Abstract
Objective
Intracranial aneurysms are sometimes presented with visual symptoms by their rupture or direct compression of the optic nerve. It is because their prevalent sites are anatomically located close to the optic pathway. Anterior communicating artery is especially located in close proximity to optic nerve. Aneurysm arising in this area can produce visual symptoms according to their direction while the size is small. Clinical importance of visual symptoms presented by aneurysmal optic nerve compression is stressed in this study.
Methods
Retrospective analysis of ruptured anterior communicating artery aneurysms compressing optic apparatus were carried out. Total 33 cases were enrolled in this study. Optic nerve compression of the aneurysms was confirmed by the surgical fields.
Results
In 33 cases among 351 cases of ruptured anterior communicating artery aneurysms treated surgically, from 1991 to 2000, the dome of aneurysm was compressed in optic pathway. In some cases, aneurysm impacted into the optic nerve that deep hollowness was found when the aneurysm sac was removed during operation. Among 33 cases, 10 cases presented with preoperative visual symptoms, such as visual dimness (5), unilateral visual field defect (2) or unilateral visual loss (3), 20 cases had no visual symptoms. Visual symptoms could not be checked in 3 cases due to the poor mental state. In 6 cases among 20 cases having no visual symptoms, optic nerve was deeply compressed by the dome of aneurysm which was seen in the surgical field. Of 10 patients who had visual symptoms, 8 showed improvement in visual symptoms within 6 months after clipping of aneurysms. In 2 cases, the visual symptoms did not recover.
Conclusion
Anterior communicating artery aneurysm can cause visual symptoms by compressing the optic nerve or direct rupture to the optic nerve with focal hematoma formation. We emphasize that cerebral vascular study is highly recommended to detect intracranial aneurysm before its rupture in the case of normal CT findings with visual symptoms and frequent headache.
Keywords: Anterior communicating artery, Intracranial aneurysm, Optic pathway, Visual Symptoms
INTRODUCTION
One of the characteristics of intracranial aneurysm is that it shows no apparent symptoms until it is ruptured. In case of giant intracranial aneurysms, clinical symptoms are manifested, even without a rupture, by the compressing the brain tissue just as in the case of brain tumor. Therefore, the presence of giant aneurysm is often confirmed through differential diagnosis for brain tumor1,21,26). Since aneurysm of the internal carotid artery and the anterior communicating artery is located closely to the optic nerve, it is frequently observed during surgery that aneurysm compresses the optic nerve6,7,18,21). In some cases, surgery reveals the rupture of aneurysm towards the optic nerve resulting in the spread of hematoma on the nerve, or tight adhesion between the nerve and the aneurysm, or an occasional presence of aneurysm sac compressing the nerve2). In general, except in the case of giant intracranial aneurysm, it is extremely rare that a patient visits hospital due to visual symptoms caused by the aneurysm compressing the optic nerve prior to its rupture. A detailed post-operative interview with the patient provides information as to pre-operative conditions marked by headache and/or dimmed vision. Visual loss due to ruptured intracranial aneurysms is, in most cases, explained by Terson's Syndrome, the condition that the visual symptoms appear from bleeding in the subhyaloid or in the cornea due to the rupture of intracranial aneurysm. Nevertheless, the precise pathological mechanism of such rupture extending the bleeding towards the eyeball and its surrounding area has yet to be examined and identified18,23,27).
Visual impairments may result from the enlargement of sac of aneurysm and the pressure applied directly onto the optic nerve. In these cases, visual impairment appears in the lesion of the optic nerve compressed by aneurysm15,19,21,22,24). Visual symptoms may also be caused by poor blood circulation due to aneurysm blocking the blood flow to the optic nerve11,16,29,30). There are also visual symptoms caused by the rupture of aneurysms which is located close to the optic nerve and the bleeding pressure from the ruptured aneurysm directly affecting the nerve. It also can be caused by the enlargement of hematoma exerting a localized pressure onto the optic nerve, in which sudden headache followed by unilateral visual defect can appear3,4,7,28). While surgical treatment for the anterior communicating artery aneurysms, we found that the aneurysm compressing the optic nerve with such force caused transformation of optic nerve. We also identified the presence of pre-rupture conditions regarding patients' vision. In this respect, the purpose of this study is to examine the clinical significance of visual symptoms as the prodromata in unruptured anterior communicating artery aneurysm.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of patients operated for ruptured anterior communicating artery aneurysm over the past ten years, from 1991 to 2000, in Neurosurgical department of Sanggye Paik hospital and categorized into sub-groups as direction of aneurysmal rupture. Presence of the aneurysm compressing the optic nerve was verified through pre-surgical radiologic assessment suspected of aneurysm protruded to the anterior inferior direction compressing the nerve. In addition, the presence of conditions affecting the patients' vision prior to the rupture was checked.
The lesion of the optic nerve compressed by intracranial aneurysm was categorized into : Type A (medial portion of the optic nerve); Type B (superior portion of the optic nerve); and Type C (the optic chiasm).
Detailed observation was made of visual symptoms of the patients in each of the three categories. Detailed interviews with the patients' direct caretakers, or in some cases, with the post-operative patients themselves who recovered consciousness, were utilized to verify the presence of the visual symptoms prior to the rupture of intracranial aneurysm. Except for those patients who visited doctor for symptoms indicating the pressure from giant intracranial aneurysm on the optic nerve, the majority of the patients were rushed into the ER due to ruptured intracranial aneurysm, with poor mentality. Though accurate examination by an ophthalmologist prior to the surgery was not available owing to the emergency requiring surgery, detailed ophthalmologic examination was provided for those patients who showed good progress and residual visual symptoms. Patients demonstrating impaired vision due to vitreous bleeding or corneal bleeding known to often accompany Terson's Syndrome were excluded from this study.
RESULTS
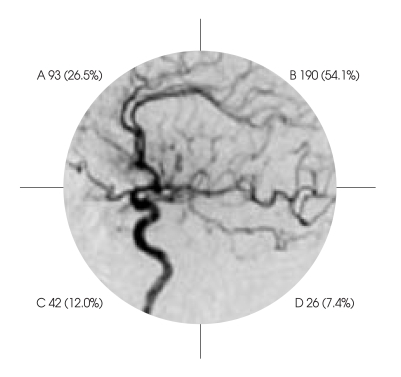
351 ruptured anterior communicating artery aneurysm patients were treated over the past ten years, from 1991 to 2000. Direction of aneurysm protrusion was categorized for surgical visibility, in connection with the direction of the distal anterior cerebral artery (A2) : 1) anterior superior, posterior superior (the same superior direction as for A2); and 2) anterior inferior, posterior inferior (the opposite inferior direction as for A2) (Fig. 1). In terms of direction-based grouping of aneurysm, aneurysm located posterior superior were found to the majority of patients (190 cases, or 50.1%), and the anterior inferior aneurysm with potential to compress the optic nerve were found in 42 cases (12.0% of the total patients) (Fig. 1).
Fig. 1.
Classification of anterior communicating artery aneurysms according to their directions related to A2. Four directios were classified; A : anterior superior, B : posterior superior, C : anterior inferior, D : posterior inferior. Center circular figure represent lateral view of carotid angiogram and numerics represents number of cases (%) of each directions.
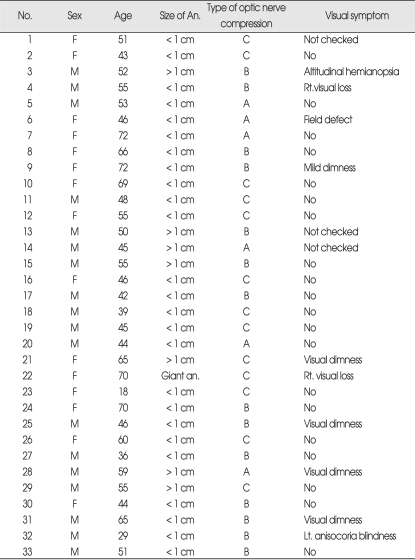
33 patients were selected from the anterior inferior intracranial aneurysm cases which were confirmed, by surgical view, that optic nerve was compressed by the aneurysm. For these cases, analysis was conducted by : 1) size of the 33 patients were selected from the anterior inferior intracranial aneurysm cases which were confirmed, by surgical view, that optic nerve was compressed by the aneurysm. For these cases, analysis was conducted by : 1) size of the aneurysm; 2) category of the optic nerve lesion compressed by the aneurysm; and 3) presence of visual symptoms prior to the aneurysm rupture. The 33 cases consisted of almost the same number of male and female (18 vs. 15), whose age ranged between 18 years old (the youngest) to 72 years old (the oldest) with mean age of 50.6 (years).
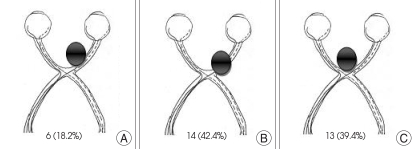
In terms of aneurysm size, the patients were categorized into : 1) less than 1 cm (the majority, or 25 cases); 2) 1cm or larger (7 cases); and 3) giant intracranial aneurysm (1 case). Out of the 33 cases, ten patients showed visual symptoms, such as vision deterioration, tunnel vision, visual loss, etc. These ten patients accounted for approximately 3% of the total of 351 anterior communicating aneurysm patients. In addition, there were three cases where the patients showed poor mentality or expired. Identification of their visual symptoms was infeasible, whereas 20 cases complained such symptoms accounting for 60.6% (Table 1). For the patient with giant intracranial aneurysm, visual loss in the left eye as well as in the right eye was apparent. Interestingly, there were also six cases, by surgical view, which intracranial aneurysm compressed the optic pathway so that deep hollowness was found on the nerve without any visual symptoms (Table 1). Based on surgical view, a categorization was made as the optic pathway lesion compressed by the intracranial aneurysm : 1) Type A (medial portion of the optic nerve) (six cases, or 18.2%); 2) Type B (superior portion of the optic nerve) (14 cases, or 42.4%); and 3) Type C (the anterior superior optic chiasm, pressure on the optic pathway) (13 cases, or 39.4%) (Fig. 2).
Table 1.
Case summary of 33 cases with the aneurysm compressing the optic nerve confirmed by surgical field
Fig. 2.
Classification of 33 anterior communicating artery aneurysms compressing optic nerve according to its compression site confirmed by surgical field. A : medial portion of optic nerve. B : superior portion of optic nerve. C : anterior superior portion of optic chiasm. Numerics represent number of cases (%).
Analysis of the lesion of the optic nerve revealed Patient Case No. 6 (Type A) with temporal hemianopsia in one eye (Fig. 3) and Patient Case No. 3 (Type B) with inferior hemianopsia (Fig. 4) but no patients in Type C with visual symptoms except one complained of dimmed vision (Fig. 5). Total visual loss in one eye was observed in two cases, namely, Patient Case No. 22 with giant intracranial aneurysm. Patient Case No. 32 with pressure on the superior portion of the optic nerve accompanying local hematoma around the nerve (Table 1). Though Patient Case No. 32 had initially visited the ER complaining headache without neurological sign, the brain CT scan showed no abnormal finding. The patient decided to return home voluntarily but was hospitalized two days later due to headache and visual field defect in the left eye. Surgery was later performed following the diagnosis of the anterior communicating artery aneurysm, during which intracranial aneurysm was found to have protruded onto the superior portion of the optic pathway with localized hematoma around the nerve (Fig. 6). Post-operative improvement of visual defects was found in eight out of ten patients in approximately three months, whereas two patients with giant intracranial aneurysm and unilateral visual loss due to hematoma did not show improvement even up to 1 year following surgery.
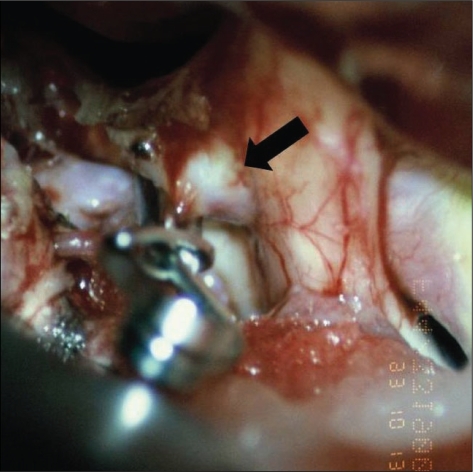
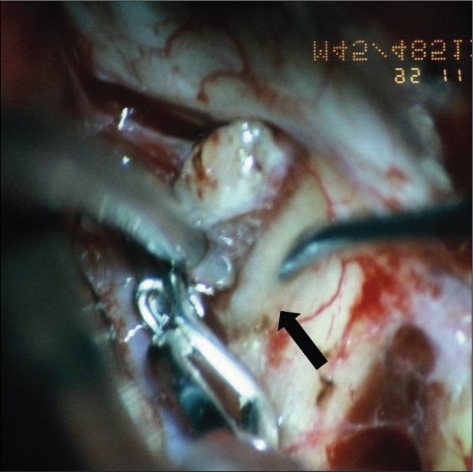
Fig. 3.
Surgical color photo shows dome of aneurysm compressing medial portion of the optic nerve (arrow)(type A).
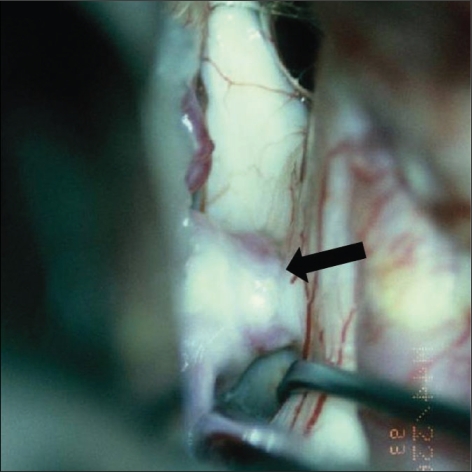
Fig. 4.
Surgical color photo shows dome of aneurysm compressing superior portion of optic nerve (type B). Color change of optic nerve due to compression by the aneurysm is seen (arrow).
Fig. 5.
Aneurysm compressing anterior superior optic chiasm (type C). Dome of aneurysm is sit deeply into the optic nerve (arrow).
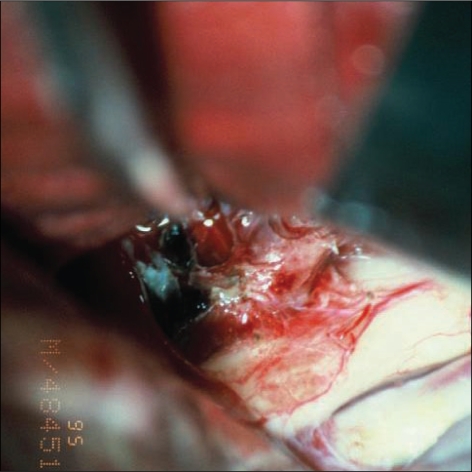
Fig. 6.
Surgical color photo shows dome of aneurysm compressing medial portion of optic nerve with focal hematoma.
DISCUSSION
Anterior communicating artery aneurysm is the most common form of intracranial aneurysm, accounting for 25-38% of total cerebral aneurysm cases.14) Visual deterioration and visual field defect induced by intracranial aneurysm can generally be classified into three categories : 1) visual symptoms caused by bleeding into the eyeball ; 2) visual symptoms due to aneurysm compressing the optic pathway ; and 3) visual symptoms resulting from poor blood circulation due to abnormality in the vessels to the optic pathway.
Usually, in patients with sudden headache and mental change by ruptured intracranial aneurysm causing SAH, show the loss of light reflex and show bleeding tendency in the fundus. Regardless of location, ruptured intracranial aneurysm may accompany SAH as well as hemorrhage in the cornea, the subhyaloid and the vitreous body. In the previous review of literature, Terson's Syndrome was defined as symptoms where visual impairment was generated by intra-globe hemorrhage due to ruptured anterior communicating artery aneurysm. Though the mechanism of post-rupture bleeding into the eyeball has not been fully explained, what is considered the most convincing is that due to sudden increase in venous pressure with the blockage in venous flow to the eyeball caused by increased intracranial pressure, small veins are ruptured and as a result cause hemorrhage18,23,27).
In most cases, visual symptoms are uncommon in patients, even though intracranial aneurysm may compress the optic pathway. However, in case of large intracranial aneurysm, such visual impairment may be observed due to the pressure from the aneurysm5,12,22), which are anatomically close to the anterior optic pathway and the arteries from the Circle of Willis (prevalent site of the intracranial aneurysm). Accompanied symptoms include unilateral scotoma or blindness owing to the pressure on the nerve, bitemporal hemianopsia caused by the pressure on the optic chiasm or homonymous hemianopsia due to compression on the optic tract. Such symptoms progress slowly, as with the increasing size of intracranial aneurysm, repeating multiple cycles of improvement and deterioration that are explained by development of a thrombus in the intracranial aneurysm as well as its expansion. The fluctuation of visual symptom is what differentiates the aneurysm from brain tumor that is characterized by consistent deterioration of visual symptoms1,21,26). The aneurysm accompanying the largest number of visual symptoms is carotid-ophthalmic aneurysm. This is due to the area being closely located to the optic nerve as well as relatively higher occurrence of giant intracranial aneurysm. In case of (atypical) binasal or altitudinal field defects, carotid-ophthalmic aneurysm must be considered9,20). According to Ferguson et al.9), 32 out of 100 patients with carotid-ophthalmic aneurysm showed visual symptom.
For the present study, we analyzed patients accompanying the visual symptom due to anterior communicating artery aneurysm. Our results show that approximately 3% of patients developed visual symptom, which is significantly smaller than in the case of the carotid-ophthalmic aneurysm.
Anterior communicating artery is a short artery connecting the left and right anterior cerebral arteries that travel towards the anterior superior portion of the brain and sit towards the anterior superior direction from the optic chiasm. Location-based analysis shows that optic chiasm is the most common site of intracranial aneurysm13). The protrusion direction of intracranial aneurysm is mostly determined by the difference of both anterior cerebral arteries' (right and left) thickness and by the direction of relatively thicker anterior cerebral artery. As demonstrated in the results, aneurysms in most cases are located posterior superior as in the direction of the distal anterior cerebral artery. In some cases, however, the relatively thicker anterior cerebral artery arches from the posterior superior portion of the brain down towards the mid inferior portion. In such cases, intracranial aneurysm should protrude in the anterior inferior direction, in close contact with the optic pathway, and compress the pathway as they increase in size21,28). Among the patients in this study, the most frequently observed cases were those with cerebral aneurysm protruded upward, parallel to the direction of the distal anterior cerebral artery, and when the direction of the anterior cerebral artery arched from the postero-superior towards the antero-inferior, the direction of the aneurysm was antero-inferior. If the aneurysm protruded more towards the anterior direction, it would compress the optic nerve. If the aneurysm is protruded more towards the posterior direction, it may compress the optic chiasm. Based on the results of this study, cerebral aneurysm Type A, compresses the middle portion of the optic nerve, which may be associated with temporal hemianopsia in unilateral eye. In type B cases with aneurysm compressing the superior optic nerve, inferior hemianopsia may show. As indicated, characteristic of visual field defect is subject to the location of cerebral aneurysm6,7,9,12,13,19,25). Aneurysms of anterior communicating artery, in general, tend to rupture while they are relatively small and cause visual symptoms. Thus, occurrence of giant cerebral aneurysm in this area is extremely rare. Nevertheless, it could be possible, that unruptured giant aneurysm with headache and visual loss may require differential diagnosis from brain tumor such as pituitary adenoma or meningioma that are frequently found in this area12). One patient with giant intracranial aneurysm had complained headache and visual field defect, requiring differential diagnosis for pituitary tumor. Giant intracranial aneurysm with visual symptom is easily differentiated from brain tumor by its repetitive, cyclic pattern of improvement and deterioration in symptom, as mentioned earlier, and by performing cerebral angiography.
When the aneurysm is ruptured, the pressure from arterial blood flow is suspected of damaging the optic nerve as much as the presence of hematoma compressing the optic nerve which may cause visual symptoms. Though the subarachnoid space is an open space, hematoma may be formed around ruptured intracranial aneurysm as the hemorrhage stanches, which could and may compress the optic pathway. In cases where there is adhesion to the surrounding brain tissues caused by a small amount of primary bleeding, localized hematoma may be formed after re-bleeding, which can compress the optic nerve strongly2-4,28).
Visual symptom due to poor blood circulation can be caused by intracranial aneurysm30). For giant intracranial aneurysm, visual symptoms appear due to by compressed optic pathway by aneurysm and also poor blood circulation resulting from the pressure upon the vessels running to the nerve as well. Hematoma may compress microvessels on and around the optic pathway and may cause poor blood circulation. Visual symptoms due to poor blood circulation also can be caused by vasospasm following SAH. We anticipate that poor blood circulation due to vasospasm causes unilateral visual symptom, and can rule out the chance of hematoma or cerebral aneurysm in which they do not compress the optic pathway11,13,16,25,29,30).
Claes et al.4) performed detailed ophthalmologic examination on the patient, who complained a minor temporary headache and suffered from sudden unilateral visual loss in a few hours, but there was no pathologic reason. After a few days patient showed mental change and lumbar puncture was done. The result confirmed that patients had SAH and cerebral angiography discovered the anterior communicating artery aneurysm. This is a case of small-scale hemorrhage of ruptured aneurysm causes visual symptom. Chan et al.2) also reported a clinical case that visual symptom was observed as early-stage symptom with ruptured aneurysm of the anterior communicating artery. It is a case that, considers optic pathway damage caused by vasospasm. There are findings that patients exhibiting sudden hemianoptic altitudinal visual field loss with normal fundus which indicates the presence of posterior ischemic optic neuropathy. Rupture of intracranial aneurysm is known to have no relationship with the symptom16,29).
However, Hara et al.11) reported that ischemic optic neuropathy took place following the rupture of anterior communicating artery. It was reported that early-stage papilloedema could cause the atrophy of the optic nerve11). Previously, pathological examination of tissue samples had revealed the ruptured anterior communicating artery aneurysm which cause hemorrhage directly by compressing the optic pathway. Cerebral vasospasm was also considered to be a possible factor. Craenen et al.5) reported a case of differential diagnosis for a 37-year-old patient with sudden painless unilateral visual loss due to the compression of optic nerve by unruptured aneurysm of the anterior communicating artery. In the report, such aneurysms of the anterior cerebral artery or of the anterior communicating artery (unruptured yet with symptoms) were referred to as paralytic aneurysm8).
Usually, treatment of the cerebral aneurysm causing visual symptom is clipping the aneurysmal neck and to removing the aneurysm sac or hematoma which may compress the optic pathway. Particular care must be taken in order not to damage the nerve. In most cases, clipping operation shows good prognosis, even for the patients with poor findings of fundus. Thus, observation up to two years after the surgery is necessary9,19). Recently, there are reports that coiling of aneurysm, which is commonly performed, has improved patients' visual symptom by reducing thrombus and in particular its size8,10,17).
CONCLUSION
There are many many factors that cause visual symptom include optic nerve compression by the aneurysm. Additional 5 cases in this study, later confirmed that there were usual experiences with headache and dimmed vision in one eye when aneurysm compressed the optic nerve. Therefore, for patients complaining of such symptoms, differential diagnosis should be made to consider the presence of intracranial aneurysm around the optic pathway. And for the differential diagnosis, cerebral angiogram, such as cerebral CT angiogram, should be considered.
References
- 1.Arseni C, Ghitescu M, Cristescu A, Mihăilă G. Intrasellar aneurysms simulating hypophyseal tumors. Eur Neurol. 1970;3:321–329. doi: 10.1159/000113996. [DOI] [PubMed] [Google Scholar]
- 2.Chan JW, Hoyt WF, Ellis WG, Gress D. Pathogenesis of acute monocular blindness from leaking anterior communicating artery aneurysms; report of six cases. Neurology. 1997;48:680–683. doi: 10.1212/wnl.48.3.680. [DOI] [PubMed] [Google Scholar]
- 3.Chong CT, Chin KJ, Yip LW, Singh K. Case series : monocular visual loss associated with subarachnoid hemorrhage secondary to ruptured intracranial aneurysms. Can J Anaesth. 2006;53:684–689. doi: 10.1007/BF03021627. [DOI] [PubMed] [Google Scholar]
- 4.Claes C, Milea D, Bodaghi B, Tran TH, LeHoang P, Blanc R. Acute retrobulbar optic neuropathy due to rupture of anterior communication artery aneurysm. Acta Ophthalmol Scand. 2006;84:145–146. doi: 10.1111/j.1600-0420.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 5.Craenen G, Brown SM, Freedman KA, Windisch TR, Corona J. Rapid, painless unilateral vision loss in an 37-year-old healthy woman. Surv Ophthalmol. 2004;49:343–348. doi: 10.1016/j.survophthal.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Cullen JF, Haining WM, Crombie AL. Cerebral aneurysms presenting with visual field defects. Br J Ophthalmol. 1966;50:251–256. doi: 10.1136/bjo.50.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Date I, Asari S, Ohmoto T. Cerebral aneurysms causing visual symptoms; their features and surgical outcome. Clin Neurol Neurosurg. 1998;100:259–267. doi: 10.1016/s0303-8467(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 8.Debrun GM, Aletich VA, Kehrli P, Misra M, Ausman JI, Charbel F. Selection of cerebral aneurysm for treatment using Guglielmi detachable coils : the preliminary university of Illinois at Chicago experience. Neurosurgery. 1998;43:1281–1295. doi: 10.1097/00006123-199812000-00011. discussion 1296-1297. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson GG, Drake CG. Carotid-ophthalmic aneurysms : visual abnormalities in 32 patients and the results of treatment. Surg Neurol. 1981;16:1–8. doi: 10.1016/s0090-3019(81)80049-3. [DOI] [PubMed] [Google Scholar]
- 10.Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Fraser KW, Smith TP, et al. The efficacy of endovasccular aneurysm occlusion in alleviating neurological deficits produced by mass effect. J Neurosurg. 1994;80:659–666. doi: 10.3171/jns.1994.80.4.0659. [DOI] [PubMed] [Google Scholar]
- 11.Hara N, Mukuno K, Ohtaka H, Shimizu K. Ischemic optic neuropathy associated with subarachnoid hemorrhage after rupture of anterior communicating artery aneurysm. Ophthalmologica. 2003;217:79–84. doi: 10.1159/000068247. [DOI] [PubMed] [Google Scholar]
- 12.Højer-Pedersen E, Haase J. Giant anterior communicating aneurysm with bitemporal hemianopsia; case report. Neurosurgery. 1981;8:703–706. doi: 10.1227/00006123-198106000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 14.Koo SK, Song YJ, Huh JT. Surgically Treated Anterior Communicating Artery Aneurysm. J Korean Neurosurg Soc. 2005;37:405–409. [Google Scholar]
- 15.Kupersmith MJ, Siegel IM, Carr RE. Reduced contrast sensitivity in compressive lesions of the anterior visual pathway. Neurology. 1981;31:550–554. doi: 10.1212/wnl.31.5.550. [DOI] [PubMed] [Google Scholar]
- 16.Lee Ag, Brazis PW, Miller NR. Posterior ischemic optic neuropathy associated with migrane. Headache. 1996;36:506–510. doi: 10.1046/j.1526-4610.1996.3608506.x. [DOI] [PubMed] [Google Scholar]
- 17.Malisch TW, Guglielmi G, Viñuela F, Duckwiler G, Gobin YP, Martin NA, et al. Unruptured aneurysms presenting with mass effect symptoms; response to endosaccular treatment with Guglielmi detachable coils. Part I. Symptoms of cranial nerve dysfunction. J Nurosurg. 1998;89:956–961. doi: 10.3171/jns.1998.89.6.0956. [DOI] [PubMed] [Google Scholar]
- 18.McCarron MO, Alberts MJ, McCarron P. A systematic review of Terson's syndrome; frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004;75:491–493. doi: 10.1136/jnnp.2003.016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino A, Sakurai Y, Arai H, Nishimura S, Suzuki S, Uenohara H. Clinical manifestations, character of aneurysms, and surgical results for unruptured cerebral aneurysms presenting with ophthalmic symptoms. Acta Neurochir Suppl. 2002;82:47–49. doi: 10.1007/978-3-7091-6736-6_9. [DOI] [PubMed] [Google Scholar]
- 20.Nonaka T, Haraguchi K, Baba T, Koyanagi I, Houkin K. Clinical manifestations and surgical results for paraclinoid cerebral aneurysms presenting with visual symptoms. Surg Neurol. 2007;67:612–619. doi: 10.1016/j.surneu.2006.08.074. discussion 619. [DOI] [PubMed] [Google Scholar]
- 21.Norwood EG, Kline LB, Chandra-Sekar B, Harsh GR., 3rd Aneurysmal compression of the anterior visual pathways. Neurology. 1986;36:1035–1041. doi: 10.1212/wnl.36.8.1035. [DOI] [PubMed] [Google Scholar]
- 22.Peiris JB, Ross Russell RW. Giant aneurysms of the carotid system presenting as visual field defect. J Neurol Neurosurg Psychiatry. 1980;43:1053–1064. doi: 10.1136/jnnp.43.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfausler B, Belcl R, Metzler R, Mohsenipour I, Schmutzhard E. Terson's syndrome in spontaneous subarachnoid hemorrhage a prospective study in 60 consecutive patients. J Neurosurg. 1996;85:392–394. doi: 10.3171/jns.1996.85.3.0392. [DOI] [PubMed] [Google Scholar]
- 24.Pico F, Biousse V, Chapot R, Bousser MG. [Aneurysm of the anterior cerebral artery disclosed by unique hemianoptic scotoma.] Rev Neurol (Paris) 2002;158:347–350. [PubMed] [Google Scholar]
- 25.Portney GL, Roth AM. Optic cupping caused by an intracranial aneurysm. Am J Ophthalmol. 1977;84:98–103. doi: 10.1016/0002-9394(77)90332-4. [DOI] [PubMed] [Google Scholar]
- 26.Raymond LA, Tew J. Large suprasellar aneurysms imitating pituitary tumor. J Neurol Neurosurg Psychiatry. 1978;41:83–87. doi: 10.1136/jnnp.41.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resnick SJ, Rabinstein AA. Neurological picture. Terson's syndrome in subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry. 2006;77:287. doi: 10.1136/jnnp.2005.077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruben S, Afshar F. Visual failure following subarachnoid hemorrhage from rupture of an anterior communication artery aneurysm. J Neurol Neurosurg Psychiatry. 1991;54:1017–1018. doi: 10.1136/jnnp.54.11.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadda SR, Nee M, Miller NR, Biousse V, Newman NJ, Kouzis A. Clinical spectrum of posterior ischemic optic neuropathy. Am J Ophthalmol. 2001;132:743–750. doi: 10.1016/s0002-9394(01)01199-0. [DOI] [PubMed] [Google Scholar]
- 30.Thygesen J, Rosenørn J. Transient bilateral blindness in relation to subarachnoid haemorrhage caused by spastic ischemic changes in retina and optic nerve. Acta Ophthalmol (Copenh) 1982;60:325–331. doi: 10.1111/j.1755-3768.1982.tb08388.x. [DOI] [PubMed] [Google Scholar]