Abstract
Melanin-concentrating hormone (MCH) is a cyclic peptide which was originally discovered in fish to lighten skin color by affecting melanosomes aggregation. This peptide is highly conserved and also found in rodents whose gene is overexpressed upon fasting. However, the site of MCH action remained obscure until its receptor was discovered in 1999 as a G protein-coupled receptor. After this receptor structure was identified, the functional domains important for MCH-MCHR interaction were revealed. Moreover, the cloning of the MCH receptor led us to identify the in vivo sites of MCH action which suggested potential physiological functions of the MCH system. Furthermore, the MCH receptor identification allow for designing surrogate molecules which can block MCH activity. Studies using these molecules revealed various physiological functions of the MCH system not only in feeding but also in other physiological responses such as stress and emotion. This review will discuss how the MCH receptor was discovered and its impact on many studies investigating the MCH receptor’s structure, signaling pathways, and expression pattern.
Keywords: Melanin-concentrating hormone, G protein-coupled receptor, Orphan receptor strategy, Reverse pharmacology
1. Introduction
In 1983, melanin-concentrating hormone (MCH) was discovered in fish as a 17 amino acid cyclic peptide [14]. MCH is secreted from the pituitary and it circulates to induce paling of the skin. In 1996, the MCH precursor gene was shown to be overexpressed upon fasting and in ob/ob mice [27]. The rat MCH consists of 19 amino acids, and is identical to the human MCH. MCH is predominantly synthesized in the brain, especially in lateral hypothalamus and zona incerta which are important regions to regulate feeding behavior. Central MCH injection increases food intake in rats suggesting that MCH is an orexigenic peptide. MCH-knockout mice were generated and shown to exhibit reduced body weight as a result of hypophagia and to have low body fat and leptin levels [29]. These results suggested that MCH is a peptide which plays a role in energy homeostasis. However, its site of action was not identified until 1999 when we and other groups discovered a receptor for MCH by studying orphan G protein-coupled receptor (GPCR) [2, 5, 18, 32, 36]. MCH receptor was found to be the previously discovered orphan GPCR, SLC-1 (now MCH1R) [16, 17]. Later it was also found that, indeed MCH binds and activates two G protein-coupled receptors, MCH1R and MCH2R but that MCH2R is functional in human but not in rodent [13, 28]. When MCH1R is expressed in in vitro cellular system, MCH1R activates second messenger systems by coupling to Gαi and Gαq proteins [32]. MCH1R has been shown to be widely expressed in various tissues, especially in the brain. This expression pattern suggests sites of MCH action and furthermore potential physiological functions of the MCH system. Most importantly, the information of MCH receptor structure initiated efforts trying to identify small molecules to block the MCH receptor. Use of these tools significantly improved our understanding of the physiological roles of the endogenous MCH system.
In this review, we will first describe the history of MCH receptor discovery. Secondly, we will summarize MCH1R’s structural domains which are important for their functional activity. Finally, the in vivo expression pattern of MCH receptors will be discussed to suggest potential functions of the MCH system.
2. Discovery of the MCH receptor
MCH was originally discovered in fish to induce paling of the skin [14] and it regulates feeding and metabolism in rats [27]. However, the receptor for MCH had not been identified and therefore MCH sites of action were unknown. Initial efforts to identify MCH receptor were based on binding assays by using radiolabeled MCH [7,8]. MCH binding was detected in mouse melanoma cells in vitro [8] and in rat brain as well as in some peripheral tissues [7]. However, the existence of MCH receptor was obscure until 1999 when it was found to be an orphan GPCR, SLC-1. SLC-1 (GPR24) had been cloned by PCR amplification as a GPCR which has 40% amino acid identity in the transmembrane (TM) regions of the five known human somatostatin receptors in rat [16, 17]. SLC-1 gene is intronless in its open reading frame, encodes a receptor of 453 amino acids in rat and maps to chromosome 22, q13.3 in human.
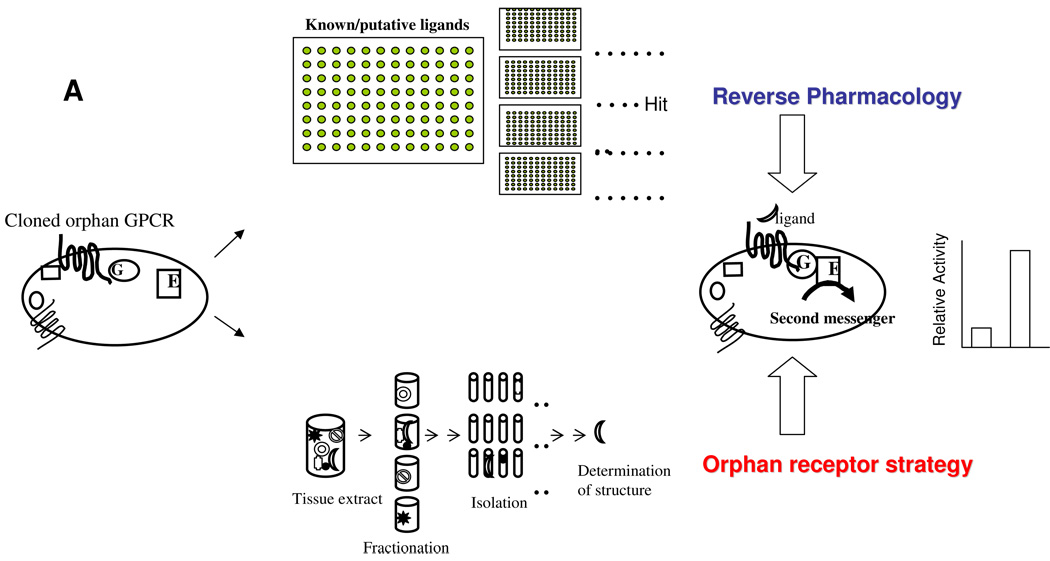
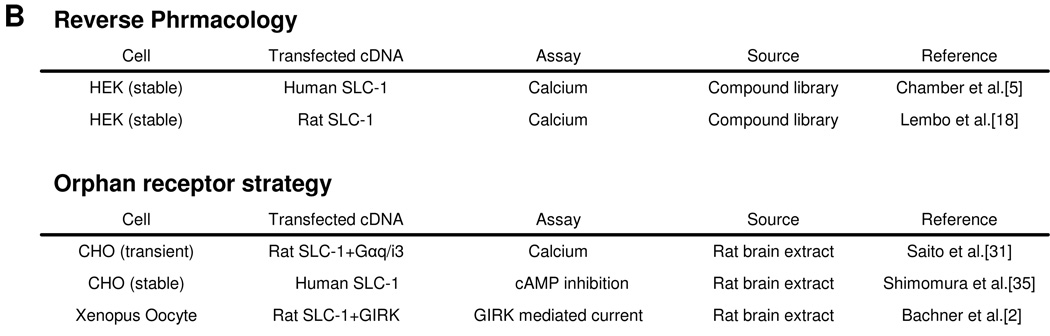
The MCH receptor was discovered by two different approaches (Fig.1A). One is to use an orphan receptor strategy [6]. In principle, an orphan GPCR is expressed in certain cell lines, and biological extracts are exposed to the receptor expressing cell lines to induce changes in intracellular second messenger levels upon receptor activation. An active component can be isolated upon monitoring any changes in the second messenger system such as intracellular calcium. Three groups used rat brain extract as material to look for the ligand to activate SLC-1 by measuring three different second messenger responses: 1) calcium influx in CHO cells transiently transfected with a chimeric Gα protein (Gαi3/q) and rat SLC-1 [32], 2) cyclic AMP (cAMP) inhibition assays in human SLC-1 expressing CHO cells [36] and 3) GIRK-mediated current in rat SLC-1 and GIRK expressing Xenopus oocytes [2] (Fig. 1B). Three groups came to the same conclusion that MCH is the only ligand from rat brain extract which activates rat or human SLC-1 receptor. Two other groups used the reverse pharmacology approach to identify a ligand for SLC-1 receptor (Fig. 1A) [5, 18]. Compound library which has more than 500 known bioactive substances were used to search for any known ligand that can activate human SLC-1 receptor [5]. Among those, MCH was the only compound which activates SLC-1 receptor with nanomolar affinity. Lembo et al. also found that MCH was the only compound which activates calcium influx in rat SLC-1 expressing HEK cells from the commercial library containing peptidic and non-peptidic ligands [18] (Fig. 1b). Collectively, all five groups’ conclusion was the same that SLC-1 is a receptor for MCH. The SLC-1 receptor is therefore referred to as MCH1R in this review.
Figure 1. A. The orphan receptor strategy and reverse pharmacology.
Orphan GPCR will be transfected in in vitro cell systems. These cells will be tested in their second messenger responses upon exposure to either known/putative ligands (reverse pharmacology) or fractions prepared from tissue extract (orphan receptor strategy) to identify a ligand for the orphan receptor. B. Strategies to identify the MCH receptor. SLC-1 refers MCH1R in this review.
Some GPCRs bind more than one ligand which is relevant and the MCH precursor generates more than one bioactive peptide, neuropeptide GE and neuropeptide EI [25]. In addition, alternative splicing of the MCH precursor gene leads to the expression of a different precursor which encodes two other bioactive peptides, MCH gene-overprinted peptides 14 and 27 [41]. These peptides were tested on MCH1R expressing cells, but were shown to be unable to increase Ca2+ levels as MCH did [5, 32].
In 2001, another receptor for MCH was identified by two groups using genomic sequence searches [13, 28]. It shares 38% amino acid identity with MCH-1R and shows high affinity MCH binding. In contrast to MCH1R, MCH2R only couples to Gαq protein; MCH2R signaling is not sensitive to pertussis toxin. The human MCH2R gene is mapped to the long arm of chromosome 6 at band 6q16.2–16.3, a region reported to be associated with cytogenetic abnormalities of obese patients [28]. MCH2R is found to be a pseudogene in rodent species, but is functional in dogs, ferrets, rhesus monkeys and humans [39]. However, the physiological importance of MCH2R remains unknown due to the lack of available animal models.
Interestingly, three MCH receptor sequences from zebrafish and two receptor sequences from fugu and barfin flounder have been identified in whole genome shotgun datasets [19, 38]. Zebrafish, fugu and barfin flounder have clear MCH1R and MCH2R orthologues. Phylogenetic analyses of these receptors have suggested that an initial duplication of the MCH receptor occurred early in evolution, giving rise to MCH1R and MCH2R. Further characterization of fish MCH receptors may provide further insights into MCH functions in fish, rodents and humans.
3. Pharmacological characteristics of the MCH Receptor
In mammalian cells transfected with the MCH1R, MCH can activate calcium mobilization and inhibit forskolin-induced cAMP accumulation [32] (Fig. 2). Study of MCH1R signaling was further investigated by Hawes et al. [11]. MCH inhibits forskolin-stimulated cAMP production in a pertussis toxin (PTX) sensitive manner, while MCH increases phosphoinositide metabolism and intracellular free Ca2+ levels which is partially decreased by PTX pretreatment. MCH also stimulates mitogen-activated protein (MAP) kinase activity which can be mediated by both Gαi and Gαo proteins, but in separate ways. Protein kinase C activity is required for Gαo dependent MAPK signaling, but is not essential for Gαi dependent MAPK signaling. MCH stimulated MAPK signaling was partially decreased by PKC inhibitor suggesting that MCH induced MAPK activity depends on both Gαi and Gαo proteins (Fig. 2). These results suggest that MCH receptor activates several diverse intracellular signaling pathways by coupling Gαq, Gαi and Gαo proteins. Activation of these various intracellular signaling pathways may contribute to the diverse physiological processes regulated by MCH.
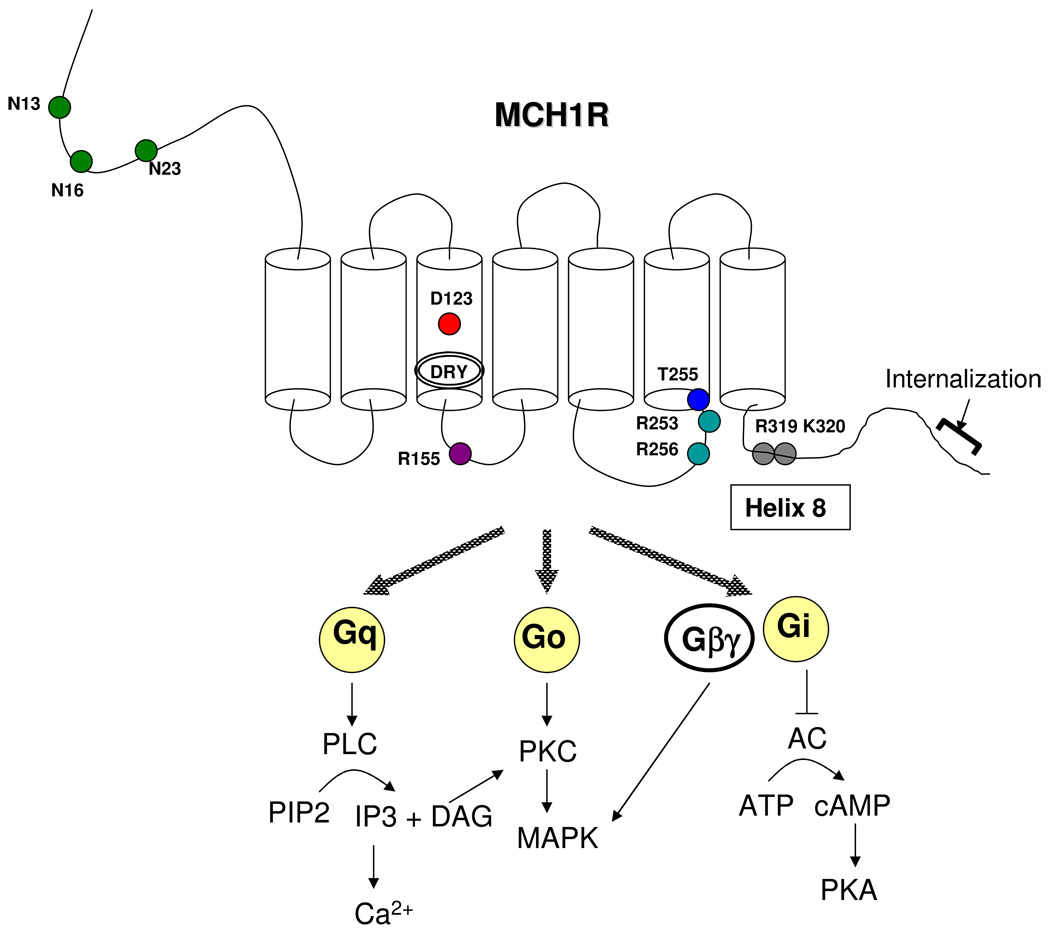
Figure 2. MCH1R signaling pathways.
Important sites for MCH1R function are indicated as circles. N13, N16 and N23 (Green circle) are three N-glycosylation sites. D123 (Red circle) is important for MCH binding and receptor activation. R155 (Purple circle) regulates signal transduction. T255 (Blue circle) regulates MCH1R cell surface expression. The proximal C-terminal tail of the MCH1R was predicted to form a helix 8 (from E316 to L324/S325), and the dibasic motif R319/K320 (Grey circle) is involved in signaling function, possibly through maintaining a proper structure of helix 8. The DRY motif may play fundamental roles in governing receptor conformation and G protein coupling in MCH1R.
MCH1R regulatory domains and amino acid residues which are important for receptor function have been analyzed. MCH1R belongs to the rhodopsin family of GPCRs; the Glu/Asp3.49-Arg3.50-Tyr3.51 (E/DRY) sequence is a highly conserved motif in this family. Although many studies have suggested that the DRY motif is involved in maintaining the receptor in its ground state, this motif in MCH1R has a distinct role in governing receptor conformation and G protein coupling/recognition [1]. Like many GPCRs, MCH1R has extracellular N-terminus three consensus sites for asparagine-linked glycosylation (Asn13, Asn16 and Asn23) [35]. All three regions are glycosylated and mutation of all these sites impaired receptor expression at the cell surface. In particular, N-linked glycosylation in Asn 23 is necessary for cell surface expression, ligand binding and signal transduction [35]. Molecular modeling of the MCH/MCH1R identified 11 potential ligand binding sites of MCH1R. In particular, Asp123 in the third transmembrane domain of the MCH1R is crucial for ligand binding and receptor activation [21]. Furthermore, Arg14 of MCH peptide is an important residue to interact with Asp123 site of MCH1R. Arg155 in intracellular loop 2 of MCH1R has been shown to play a critical role in receptor function since Arg155 mutation led to a drastic loss of MCH1R signal transduction without changing the high affinity constant (Kd) value [34]. Also, a point mutation in Thr255 which is located at the junction of intracellular loop 3 and transmembrane domain 6 resulted in the receptor being retained in the endoplasmic reticulum; it dramatically reduced the MCH1R cell surface expression level. This indicates that this region is important for receptor folding and correct trafficking to the cell surface [9]. Two dibasic amino acids (Arg319 and Lys320) in helix 8 of MCH1R, a common structural domain in the rhodopsin family of GPCRs, seem to be essential to constitute a proper structure of helix 8 and therefore important for receptor signaling [40], while the distal portion of the C-terminal tail is necessary for the receptor internalization process [33].
MCH1R-binding proteins have been detected and described. Periplakin and neurochondrin, which interact with the proximal C-terminus of MCH1R, reduce the capacity to initiate calcium mobilization [10, 24]. RGS8, one of the GTPase-activating proteins for Gα subunits, is shown to be a negative regulator. Indeed, Arg253 and Arg256 at the distal end of the third cytoplasmic loop were found to be structurally important sites for the functional interaction with RGS8, since coexpression of RGS8 with R253Q/R256Q mutant MCH1R led to a loss of function in MCH-stimulated calcium mobilization [22].
4. MCH receptor distribution pattern
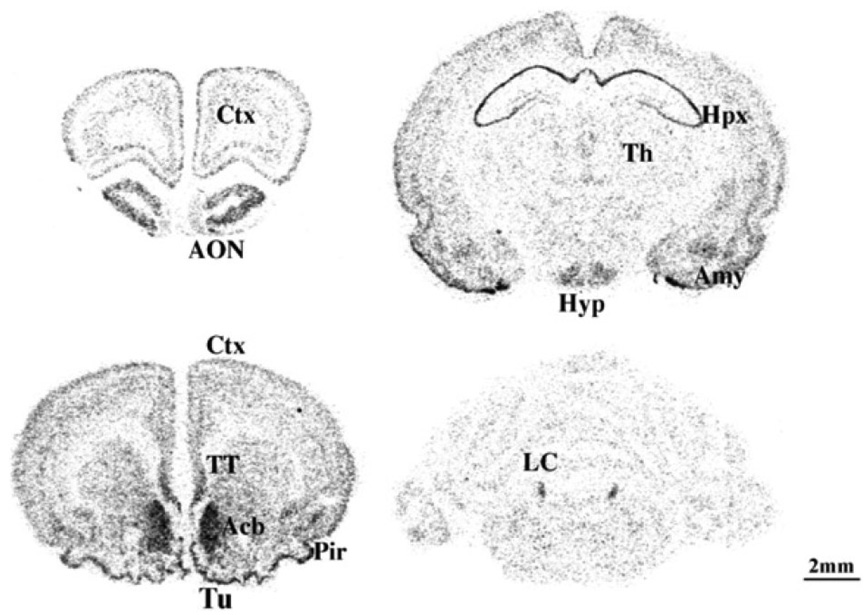
Initially, MCH1R expression pattern in adult rat was examined by using a Northern blot analysis [32]. MCH1R mRNA was detected at high levels in brain, at moderate levels in eye and skeletal muscle, and at low levels in tongue and pituitary [32]. Since MCH1R is a receptor which is predominantly expressed in the brain, in situ hybridization was carried out to further examine MCH1R expression pattern in the rat brain [12, 31] (Fig.3). The highest expression of MCH1R is detected in most anatomical areas implicated in the control of olfaction, such as the olfactory nerve layer, olfactory nucleus and tubercle. Strong labeling is also detected in several limbic structures such as hippocampal formation, septum and amygdala; all of them are implicated in the regulation of stress and emotional processing. Furthermore, MCH1R is abundantly expressed in the nucleus accumbens shell where it may play a role in the regulation of motivation and reward. Moreover, MCH1R is detected in monoaminoergic nuclei such as the locus coeruleus and dorsal raphe nucleus; therefore it might play a role in regulating serotonergic and noradrenergic transmission. Moderate MCH1R mRNA expression is found in various hypothalamic regions including the arcuate nucleus, ventromedial hypothalamic nucleus and paraventricular nucleus (PVN) that are involved in the neuronal circuitry of feeding and energy homeostasis. These localizations clearly imply the central MCH system’s diverse physiological functions. This assumption is supported by recent findings using MCH1R-selective antagonists and genetically engineered animals [20, 30].
Figure 3. MCH1R expression pattern in the brain.
Ctx: cortex; AON: anterior olfactory nucleus; TT: taenia tecta; Tu: olfactory tubercle; Acb: nucleus accumbens; Pir: piriform cortex; Hpx: hippocampus; Th: thalamus; Hyp: hypothalamus; Amy: amygdala; LC: locus coeruleus. Adapted from [32]
Recent studies reveal that functional MCH1R is present in several peripheral tissues. It has shown that MCH1R is expressed in adipose tissues and regulates leptin release via transiently increasing ERK1/2 and pp70 S6 kinase [3, 4]. Also, both MCH and MCH1R are present in clonal β cell lines and in mouse and human islets of Langerhans, where MCH stimulates insulin secretion, possibly via an autocrine mechanism [37]. Furthermore, MCH knock out mice showed reduced β cell mass and altered expression of islet-enriched genes in their islet, suggesting that MCH regulates islet function as being part of a hypothalamic-islet axis [26]. These data provide novel evidence that MCH1R can regulate energy homeostasis peripherally. MCH1R is also found in human colonic epithelial cells and regulates inflammatory processes in the intestine [15].
Localization of MCH1R and MCH2R in human tissues was examined by using TaqMan RT-PCR [13, 23]. Both MCH1R and MCH2R are predominantly expressed in the brain. MCH1R is predominantly expressed in the brain and the pituitary, whereas the expression level is relatively low in peripheral tissues [13]. MCH2R is also highly expressed in the brain, but not in the pituitary [13]. Human MCH1R is widely expressed in brain areas such as the cerebral cortex, amygdala, hippocampus, hypothalamus, substantia nigra. The widespread distribution of human MCH1R is in agreement with that reported in the rat brain [18, 32]. MCH2R distribution in human is similar to MCH1R, but there are subtle differences. For example, MCH1R expression is higher than MCH2R in several brain regions, the hypothalamus, locus coeruleus, medulla oblongata and cerebellum [13, 23]. Interestingly, in situ hybridization of monkey brain shows that MCH2R is highly expressed in the arcuate nucleus and ventromedial hypothalamic nucleus [28]. At present, the functional importance of MCH2R in feeding remains unknown due to the lack of available animal models.
5. Conclusion
The hypothalamus has been regarded as the brain center in regulating feeding behavior. Several neuropeptides which are made in the hypothalamus such as neuropeptide Y (NPY) have been studied in how they regulate energy homeostasis. Discovery of the MCH added one more candidate in this field as a hypothalamic neuropeptide which can regulate feeding and energy homeostasis. However, the site of action was not evident until the receptor for MCH was discovered in 1999. MCH1R discovery immediately expedited our understanding of the MCH system and this is an example of how deorphanized receptors can reveal more detailed physiological functions. Studies of MCH1R structure identified important structural domains for MCH-MCHR interaction and its impact on second messenger system. Most importantly, MCH1R discovery revealed in vivo sites of MCH action. Also, many efforts to identify MCH1R antagonists started in pharmaceutical companies and its use have suggested many therapeutic indications in regulating important physiological responses not only in energy homeostasis but also in stress, emotion and anxiety. Therefore, although MCH1R discovery through the orphan receptor strategy and the reverse pharmacology approach had been demanding work, it significantly improved our understanding of various physiological functions of the MCH system and suggested a new target to treat human disorders
Acknowledgements
We thank Lily Tamura for reviewing our manuscript. This work was supported by grants MH60231, DK63001, BIO05-10485 to OC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aizaki Y, Nakano-Tetsuka M, Maruyama K, Saito Y. Distinct role of the DRY motif in rat melanin-concentrating hormone receptor 1 in signaling control. doi: 10.1016/j.peptides.2009.01.017. Peptides in press. [DOI] [PubMed] [Google Scholar]
- 2.Bachner D, Kreienkamp H, Weise C, Buck F, Richter D. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1) FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- 3.Bradley RL, Kokkotou EG, Maratos-Flier E, Cheatham B. Melanin-concentrating hormone regulates leptin synthesis and secretion in rat adipocytes. Diabetes. 2000;49:1073–1077. doi: 10.2337/diabetes.49.7.1073. [DOI] [PubMed] [Google Scholar]
- 4.Bradley RL, Mansfield JP, Maratos-Flier E, Cheatham B. Melanin-concentrating hormone activates signaling pathways in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2002;283:E584–E592. doi: 10.1152/ajpendo.00161.2002. [DOI] [PubMed] [Google Scholar]
- 5.Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, Dytko GM, Foley JJ, Martin J, Liu WS, Park J, Ellis C, Ganguly S, Konchar S, Cluderay J, Leslie R, Wilson S, Sarau HM. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 6.Civelli O, Nothacker HP, Saito Y, Wang Z, Lin SH, Reinscheid RK. Novel neurotransmitters as natural ligands of orphan G-protein-coupled receptors. Trends Neurosci. 2001;24:230–237. doi: 10.1016/s0166-2236(00)01763-x. [DOI] [PubMed] [Google Scholar]
- 7.Drozdz R, Eberle AN. Binding sites for melanin-concentrating hormone (MCH) in brain synaptosomes and membranes from peripheral tissues identified with highly tritiated MCH. J Recept Signal Transduct Res. 1995;15:487–502. doi: 10.3109/10799899509045235. [DOI] [PubMed] [Google Scholar]
- 8.Drozdz R, Siegrist W, Baker BI, Chluba-de Tapia J, Eberle AN. Melanin-concentrating hormone binding to mouse melanoma cells in vitro. FEBS Lett. 1995;359:199–202. doi: 10.1016/0014-5793(95)00043-9. [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Perry SJ, Gao Y, Schwarz DA, Maki RA. A point mutation in the human melanin concentrating hormone receptor 1 reveals an important domain for cellular trafficking. Mol Endocrinol. 2005;19:2579–2590. doi: 10.1210/me.2004-0301. [DOI] [PubMed] [Google Scholar]
- 10.Francke F, Ward RJ, Jenkins L, Kellett E, Richter D, Milligan G, Bachner D. Interaction of neurochondrin with the melanin-concentrating hormone receptor 1 interferes with G protein-coupled signal transduction but not agonist-mediated internalization. J Biol Chem. 2006;281:32496–32507. doi: 10.1074/jbc.M602889200. [DOI] [PubMed] [Google Scholar]
- 11.Hawes BE, Kil E, Green B, O'Neill K, Fried S, Graziano MP. The melanin-concentrating hormone receptor couples to multiple G proteins to activate diverse intracellular signaling pathways. Endocrinology. 2000;141:4524–4532. doi: 10.1210/endo.141.12.7833. [DOI] [PubMed] [Google Scholar]
- 12.Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, Leslie RA. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 13.Hill J, Duckworth M, Murdock P, Rennie G, Sabido-David C, Ames RS, Szekeres P, Wilson S, Bergsma DJ, Gloger IS, Levy DS, Chambers JK, Muir AI. Molecular cloning and functional characterization of MCH2, a novel human MCH receptor. J Biol Chem. 2001;276:20125–20129. doi: 10.1074/jbc.M102068200. [DOI] [PubMed] [Google Scholar]
- 14.Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- 15.Kokkotou E, Moss AC, Torres D, Karagiannides I, Cheifetz A, Liu S, O'Brien M, Maratos-Flier E, Pothoulakis C. Melanin-concentrating hormone as a mediator of intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105:10613–10618. doi: 10.1073/pnas.0804536105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolakowski LF, Jr, Jung BP, Nguyen T, Johnson MP, Lynch KR, Cheng R, Heng HH, George SR, O'Dowd BF. Characterization of a human gene related to genes encoding somatostatin receptors. FEBS Lett. 1996;398:253–258. doi: 10.1016/s0014-5793(96)01160-x. [DOI] [PubMed] [Google Scholar]
- 17.Lakaye B, Minet A, Zorzi W, Grisar T. Cloning of the rat brain cDNA encoding for the SLC-1 G protein-coupled receptor reveals the presence of an intron in the gene. Biochim Biophys Acta. 1998;1401:216–220. doi: 10.1016/s0167-4889(97)00135-3. [DOI] [PubMed] [Google Scholar]
- 18.Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, St-Onge S, Pou C, Labrecque J, Groblewski T, O'Donnell D, Payza K, Ahmad S, Walker P. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 19.Logan DW, Bryson-Richardson RJ, Pagan KE, Taylor MS, Currie PD, Jackson IJ. The structure and evolution of the melanocortin and MCH receptors in fish and mammals. Genomics. 2003;81:184–191. doi: 10.1016/s0888-7543(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 20.Luthin DR. Anti-obesity effects of small molecule melanin-concentrating hormone receptor 1 (MCHR1) antagonists. Life Sci. 2007;81:423–440. doi: 10.1016/j.lfs.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald D, Murgolo N, Zhang R, Durkin JP, Yao X, Strader CD, Graziano MP. Molecular characterization of the melanin-concentrating hormone/receptor complex: identification of critical residues involved in binding and activation. Mol Pharmacol. 2000;58:217–225. doi: 10.1124/mol.58.1.217. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto-Matsubara M, Saitoh O, Maruyama K, Aizaki Y, Saito Y. Regulation of melanin-concentrating hormone receptor 1 signaling by RGS8 with the receptor third intracellular loop. Cell Signal. 2008;20:2084–2094. doi: 10.1016/j.cellsig.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Mori M, Harada M, Terao Y, Sugo T, Watanabe T, Shimomura Y, Abe M, Shintani Y, Onda H, Nishimura O, Fujino M. Cloning of a novel G protein-coupled receptor, SLT, a subtype of the melanin-concentrating hormone receptor. Biochem Biophys Res Commun. 2001;283:1013–1018. doi: 10.1006/bbrc.2001.4893. [DOI] [PubMed] [Google Scholar]
- 24.Murdoch H, Feng GJ, Bachner D, Ormiston L, White JH, Richter D, Milligan G. Periplakin interferes with G protein activation by the melanin-concentrating hormone receptor-1 by binding to the proximal segment of the receptor C-terminal tail. J Biol Chem. 2005;280:8208–8220. doi: 10.1074/jbc.M405215200. [DOI] [PubMed] [Google Scholar]
- 25.Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 26.Pissios P, Ozcan U, Kokkotou E, Okada T, Liew CW, Liu S, Peters JN, Dahlgren G, Karamchandani J, Kudva YC, Kurpad AJ, Kennedy RT, Maratos-Flier E, Kulkarni RN. Melanin concentrating hormone is a novel regulator of islet function and growth. Diabetes. 2007;56:311–319. doi: 10.2337/db06-0708. [DOI] [PubMed] [Google Scholar]
- 27.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 28.Sailer AW, Sano H, Zeng Z, McDonald TP, Pan J, Pong SS, Feighner SD, Tan CP, Fukami T, Iwaasa H, Hreniuk DL, Morin NR, Sadowski SJ, Ito M, Bansal A, Ky B, Figueroa DJ, Jiang Q, Austin CP, MacNeil DJ, Ishihara A, Ihara M, Kanatani A, Van der Ploeg LH, Howard AD, Liu Q. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc Nat Acad Sci U S A. 2001;98:7564–7569. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 30.Shimazaki T, Yoshimizu T, Chaki S. Melanin-concentrating hormone MCH1 receptor antagonists: a potential new approach to the treatment of depression and anxiety disorders. CNS drugs. 2006;20:801–811. doi: 10.2165/00023210-200620100-00002. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 33.Saito Y, Tetsuka M, Li Y, Kurose H, Maruyama K. Properties of rat melanin-concentrating hormone receptor 1 internalization. Peptides. 2004;25:1597–1604. doi: 10.1016/j.peptides.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Saito Y, Tetsuka M, Saito S, Imai K, Yoshikawa A, Doi H, Maruyama K. Arginine residue 155 in the second intracellular loop plays a critical role in rat melanin-concentrating hormone receptor 1 activation. Endocrinology. 2005;146:3452–3462. doi: 10.1210/en.2005-0115. [DOI] [PubMed] [Google Scholar]
- 35.Saito Y, Tetsuka M, Yue L, Kawamura Y, Maruyama K. Functional role of N-linked glycosylation on the rat melanin-concentrating hormone receptor 1. FEBS Lett. 2003;533:29–34. doi: 10.1016/s0014-5793(02)03744-4. [DOI] [PubMed] [Google Scholar]
- 36.Shimomura Y, Mori M, Sugo T, Ishibashi Y, Abe M, Kurokawa T, Onda H, Nishimura O, Sumino Y, Fujino M. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- 37.Tadayyon M, Welters HJ, Haynes AC, Cluderay JE, Hervieu G. Expression of melanin-concentrating hormone receptors in insulin-producing cells: MCH stimulates insulin release in RINm5F and CRI-G1 cell-lines. Biochem Biophys Res Commun. 2000;275:709–712. doi: 10.1006/bbrc.2000.3357. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi A, Kosugi T, Kobayashi Y, Yamanome T, Schioth HB, Kawauchi H. The melanin-concentrating hormone receptor 2 (MCH-R2) mediates the effect of MCH to control body color for background adaptation in the barfin flounder. Gen Comp Endocrinol. 2007;151:210–219. doi: 10.1016/j.ygcen.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van der Ploeg LH, Howard AD. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- 40.Tetsuka M, Saito Y, Imai K, Doi H, Maruyama K. The basic residues in the membrane-proximal C-terminal tail of the rat melanin-concentrating hormone receptor 1 are required for receptor function. Endocrinology. 2004;145:3712–3723. doi: 10.1210/en.2003-1638. [DOI] [PubMed] [Google Scholar]
- 41.Toumaniantz G, Bittencourt JC, Nahon JL. The rat melanin-concentrating hormone gene encodes an additional putative protein in a different reading frame. Endocrinology. 1996;137:4518–4521. doi: 10.1210/endo.137.10.8828517. [DOI] [PubMed] [Google Scholar]