI] [PubMed] [Google Scholar]
- 87.de Lorenzo V. Systems biology approaches to bioremediation. Curr Opin Biotechnol. 2008;19:579–589. doi: 10.1016/j.copbio.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Farmer WR, Liao JC. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat Biotechnol. 2000;18:533–537. doi: 10.1038/75398. [DOI] [PubMed] [Google Scholar]