Abstract
Objective
Several surgical procedures have been reported for the treatment of chronic subdural hematoma (CSDH). We compared the results of treatments for CSDH obtained from one burr-hole craniostomy with closed system drainage with or without irrigation, two burr-hole craniostomy with closed system drainage with irrigation, and small craniotomy with irrigation and closed-system drainage.
Methods
Eighty-seven patients with CSDH underwent surgery at our institution from January 2004 to December 2008. Our patients were classified into three groups according to the operative procedure; group I, one burr-hole craniostomy with closed system drainage with or without irrigation (n = 25), group II, two burr-hole craniostomy with closed system drainage with irrigation (n = 32), and group III, small craniotomy with irrigation and closed-system drainage (n = 30).
Results
Age distribution, male and female ratio, Markwalder's grade on admission and at the time of discharge, size of hematoma before and after surgery, duration of operation, Hounsfield unit of hematoma before and after surgery, duration of hospital treatment, complication rate, and revision rate were categories that we compared between groups. Duration of operation and hospitalization were only two categories which were different. But, when comparing burr hole craniostomy group (group I and group II) with small craniotomy group (group III), duration of post-operative hospital treatment, complication and recurrence rate were statistically lower in small craniotomy group, even though operation time was longer.
Conclusion
Such results indicate that small craniotomy with irrigation and closed-system drainage can be considered as one of the treatment options in patients with CSDH.
Keywords: Chronic subdural hematoma, Burr hole craniostomy, Small craniotomy
INTRODUCTION
Chronic subdural hematoma (CSDH) is one of the most common clinical entities encountered in daily neurosurgical practice. The incidence of CSDH is 1-2 cases per 100,000 populations per year20), and more common in the old age group. As we are now living in a society that is aging, CSDH cases seem to be on the rise. However, in spite of this increase, the optimal treatment for CSDH is not well defined11).
There are many operation techniques for CSDH; one or two burr hole craniostomy with or without saline irrigation and closed-system drainage4,13), twist drill craniostomy with or without irrigation and with or without drainage14), craniotomy and excision of the subdural membranes, reservoir shunting for continuous irrigation and drainage1), percutaneous needle trephination and open system drainage with repeated saline rinsing17), replacement of the hematoma with oxygen via percutaneous subdural tapping without irrigation and drainage16), continuous subgaleal suction drainage3), etc. Which one can be the treatment of choice? Burr-hole craniostomy is accepted as the most common treatment, but no one can say this is the best surgical technique for CSDH.
In this article, we compared three different types of operative procedures.
MATERIALS AND METHODS
Eighty-seven patients have undergone surgery, and were analyzed retrospectively for CSDH at our institute from January 2004 to December 2008. There were 65 males and 22 females (M : F ratio 3 : 1) in the study group. Median age was 65.2 with a range from 56 days old to 83 years old. We classified our patients into three groups according to the operative procedure; group I, one burr-hole craniostomy with closed system drainage with or without irrigation (n = 25), group II, two burr-hole craniostomy with closed system drainage with irrigation (n = 32), and group III, small craniotomy (about 3-4 cm in diameter) with irrigation and closed-system drainage (n = 30).
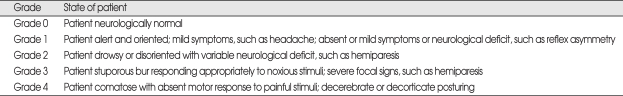
The neurologic performance of the patients was evaluated with the "Markwalder's Neurological Grading System" preoperatively and post-operatively which is the most commonly used neurological grading system for CSDH9) (Table 1). Days of post-operative hospitalization, reoperation and complication rate were also used for post-operative assessment.
Table 1.
Markwalder's neurologic grading system
Diagnosis was confirmed by computed tomography (CT) [SOMATOME SENSATION (64-slice), Siemens, Munich, Germany] in 80 patients (92.0%), and by magnetic resonance imaging (MRI) [Gyroscan Intergra 1.5T (R10), Philips, Best, Netherlands] in 7 patients (8.0%). In all cases, CT was used for post-operative assessment. With these imaging studies, we measured size and mean Hounsfield unit of the hematoma by STARPACS (PiViewSTAR™, ver. 5.0.5.2., INFINITT. Co., Ltd., Seoul, Korea) to evaluate differences on hematoma.
All operations were performed under general anesthesia. In group I and II, the size of burr holes was about 10 mm in diameter, and in group III, the size of craniotomy was about 30 mm in diameter (Fig. 1). A burr-hole, two burr-holes or small craniotomy were made over the maximum thickness of the hematoma. Subdural fluid was evacuated by repeated irrigation with physiologic isotonic saline except 12 cases in group I. Especially in group III, chronic subdural membrane was removed about 20 mm in diameter for the pathologic study. All patients were kept with catheter for the closed-system drainage for 3-7 days. Duration of the closed-system drainage was decided based on the amount of drained subdural fluid and on brain re-expansion verified by follow-up CT scans.
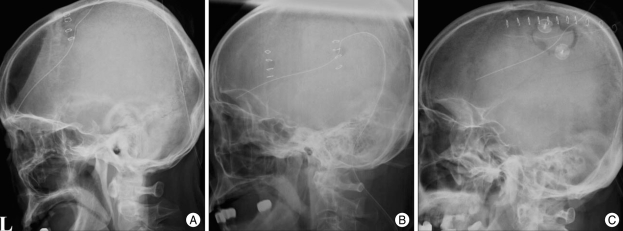
Fig. 1.
Postoperative simple X rays. A : One burr-hole craniostomy. B : Two burr-hole craniostomy. C : Small craniotomy.
Results were analyzed using ANOVA with p < 0.05 considered significant (SPSS 11.0 for Windows, SPSS Inc., Chicago, IL, USA).
RESULTS
Pre-operative evaluation
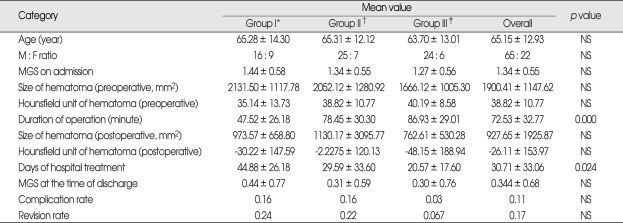
Three groups were similar with preoperative clinical data. No statistical differences were found in the distribution of males and females, age, laterality, and Markwalder's grade on admission. Distribution of males and females in each group was 16 : 9 in group I, 27 : 7 in group II, and 24 : 6 in group III. The average age of each group was 65.3 ± 14.3, 65.3 ± 12.1 and 63.7 ± 13.0 in group I, II, and III, respectively. In group I, there were 9 patients who were graded 2 by Markwalder's neurologic grading system, 1 patient graded 3, and other 15 patients were graded 1. Median value of Markwalder's grade of group I was 1.44 ± 0.58. Median value of Markwalder's grade of group II was 1.34 ± 0.55, and group III was 1.27 ± 0.56. Also, in the preoperative CT parameters, such as size and Hounsfield units of hematoma, there were no significant differences between each group. Mean value of hematoma size from preoperative CT scans was 2131.50 ± 1117.78 mm2 (group I), 2052.12 ± 1280.91 mm2 (group II), and 1661.12 ± 1005.30 mm2 (group III) in each group. Mean value of Hounsfield unit was 35.14 ± 13.73, 40.40 ± 9.60, and 40.19 ± 8.58 in each group.
Efficiency of operation
Duration of operation, mean Hounsfield unit and hematoma size measured from postoperative CT scans were three clinical data to evaluate the efficiency of operation. The mean value for duration of operation was 47.52 ± 26.18 (group I), 78.56 ± 30.30 (group II), and 86.93 ± 29.01 (group III) minutes in each group. There was statistical difference in duration of operation by ANOVA (p value = 0.000). Using multiple comparison study, we found that duration of operation of group III is longer than group I, and also group II is longer than group I, but showed no difference between group II and III. From post-operative CT scans, we evaluated the size of hematoma and the mean Hounsfield unit of hematoma. Mean size of hematoma was 973 ± 658.80, 1130.17 ± 3095.77, and 762.62 ± 530.28 in group I, II, and III, respectively. Mean value of the Hounsfield unit of hematoma was -30.22 ± 147.59, -2.23 ± 120.13, -48.15 ± 188.94 in each group. No difference was in these two categories.
Post-operative results
Four categories were used to compare post-operative clinical data. Categories were Markwalder's grade at the time of discharge from the hospital, duration of hospitalization, re-operation rate and complications after operation. At the time of discharge, there were three Markwalder's grade 3 patients; two in group I, and one in group III. Clinical course of two out of three patients was due to pneumonia that developed during post-operative period. Only one patient was getting worse due to the recurrent CSDH. Despite these entire factors, no difference was found in this category. Median value of Markwalder's grade at the time of discharge was 0.44 ± 0.77, 0.31 ± 0.59, and 0.30 ± 0.76 in each group. Duration of hospitalization was 44.88 ± 42.19 days in group I, 29.59 ± 33.61 days in group II, and 20.57 ± 17.60 days in group III. Using ANOVA study, p value was 0.024. More details from multiple comparison study showed that group I patients stayed more days in the hospital than group III patients. No difference was found between group II and III, as well as group I and II. Eleven patients (12.6%) developed complications. Four patients from group I (4 out of 25 patients, 25%) developed complications such as wound infection, decreased mentality, hemothorax, and pneumonia. Six out of thirty-two patients (18.8%) developed complications in group II. Each of four patients had partial seizure attack, general-tonic-clonic seizure attack, hematochezia, pneumonia, and two developed left side motor weakness. One out of thirty patients in group III (3.3%) developed pneumonia. Fifteen patients underwent operation again because of CSDH recurrence. Revision rate was 0.24 in group I, 0.22 in group II, and 0.07 in group III (Table 2, 3).
Table 2.
Mean values and ANOVA study of three groups in each category
*Group I : one burr-hole craniostomy with closed system drainage with or without irrigation, †Group II : two burr-hole craniostomy with closed system drainage with irrigation, ‡Group III : small craniotomy with irrigation and closed-system drainage (n = 30). MGS : Markwalder's grade scale
Table 3.
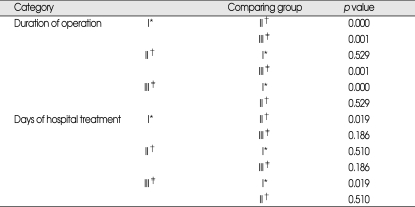
Comparison study of each category with statistic difference
*Group I : one burr-hole craniostomy with closed system drainage with or without irrigation, †Group II : two burr-hole craniostomy with closed system drainage with irrigation, ‡Group III : small craniotomy with irrigation and closed-system drainage (n = 30)
DISCUSSION
The surgical treatment of CSDH is a controversial topic, although most widely used technique in last 20 years seems to be burr-hole craniostomy2,6-8,10,12,13,15,18,19). Many other surgical techniques were reported, such as twist drill craniostomy14), craniotomy and excision of the subdural membranes, reservoir shunting for continuous irrigation and drainage1), percutaneous needle trephination and open system drainage with repeated saline rinsing17), etc. But, these techniques are neither used worldwide, nor performed recently. According to Isobe et al.5), craniotomy is effective in cases with organized hematoma. They performed 4 small craniotomy and 2 enlarged craniotomy. From recent review on CSDH, most of them were about burr-hole craniostomy, and there were neither analysis of small craniotomy, nor comparative study using one- or two-burr hole craniostomy.
Herewith, we report preliminary results of a retrospective study which compare burr-hole craniostomy with small craniotomy. The preliminary results are based on a population of 87 patients who were divided into 3 groups. Comparison of preoperative evaluation showed no significant difference, but in the categories regarding operation, there was difference in duration of the operation between group I and II, I and III. Between group II and III, there was statistically no significant difference. It is obvious that making one burr-hole does take less time than to make two, and to perform small craniotomy. The fact that more irrigation was done during operation in group II and III than group I can also make duration of operation longer. In comparing group II with group III, there was no difference. Performing craniotomy and using larger incision would take more time than just make burr-holes. Because the chance of epidural hematoma is higher, more careful bleeding control with tight dural closure, delicate and careful control of CSDH membrane, and autologues bone flap fixation are needed. There should be some reasons for shallow gap of operation time between group II and III. Time for irrigation can be one big reason. Irrigation through burr hole needs quite long time, because high pressure during irrigation can lead to brain cortical injury or cortical vessel injury, therefore it requires more time with less amount of saline. On the contrary, irrigation after small craniotomy can guarantee larger inlet for saline irrigation, and perform irrigation more effectively, if organized hematoma is present.
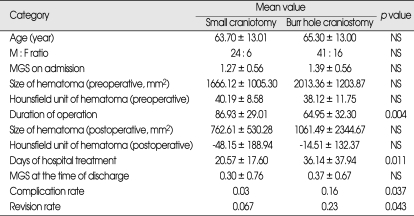
Postoperative results were more interesting. Days of hospitalization were significantly longer in group I than group III, but group II was not different from group I and III statistically. The p value of postoperative complication and revision rate was 0.162 and 0.167, which means no statistical significance. At this point, we questioned ourselves about the relationship between the duration of hospital treatment, complication and revision rate. If a patient developed complications or needs to undergo operation again, the duration of hospital treatment gets longer as well. Therefore, we have put group I and II together, and compared with group III patients who underwent small craniotomy. There were no differences in categories evaluating the preoperative condition and the operation, except the duration of operation (p = 0.004, 86.4 ± 27.5 minutes in small craniotomy group, 66.2 ± 32.3 minutes in burr hole craniostomy group). However, the days of hospital treatment, complication and revision rate showed significant difference between group I, II patients with group III patients. The mean value for days of hospital treatment was 20.6 ± 17.6 days in small craniotomy group, and 36.1 ± 37.9 days in burr-hole group. Complication and revision rate were 6.7% and 3.3% in small craniotomy group, 22.8% and 17.5% in burr-hole group, respectively. P value for complication rate between two groups was 0.037, and for revision rate was 0.043. These results suggest that small craniotomy group shows better prognosis than burr-hole group, although the duration of operation is longer (Table 4). Considering that general anesthesia is needed for all small craniotomy and burr-hole craniostomy, the difference of mean values of the duration of the operation, 20.2 minutes, is not enough to be considered as better prognosis.
Table 4.
ANOVA study comparing small craniotomy with burr hole craniostomy
MGS : Markwalder's grade scale
CONCLUSION
One burr-hole craniostomy requires less time, but duration of hospital treatment is longer than two-burr hole craniostomy and small craniotomy. Performing small craniotomy for the CSDH patients needs more time and more invasive, but has some benefits. In this group, most of all complications and reoperation rate were lower. We can remove organized hematomas or clots by irrigation much more easily and effectively. Sometimes, fenestrating multiple CSDH layers can make the prognosis better, and also can lower the recurrence and complication rate. In our cases, three patients who needed revision after burr-hole craniostomy underwent small craniotomy, and there were no more recurrences. That might be related with multi-layers of CSDH and remained blood clots. Also, we can make subdural catheter directing to the place where operator wants more easily. In our opinion, there is no debate that burr-hole craniostomy with or without irrigation is effective technique for CSDH. However, small craniotomy also needs to be considered as one of the surgical techniques of choice, and can be a better technique if there are layers or clots shown on CT scan or MRI.
References
- 1.Aydin MD. The use of reservoir shunt in chronic subdural hematoma. Neurol India. 2004;52:121–122. [PubMed] [Google Scholar]
- 2.Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma : surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48:220–225. doi: 10.1016/s0090-3019(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 3.Gazzeri R, Galarza M, Neroni M, Canova A, Refice GM, Esposito S. Continuous subgaleal suction drainage for the treatment of chronic subdural haematoma. Acta Neurochir (Wien) 2007;149:487–493. doi: 10.1007/s00701-007-1139-8. discussion 493. [DOI] [PubMed] [Google Scholar]
- 4.Gurelik M, Aslan A, Gurelik B, Ozum U, Karadag O, Kars HZ. A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci. 2007;34:84–87. doi: 10.1017/s0317167100005849. [DOI] [PubMed] [Google Scholar]
- 5.Isobe N, Sato H, Murakami T, Kurokawa Y, Seyama G, Oki S. [Six cases of organized chronic subdural hematoma.] No Shinkei Geka. 2008;36:1115–1120. [PubMed] [Google Scholar]
- 6.Kotwica Z. Treatment of chronic subdural hematoma by burr holes and closed-system drainage. Neurosurg Clin N Am. 2000;11:503–505. [PubMed] [Google Scholar]
- 7.Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien) 2001;143:1041–1044. doi: 10.1007/s007010170010. [DOI] [PubMed] [Google Scholar]
- 8.Lind CR, Lind CJ, Mee EW. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J Neurosurg. 2003;99:44–46. doi: 10.3171/jns.2003.99.1.0044. [DOI] [PubMed] [Google Scholar]
- 9.Markwalder TM, Steinsiepe KF, Rohner M, Reichenbach W, Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55:390–396. doi: 10.3171/jns.1981.55.3.0390. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto K, Akagi K, Abekura M, Ryujin H, Ohkawa M, Iwasa N, et al. Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage. Neurol Res. 1999;21:277–280. doi: 10.1080/01616412.1999.11740931. [DOI] [PubMed] [Google Scholar]
- 11.Muzii VF, Bistazzoni S, Zalaffi A, Carangelo B, Mariottini A, Palma L. Chronic subdural hematoma: comparison of two surgical techniques. Preliminary results of a prospective randomized study. J Neurosurg Sci. 2005;49:41–46. discussion 46-47. [PubMed] [Google Scholar]
- 12.Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791–795. doi: 10.3171/jns.2000.93.5.0791. [DOI] [PubMed] [Google Scholar]
- 13.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma-burr hole drainage versus burr hole irrigation. Surg Neurol. 2002;57:405–409. doi: 10.1016/s0090-3019(02)00720-6. discussion 410. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Ditto RA, Santos-Franco JA, Pinos-Gavilanes MW, Mora-Benítez H, Saavedra T, Martínez-Gonzáles V. [Management of chronic subdural hematoma with twist-drill craniostomy. Report of 213 patients.] Gac Med Mex. 2007;143:203–208. [PubMed] [Google Scholar]
- 15.Suzuki K, Sugita K, Akai T, Takahata T, Sonobe M, Takahashi S. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998;50:231–234. doi: 10.1016/s0090-3019(97)00339-x. [DOI] [PubMed] [Google Scholar]
- 16.Takeda N, Sasaki K, Oikawa A, Aoki N, Hori T. A new simple therapeutic method for chronic subdural hematoma without irrigation and drainage. Acta Neurochir (Wien) 2006;148:541–546. doi: 10.1007/s00701-005-0689-x. [DOI] [PubMed] [Google Scholar]
- 17.van Eck AT, de Langen CJ, Börm W. Treatment of chronic subdural haematoma with percutaneous needle trephination and open system drainage with repeated saline rinsing. J Clin Neurosci. 2002;9:573–576. doi: 10.1054/jocn.2001.1061. [DOI] [PubMed] [Google Scholar]
- 18.Wakai S, Hashimoto K, Watanabe N, Inoh S, Ochiai C, Nagai M. Effi-cacy of closed-system drainage in treating chronic subdural hematoma : a prospective comparative study. Neurosurgery. 1990;26:771–773. doi: 10.1097/00006123-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74:937–943. doi: 10.1136/jnnp.74.7.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilberger JE. Pathophysiology of evolution and recurrence of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:435–438. [PubMed] [Google Scholar]