Abstract
A 54-year-old man experienced injury to the second finger of his left hand due to damage from a paintball gun shot 8 years prior, and the metacarpo-phalangeal joint was amputated. He gradually developed mechanical allodynia and burning pain, and there were trophic changes of the thenar muscle and he reported coldness on his left hand and forearm. A neuroma was found on the left second common digital nerve and was removed, but his symptoms continued despite various conservative treatments including a morphine infusion pump on his left arm. We therefore attempted median nerve stimulation to treat the chronic pain. The procedure was performed in two stages. The first procedure involved exposure of the median nerve on the mid-humerus level and placing of the electrode. The trial stimulation lasted for 7 days and the patient's symptoms improved. The second procedure involved implantation of a pulse generator on the left subclavian area. The mechanical allodynia and pain relief score, based on the visual analogue scale, decreased from 9 before surgery to 4 after surgery. The patient's activity improved markedly, but trophic changes and vasomotor symptom recovered only moderately. In conclusion, median nerve stimulation can improve chronic pain from complex regional pain syndrome type II.
Keywords: Complex regional pain syndrome, Median nerve, Peripheral nerve stimulation
INTRODUCTION
Chronic pain due to peripheral nerve injury often results in significant suffering and can be challenging to treat both medically and surgically. Complex regional pain syndrome type II (CRPS II), referred to as causalgia, is a chronic painful condition that develops after trauma affecting an arm or a leg with nerve injury. It worsens over time, and may spread to other parts of the body. Use of peripheral nerve stimulation (PNS) for pain is based on the gate control theory of pain introduced by Melzack and Wall in 1965; it is based on the premise that stimulation of large-diameter afferent fibers can interrupt the transmission of nociceptive input10,18). Peripheral nerve stimulator implants have therefore been used to treat patients with chronic peripheral nerve pain.
We report the application of median nerve stimulation in a patient with intractable pain from CRPS II.
CASE REPORT
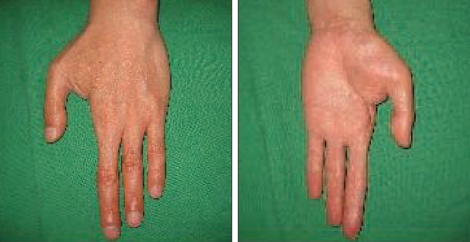
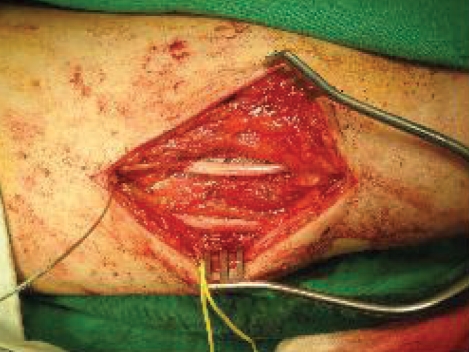
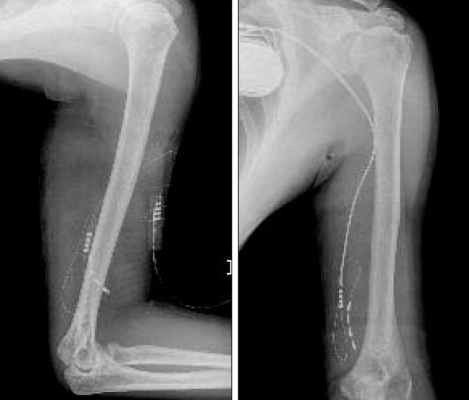
A 54-year-old man experienced injury to the second finger of his left hand due to damage from a paintball gun shot 8 years prior, and the metacarpo-phalangeal joint was amputated. He gradually developed mechanical allodynia and burning pain, and there were trophic changes of the thenar muscle and he reported coldness on his left hand and forearm (Fig. 1). A neuroma was found on the left second common digital nerve and was removed, but his symptoms persisted. The patient underwent a sympathetic nerve block and a morphine infusion pump was placed on his arm to treat his diffuse finger-to-shoulder arm pain in another medical department. The pain improved mildly and temporarily, but it soon recurred. When the medical history of the patient was first taken at our department, the patient reported awakening frequently every night due to pain and he had a very low level of activity. He was taking oral oxycontin® (80 mg/day), IR-codon®, antidepressants, and neurontin® (3600 mg/day). His visual analogue scale (VAS) score for pain was 9/10 and he reported left frozen shoulder pain. We applied median nerve stimulation to treat the chronic pain. The procedure for implantation was performed in two stages. The first procedure involved exposure of the median nerve on the mid-humerus level and placement of the electrode (Fig. 2). The trial stimulation lasted for 7 days, and the improvement in the VAS score from 9 to 5 indicated that this treatment was effective. Therefore, we performed a second procedure that involved implantation of a battery/generator unit at the left pectoral area. Connecting leads were placed subcutaneously through the axilla and medial aspect of the left arm (Fig. 3). The mechanical allodynia and pain VAS score improved from 9 before surgery to 4 after surgery and was maintained at this level at the 10 month follow-up. The patient's activity level improved, and the trophic changes and vasomotor symptoms showed moderate and slow recovery. The left frozen shoulder pain, however, did not change.
Fig. 1.
Digital photograph showing the patient's left hand with amputation on second metacarpo-phalangeal joint and trophic changes.
Fig. 2.
Intraoperative digital photograph showing that paddle type electrode placed on median nerve in the middle humeral level.
Fig. 3.
Simple x-ray showing implantation of electrode and pulse generator.
DISCUSSION
Chronic regional pain disorders are associated with sudomotor or vasomotor changes, and are notoriously difficult to treat. The pathophysiological mechanism underlying CRPS is not completely understood. The International Association for the Study of Pain uses the following clinical findings to define CRPS type I (reflex sympathetic dystrophy) : regional pain, sensory changes, temperature abnormalities, abnormal sudomotor activity, skin color, edema, and onset after a noxious event. CRPS type II includes all of above symptoms plus peripheral nerve lesions. The most common type of CRPS is type I, which occurs in about 90% of all cases. CRPS type II may be the result of a neuroma and is generally associated with scarring of and altered sensation in the injured nerve. Because of the severe pain sometimes associated with a neuroma, patients are often significantly disabled. Our patient was diagnosed as CRPS type II.
Because there is no cure for CRPS, treatment is aimed at relieving painful symptoms, functional recovery, and psychological improvement. The general strategy in CRPS treatment is often multi-disciplinary; different types of medications are combined with distinct physical therapies. Most physicians generally prescribe a variety of drugs to relieve pain, including topical analgesics, antidepressants, anti-inflammatories such as corticosteroids, COX-inhibitors such as piroxicam, vasodilators, GABA analogs such gabapentin and pregabalin, opioids, and alpha- or beta-adrenergic-blocking compounds. However, no single drug or combination of drugs produces consistent long-lasting improvement in symptoms. Other treatment options may include sympathetic nerve blocks, intrathecal drug pumps to deliver opioids, and neurostimulation therapy. In this case, various treatments including oral medications, a sympathetic nerve block, a morphine infusion pump, and the removal of the neuroma failed to alleviate the patient's chronic pain.
The neurostimulation techniques proposed for treating intractable pain are PNS, spinal cord stimulation (SCS), deep brain stimulation (DBS), motor cortex stimulation (MCS), and repetitive transcranial magnetic stimulation (rTMS). SCS for CRPS type I and PNS for CRPS type II have been considered late in the treatment of patients who experience severe pain and disability despite all other therapies2,4,6). MCS and DBS are considered experimental options. A review of the DBS literature indicated that the intractable neuropathic pain of 30-40% of patients was adequately controlled with this therapy14). Nguyen et al.12) reported a prospective study of patients with central and neuropathic facial pain who were treated with chronic stimulation of the motor cortex. rTMS has been proposed as a non-invasive pre-operative therapeutic test for patients with drug-resistant chronic pain who are candidates for surgically implanted chronic MCS9). More data and research are required to verify the efficacy of brain stimulation, as DBS and MCS can cause adverse events including lead migration or battery depletion, and rarely, aseptic meningitis, transient paraparesis, epidural hematoma, epileptic seizure, and skin reactions.
SCS or PNS are alternative treatment options for chronic pain in cases when less invasive procedures have failed or are contraindicated. SCS has been used to treat lower limb pain syndrome5,8,15). Severe CRPS start frequently at a focal point but then spreads to involve other limbs, and can become an almost systemic disease. Because SCS provides more generalized coverage than PNS, it is preferred over PNS in severe CRPS where symptoms have spread to other limbs. SCS appears to be a cost-effective therapy for the management of patients with CRPS type I17).
The hypothesis of PNS is based on the gate control theory of pain10,16,18). In 1967, Wall and Sweet19) reported the first case of implantation of electrodes on the median and ulnar nerves of a patient. Electrical stimulation of the median nerve produced a "pleasant tingling" sensation in the patient's fingers, and the patient's burning pain subsided. Patients with chronic peripheral nerve injury showed the best response1,13,16). Patients suitable for this form of neuromodulation include those with peripheral mononeuropathy (traumatic, idiopathic, or iatrogenic), a chronic entrapment syndrome, and CRPS3,4,11,15). The best predictor for success using a PNS implant to treat intractable pain is accurate patient selection.
PNS implant selection criteria should include the following : 1) a demonstrated injury for the pain complaint, 2) failure of more conservative treatment therapies, including surgery, 3) no significant drug dependence issue, 4) adequate patient motivation and intelligence, 5) clear understanding that PNS neuromodulation is designed to help control chronic pain but not to cure the underlying disease process, 6) successful trial stimulation, 7) identification of the specific injured and painful nerve using a selective nerve root-blocking technique20). PNS can provide relief for patients with CRPS type II localized in the distribution of only one major peripheral nerve, and improve clinical findings2,4).
The advantages of PNS include the simplicity of the surgical procedure, and the fact that it is non-destructive and reversible when the patient turns the stimulator off. Patients can also be trialed prior to implantation of the complete system, thus reducing the cost of permanent implantation if unsuccessful. Furthermore, PNS has less of a positional effect than SCS, because of the minimal positional change of the paddle to the nerve7). The disadvantages of PNS includes the risk of infection, which can be as high as 5%, the risk that the stimulator may cease to be effective in some patients soon after implantation, battery failure or exchange, and the high cost of the implantation device. Most peripheral nerves have sensory and motor components with similar thresholds, and motor responses could be induced by nerve stimulation7). There is also the possibility of nerve entrapment by the stimulator, which necessitates removal of the device11).
In our case, median nerve stimulation for treatment of CRPS type II was suitable and successful, as evidenced by pain relief, improvements in sleep-wake cycles, and reduction of narcotics intake by our patient. No complications occurred. Careful long-term follow-up and prospective studies are needed to verify the effectiveness of PNS for CRPS type II.
CONCLUSION
PNS appears to be an effective treatment option for the management of patients with CRPS type II, and this procedure is simple, non-destructive, reversible and safe. However, accurate patient selection is important for a successful treatment outcome.
References
- 1.Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976;45:692–699. doi: 10.3171/jns.1976.45.6.0692. [DOI] [PubMed] [Google Scholar]
- 2.Ebel H, Balogh A, Volz M, Klug N. Augmentative treatment of chronic deafferentation pain syndromes after peripheral nerve lesions. Minim Invasive Neurosurg. 2000;43:44–50. doi: 10.1055/s-2000-8413. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg E, Waisbrod H, Gerbershagen HU. Long-term peripheral nerve stimulation for painful nerve injuries. Clin J Pain. 2004;20:143–146. doi: 10.1097/00002508-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hassenbusch SJ, Stanton-Hicks M, Schoppa D, Walsh JG, Covington EC. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415–423. doi: 10.3171/jns.1996.84.3.0415. [DOI] [PubMed] [Google Scholar]
- 5.Jang HD, Kim MS, Chang CH, Kim SW, Kim OL, Kim SH. Analysis of failed spinal cord stimulation trials in the treatment of intractable chronic pain. J Korean Neurosurg Soc. 2008;43:85–89. doi: 10.3340/jkns.2008.43.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy : two years' follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13–18. doi: 10.1002/ana.10996. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH. Interventional and Surgical Treatment of Pain. Seoul: Youngchang medical publisher; 2005. Peripheral nerve stimulation in Korean Society of Stereotactic and Functional Neurosurgery; pp. 598–609. [Google Scholar]
- 8.Kim SH, Tasker RR, Oh MY. Spinal cord stimulation for nonspecific limb pain versus neuropathic pain and spontaneous versus evokec pain. Neurosurgery. 2001;48:1056–1064. doi: 10.1097/00006123-200105000-00017. discussion 1064-1065. [DOI] [PubMed] [Google Scholar]
- 9.Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75:612–616. doi: 10.1136/jnnp.2003.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 11.Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14:216–221. doi: 10.1016/j.jocn.2005.11.007. discussion 222-223. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier A, Fontaine D, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245–251. doi: 10.1016/S0304-3959(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 13.Novak CB, Mackinnon SE. Outcome following implantation of a peripheral nerve stimulator in patients with chronic nerve pain. Plast Reconstr Surg. 2000;105:1967–1972. doi: 10.1097/00006534-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Rezai AR, Rozano AM. Burchiel KJ. Surgical management of pain. New York: Thieme medical publishers; 2002. Deep brain stimulation (DBS) for pain; pp. 565–576. [Google Scholar]
- 15.Stanton-Hicks M, Salamon J. Stimulation of the central and peripheral nervous system for the control of pain. J Clin Neurophysiol. 1997;14:46–62. doi: 10.1097/00004691-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Strege DW, Cooney WP, Wood MB, Johnson SJ, Metcalf BJ. Chronic peripheral nerve pain treated with direct electrical nerve stimulation. J Hand Surg Am. 1994;19:931–939. doi: 10.1016/0363-5023(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome : a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91–101. doi: 10.1016/j.ejpain.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Wall PD. The gate control theory of pain mechanisms. A re-examination and re-statement. Brain. 1978;101:1–18. doi: 10.1093/brain/101.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108–109. doi: 10.1126/science.155.3758.108. [DOI] [PubMed] [Google Scholar]
- 20.Weiner RL. Peripheral nerve neurostimulation. Neurosurg Clin N Am. 2003;14:401–408. doi: 10.1016/s1042-3680(03)00037-8. [DOI] [PubMed] [Google Scholar]