Abstract
The literature abounds with reports of malformation syndromes in which human external ears are variously described as dysplastic, abnormal, large/small, low set, typical, or in some way unusual. Rarely is the ear well illustrated or described in meaningful detail. With few exceptions, such as Down syndrome, there is no real understanding of the degree to which ear morphology is affected in a specific syndrome. This paper describes a retrospective attempt to apply the recently published Elements of Morphology: Standard Terminology of the ear to compare a control sample of convenience with a group of patients with Cornelia de Lange syndrome (CdLS) (all six papers in this issue are available online, open access at http://www3.interscience.wiley.com/journal/121641055/issue).
Although this study has a number of limitations, it demonstrates that the method can be successfully applied and is capable of producing data that can be subjected to statistical analysis. The ears of the patients with CdLS were significantly different from the controls over a number of descriptors, the most significant of which included more frequent apparent posterior rotation, a shorter more serpiginous antihelical stem and sharper antihelical to inferior crus angle, a shorter crus helix, a more V-shaped incisura, and a smaller lobe.
Keywords: Cornelia de Lange syndrome, ear dysmorphology, minor ear variants, anthropometry
INTRODUCTION
In 2005, Hunter and Yotsuyanagi suggested that greater attention to the specific anatomy of the ear might aid in the diagnosis of certain syndromes. At that time, a group of dysmorphologists/medical geneticists began an effort to standardize the description of clinical morphology and the respective terminology used to report syndromes and dysmorphic signs; so-called dysmorphic features. This group of experienced clinical dysmorphologists from many countries standardized definitions and descriptions for the individual components of the craniofacies, hands, and feet that are used in the delineation and diagnosis of syndromes [Allanson et al., 2009; Biesecker et al., 2009; Carey et al., 2009; Hall et al., 2009; Hennekam et al., 2009]. That work, included one paper dedicated to the external ear [ Hunter et al., 2009]. With the exception of the incisura, this study used those standardized descriptions of the ear to compare a sample of ears from the general population (controls) to the ears from patients with a single syndrome. Signs that could be assessed quantitatively on all ears using the photographic scales developed by the Ear Dysmorphology Subgroup (EDS) [Hunter et al., 2009], such as antihelical stem length, tragal and antitragal size were selected for evaluation with other anomalies and variants recorded as they occurred. Cornelia de Lange syndrome (CdLS) was chosen as a sample of convenience because lateral facial photographs from a large group of well evaluated patients were available. This syndrome is not known for significant anatomical variation in the ear. Indeed the only comment on the ear in CdLS in the most widely used text on human syndromes is to simply mention low-set ears as an ‘occasional finding’ [Jones, 2006].
The sample of control ears had two purposes. First, by systematically applying the photographic scales developed by the EDS, the study would test the distribution of scores and determine whether what was considered average by the EDS actually was the common score in a sample population. Secondly, it provided a comparison group for the CdLS patient population. Studying the patients provided a test of whether the approach would find any ear differences in a syndrome not traditionally known for ear anomalies.
METHODS
One individual (AH) scored the photographs of ears for both patients and controls. The photographs of CdLS ears were not taken specifically for purposes of this study, although the control photographs were taken for purposes of the EDS. A single ear was used from each patient and from each control. No photographs were marked with a measuring tape or reference to the facial plane, which limited some of the potential assessments of size and orientation. The quality of the photograph meant that on some ears in the patient group it was not possible to assess some of the pertinent features. The assessment was not blinded as to patient versus control ear but the patient’s mutation status was not revealed until the assessments were complete.
Controls
The 57 control ears were a sample of convenience taken of individuals of both sexes, ranging in age from 6 to 65 years of age that worked in the Genetics department at The Children’s Hospital of Eastern Ontario and/or those of family, friends or colleagues. Ages were not generally recorded but the majority fell into the 30 to 45 year age group. The same SLR camera with a macro lens, held at 90° to the subject, was used from a distance of about 15 inches. The photograph was limited to the ear and minimal surrounding lateral skull; the face was not included and voluntary turning of the head to an appropriate position was taken as consent. The request for a personal ear photograph was not subject to Research Ethics Board review. (As an aside, very few people can recognize their own ears.) The control ears were the first to be analyzed.
CdLS patient group
The 119 ear photographs of the CdLS individuals were from those evaluated and studied molecularly at The Children’s Hospital of Philadelphia. Ages ranged from 3 days to 45 years with a mean of 8.3 years; 24 were older than 10 years and six over 25 years-of-age. The photographs were coded and forwarded without any data as to mutation status to AH for dysmorphology assessment. They were cropped to eliminate the face and any identification of the individual. Once that analysis was complete, the mutation status was supplied so that the groups could be examined by specific gene involvement, including “no mutation found.” Of the patients, 54 had mutations in NIPBL, 7 in SMC1A, 1 in SMC3, no mutation was found in 39, and 18 were not tested. The patients with SMC1A and SMC3 mutations were combined for analysis as both involve the core Cohesin complex, both result in a mild phenotype, and each group had small numbers.
Assessment of the ears
Each morphologic ear sign (EMS) was evaluated on all ears in succession before moving on to the next sign. With the exception of Rotation, Length/Width (L/W) ratio, and the Incisura, the definitions and assessment categories were those now published by the EDS [Hunter et al., 2009]. A numerical Likert scale was applied to the categories (Table I), and a scale for the Incisura was developed for this study [Hunter, 2009]. In addition, an attempt was made similarly to score the length of the antihelical stem (Fig 1) and its angulation [Hunter et al., 2009] (Fig 2), as well as the width of the superior crus of the antihelix (Fig 3). The EMSs that were assessed are listed in column 1 of Appendices 1 to 6. Although the comparisons use a photographic scale, it is important to note that most of the scoring remains a subjective evaluation of the ear against this scale.
Table I.
Scoring of the various components of the ear as used in the study
| Antihelix, Stem | |||||
| Length * | Absent | Short | Average | Long | |
| Prominence | “ | Underdeveloped | “ | Prominent | |
| Angulated ** | No angle | Slight | ~90° | >90° | |
| Serpiginous | No | Yes | - | - | |
| Score | 0 | 1 | 2 | 3 | |
| Antihelix, Inferior Crus | |||||
| Length | Absent | Short | Average | Long | |
| Width | “ | Narrow | “ | Broad | |
| Prominence | “ | Underdeveloped | “ | Prominent | |
| Angle | “ | Anterosuperior | Horizontal | Anteroinferior | |
| Score | 0 | 1 | 2 | 3 | |
| Antihelix, Superior Crus | |||||
| Width * | “ | Narrow | “ | Broad | |
| Prominence | “ | Underdeveloped | “ | Prominent | |
| Score | 0 | 1 | 2 | 3 | |
| Antitragus | |||||
| Size | Absent | Underdeveloped | Average | Prominent | |
| Score | 0 | 1 | 2 | 3 | |
| Crus Helix | |||||
| Length * | Absent | Short | Average | Long | |
| Prominence | “ | Underdeveloped | “ | Prominent | |
| Extension | No | Yes | |||
| Score | 0 | 1 | 2 | 3 | |
| Incisura *** | |||||
| Length | Short | Average | Long | - | |
| Width | Wide | “ | Narrow | Slit | |
| Shape | U | V | Λ | – | |
| Score | 1 | 2 | 3 | 4 | |
| Lobe Size | Very small | Slightly Small | Average | Large | Very large |
| Score | 1 | 2 | 3 | 4 | 5 |
| Tragus | |||||
| Size | Absent | Underdeveloped | “ | Prominent | |
| Score | 0 | 1 | 2 | 3 | |
Newly defined in this manuscript
Expanded definition from present/absent in Hunter et al., 2009, Figure 7
Figure 1.
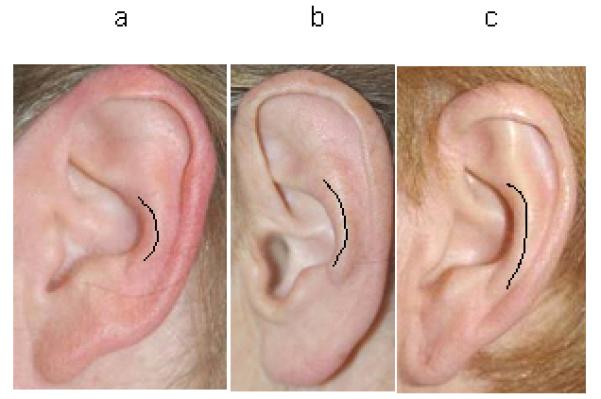
Scale for assessment of antihelical stem length; 1a is short, 1b average, and 1c long.
Figure 2.
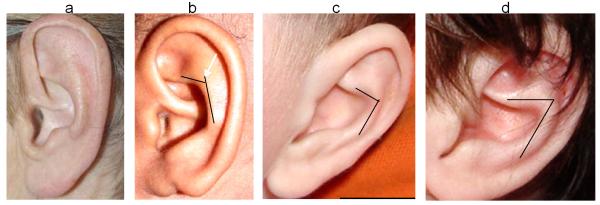
Method used to assess antihelical to inferior antihelical crus angle. Usually there is a smooth transition from the antihelical stem to its crus (no angle). 2a shows a mild angle greater than 90° (scored anterosuperior), 2b shows an angle of about 90° (scored horizontal) and 2c the angle is acute and clearly less than 90 (scored anteroinferior).
Figure 3.
Scale for assessment of width of the superior crus of the antihelix; 3a is narrow, 3b average, and 3c wide.
Two scores require special explanation. First, the lack of actual direct length or width measurements, or of a metric scale pre-placed on the photographs, meant that no direct assessment of ear size was possible. However, it seemed possible that the ratio of length to width would be an interesting variable to examine and the method used is illustrated in Figure 4. A line was first drawn through the long axis of the ear; a second line, originating at the insertion of the ear at the anterior edge of the tragus, judged as the most anterior beginning of the tragal elevation, was then drawn perpendicular to that line and extending beyond the posterior margin of the ear. Finally, a line parallel to the long axis but at the extreme lateral edge of the ear was drawn to intersect the perpendicular line. The longitudinal axis was taken as length, and the distance of the perpendicular line from the anterior tragus to the intersection with the second of the parallel lines as the width. In the absence of a sizing standard on the photograph these distances do not represent the true size of the ear and so the photographic measurements were expressed as a L/W ratio. Secondly, the lack of a true reference plane precluded proper assessment of ear rotation. However, since general observation showed that sideburn hair tends to grow straight down and parallel to the height axis of the body, a sense of the rotation of the ear could be obtained by comparison to the “vertical” sideburn hair. If the insertion of the anterior edge of the ear at the face, assessed using a protractor, was rotated posteriorly more than 20° to the hair it was scored as rotated (Fig. 5). Clearly this approach is not rigorous, but it was applied equally to both patient and control samples. Furthermore, it serves as an appropriate tool, since current methods of assessing ear rotation continue to be imperfect [Hunter et al., 2009].
Figure 4.
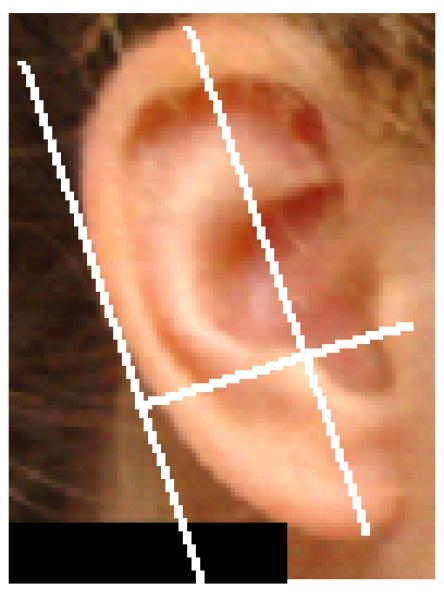
An ear marked with a line on the longitudinal axis of the ear (length) and a line parallel to that drawn touching the extreme posterior of the ear. The width was taken as the perpendicular distance from the anterior tragus to intersect with the posterior parallel line.
Figure 5.
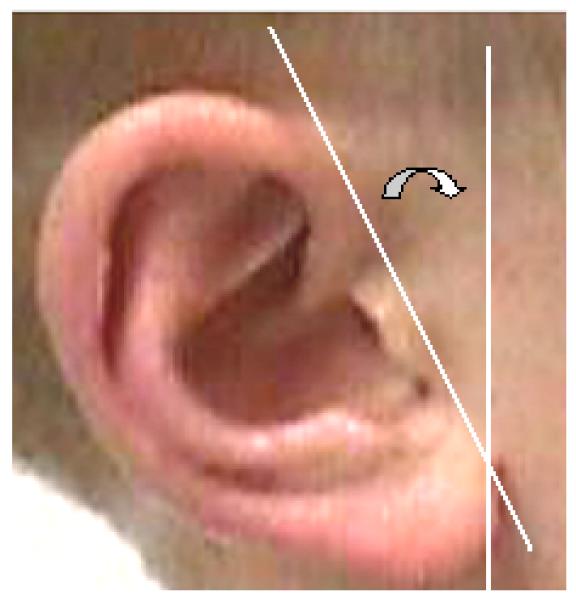
Ear showing how the rotation was estimated as the angle (curved arrow) between a vertical line parallel to the sideburn hair and a line along the insertion of the ear at the face.
RESULTS
The total numbers in each scoring category (usually scaled from 0 to 3) for each assessed EMS (e.g. tragal prominence) for the controls and for the patients subdivided by mutation category were tabularized and are provided as Appendices 1-5. All patients are combined in Appendix 6. The L/W ratios totaled by 0.10 intervals between 1.30 and 3.00 are summarized as Appendix 7. Underneath each appendix is a list of additional descriptive variations (such as everted antitragus [Hunter et al., 2009] that were recorded for each group). The summarized data were transcribed to a graphical format for ease of visualization (available on request from AH). For most variants there were examples where a trait could not be assessed because it was obscured by hair, ear position, or simply poor quality of the photograph. This was more common in the patient group where the photographs were not taken specifically to illustrate the ear but were cropped from lateral facial views. The number of missing data per EMS is recorded on the appendices.
As the first step, the distributions of the control data scores (Appendix 1) were examined to see whether most scores indeed fell into the expected average range (Table I). In 12 of the 14 items scored in this fashion, the defined average for each EMS in Hunter et al. [2009] and each EMS added for this study (Table I) was indeed the most frequent score, with the defined average ranging from 43.9% to 91.2% of controls. In 10 of the 13 examples scored as 0 to 3 the defined average score represented greater than 70% of the ears. In some cases, the spread in scores was relatively even on either side of the mean, whereas in others there was significant skewing either above or below the mean. The two exceptions, where the defined average was not the most common choice, were the tragus, where 62.5% were judged as underdeveloped, and the antitragus, where 50.9 % where judged as underdeveloped and 17.5% as absent. Extension of the crus helix, serpentine shape to the antihelix, and posterior rotation of the ear were all scored as absent (No=0) or present (Yes=1), with absent considered the typical form. This proved consistent in all three of these EMSs (89.3% to 98.2%). The lobe size had 55.6% considered average with the distribution skewed towards the ‘larger than average’ in this adult control group. For the antihelix angle, a score of ‘0’ indicated the usual pattern and was recorded in 91.2% of the controls. Incisura shape was simply scored as being ‘U’, ‘V’, or ‘Λ’, and 70% were the ‘U’ form where the sides are parallel. The distribution of L/W ratio is shown in Appendix 7. Thirty-eight of the 57 controls (64%) lay between 2.0 and 2.29 with a range of 1.80 to 2.89.
Observational assessment
Although the 119 CdLS patients are genotypically heterogeneous, they provide a large patient group diagnosed because they share significant clinical signs, and so were first combined as a group for comparison with controls. Visual comparison of the data sets in Appendices 1 and 2 immediately show differences in the distribution of scores. Four of the 18 scores that met the average or present/absent expectations in the controls, did not do so in the patient group; the length of the crus helix was most commonly scored as short, the angle of the inferior crus of the antihelix and of the antihelix was reduced, and three-quarters of the ears were judged as posteriorly rotated by the method used, as compared to only one of 57 controls. Furthermore, virtually all of the remaining 14 scores, where average or present/absent remained the most common score, showed much more skewing with the range of a low of 35.5 % to a high of 70.2%, with most having 50% or less scored as average.
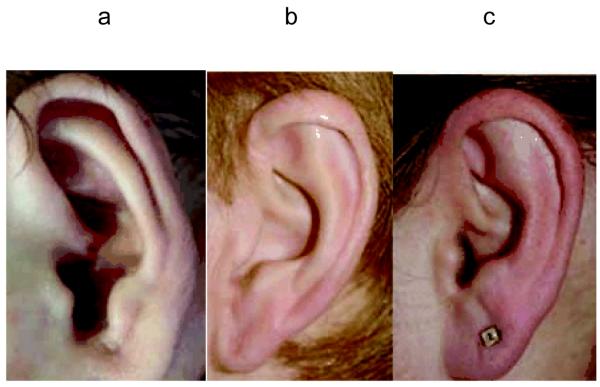
A serpiginous shape to the antihelix was much more common in patients (29.2% versus 7% of controls). Almost one-in-six patients had a wide open incisura to the point where it was difficult to judge where the concha began; 12 patients, but only one control, lacked a substantive portion of the ear forming the lower incisural margin between the tragus and antitragus (Figure. 6), so that it essentially ended at the side of the face [Hunter, 2009]. Another striking difference in the CdLS ears, as compared to the controls, was that the L/W ratio (Appendix 7) demonstrated a much squarer ear shape with 72 of the 112 measurable patients falling at or below the lowest value recorded for any control (1.80); 60 fell between 1.60 and 1.79 and their range was 1.30 to 2.39.
Figure 6.
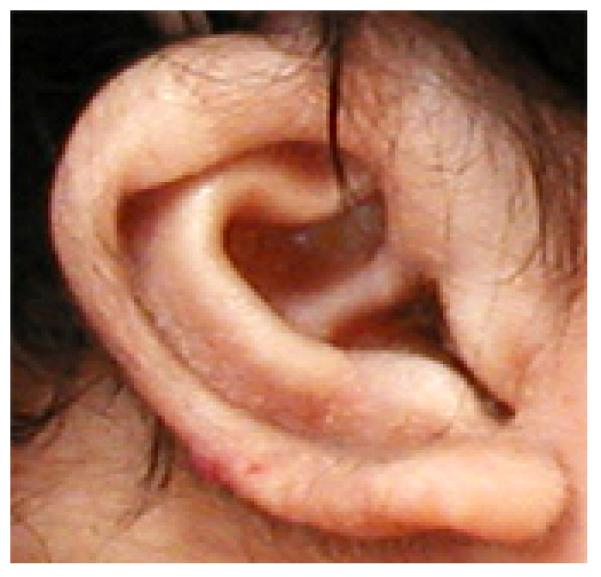
Example of an ear showing the open incisura (described in detail in Hunter, 2009, Am J Med Genet, in press) found to be relatively common in CdLS.
Statistical assessment
The CdLS group was compared against controls using the Pearson Chi Square test and the Yates corrected Chi Square test was used when appropriate for small numbers. Cells containing less than 5 were lumped with the next cell(s) in ascending value as required. The data and results are summarized in Table II. Data was managed using Microsoft Excel (Redmond, OR) and analyzed in Excel and Epi Info™ (Atlanta, GA). Fourteen of the 20 Chi Square tests were significant; all but three at less than the 0.01 level (Table II).
Table II.
Chi square values for comparison between total patients and the controls for each of the ear characteristic examined. Scoring as outlined in Table I, after the Ear Morphology Subcommittee [Hunter et al., 2009]). Where numbers were too small in categories to allow statistical comparison they were grouped with the adjacent score as indicated in the Table.
| Variable name | Score-patient/controls | χ2 value |
P value | ||
|---|---|---|---|---|---|
| Score | Yes | No | |||
| Crus helix, extended | 88/50 | 23/6 | 2.6 | 0.1071 | |
| Antihelix, serpiginous | 33/4 | 80/53 | 9.7* | 0.0019 | |
| Ear rotation | 27/56 | 79/1 | 75.7* | <0.0001 | |
| Score | 0 | 1 | 2-3 | ||
| Antitragus size | 9/10 | 77/29 | 28/18 | 5.6 | 0.0613 |
| Score | 0-1 | 2-3 | |||
| Tragus size | 100/36 | 14/20 | 12.9 | 0.0003 | |
| Crus helix, prominence | 48/1 | 54/56 | 33.1* | <0.0001 | |
| Inferior crus, length | 12/12 | 86/45 | 2.1 | 0.1438 | |
| Inferior crus, width | 37/10 | 64/47 | 6.4 | 0.0117 | |
| Inferior crus, angle | 44/55 | 60/2 | 43.4* | <0.0001 | |
| Antihelix, length | 55/12 | 58/45 | 12.1 | 0.0005 | |
| Score | 0-1 | 2 | 3 | ||
| Crus helix, length | 65/6 | 31/41 | 8/11 | 40.4 | <0.0001 |
| Antihelix, prominence | 6/6 | 80/45 | 28/6 | 5.7 | 0.0589 |
| Score | 0-1 | 2 | 3-4 | ||
| Superior crus, prominence | 43/15 | 37/25 | 13/17 | 8.2 | 0.0165 |
| Score | 0-2 | 3 | |||
| Superior crus, width | 79/43 | 14/12 | 1.1 | 0.2960 | |
| Inferior crus, prominence | 71/50 | 30/7 | 6.2 | 0.0130 | |
| Score | 1 | 2-3 | |||
| Antihelix, angle | 70/52 | 42/5 | 15.5 | <0.0001 | |
| Incisura, length | 33/11 | 82/46 | 1.8 | 0.1836 | |
| Incisura, shape | 56/40 | 59/17 | 7.1 | 0.0076 | |
| Score | 1 | 2 | 3-4 | ||
| Incisura, width | 10/7 | 47/41 | 58/9 | 19.4 | <0.0001 |
| Score | 1-2 | 3 | 4-5 | ||
| Lobe, size | 38/6 | 54/30 | 26/21 | 10.7 | 0.0048 |
Yates correction applied
The L/W ratios were transformed using their natural log to make the distributions for each variable more normal, and a two-sided, unpaired Student’s t-test was used to compare groups. An F-test was run before the t-test to check if the variances were equal, and this information was used to run the t-test. The L/W ratios between the case and control groups were highly significantly different (p=1.84E-24).
With the exception of the NIPBL mutation-positive and the mutation-negative CdLS patients, the individual mutation groups were too small to be compared with each other or with the controls. When comparing the NIPBL mutation-positive patients to the mutation-negative CdLS patients (Table III), two measures showed a significant difference; the degree of definition of the superior crus of the antihelix and the length of the crus helix. The L/W ratio did not differ significantly (p=0.2367) between the NIPBL mutation-positive and the mutation-negative CdLS patients.
Table III.
Chi square values for comparison between the NIPBL mutation positive probands and those in whom no mutation was found for each of the ear characteristic examined. Scoring is as outlined in Table I, after [Hunter et al., 2009]. Where numbers were too small in categories to allow statistical comparison they were grouped with the adjacent score as indicated in the Table.
| Variable name | Score- NIPBL/No mutation |
χ2 value |
P value | ||
|---|---|---|---|---|---|
| Score | Yes | No | |||
| Crus helix, extended | 9/13 | 41/23 | 3.61 | 0.576 | |
| Antihelix, serpiginous | 13/14 | 40/22 | 2.09 | 0.148 | |
| Ear rotation | 36/25 | 13/8 | 0.05 | 0.816 | |
| Score | 0-1 | 2-3 | |||
| Tragus size | 43/34 | 9/5 | 0.34 | 0.5571 | |
| Antitragus size | 34/30 | 16/8 | 1.30 | 0.2534 | |
| Crus helix, length | 33/20 | 11/17 | 3.90 | 0.0483 | |
| Crus helix, prominence | 26/15 | 17/21 | 2.767 | 0.0958 | |
| Superior crus, width | 17/9 | 27/19 | 0.31 | 0.5670 | |
| Inferior crus, length | 5/5 | 43/29 | 0.34 | 0.5587 | |
| Inferior crus, width | 16/13 | 30/21 | 0.10 | 0.7508 | |
| Inferior crus, angle | 19/15 | 30/21 | 0.07 | 0.7880 | |
| Antihelix, length | 27/19 | 25/18 | 0.00 | 0.9576 | |
| Antihelix, angle | 30/25 | 23/10 | 1.98 | 0.1597 | |
| Score | 0-1 | 2 | 3-4 | ||
| Superior crus, prominence | 21/6 | 15/5 | 8/17 | 13.69 | 0.0011 |
| Score | 0-2 | 3 | |||
| Antihelix, prominence | 41/26 | 12/11 | 0.58 | 0.4481 | |
| Inferior crus, prominence | 33/24 | 14/10 | 0.00 | 0.9709 | |
| Score | 1 | 2-3 | |||
| Incisura, length | 15/9 | 36/30 | 0.45 | 0.5007 | |
| Incisura, shape | 24/18 | 27/21 | 0.01 | 0.9320 | |
| Score | 1-2 | 3-4 | |||
| Incisura, width | 23/23 | 28/16 | 1.70 | 0.1919 | |
| Score | 1-2 | 3 | 4-5 | ||
| Lobe, size | 12/12 | 28/16 | 13/11 | 1.34 | 0.5117 |
DISCUSSION
The task of the Dysmorphology Working Group and its various subcommittees was to provide an acceptable definition, describe the variability in the morphology, and standardize a name for each of the surface markers used in the current practice of Clinical Dysmorphology. In instances where a clear variant was either present or absent (e.g. preauricular pit), this job was fairly easy and objective. Of greater challenge were variations that are continuous such as absent to large or prominent. In these cases, a photographic scale was developed to illustrate the range of variation in the population. The scoring (for example as underdeveloped, average, or prominent) was not based on a formal assessment of a good sized sample of the population. It was subjectively assigned based upon a group’s consensus as to what they thought was usual or average. Thus it is somewhat reassuring that for 18 of the 20 variations assessed on the control ears using the EDS standards and some additional quantification, the majority of control scores were assigned as average or present/absent as expected. The poorest correlation of those 18 was the Superior Crus of the Antihelix. This is not unexpected as it is one of the most variable and difficult to assess features in ear morphology. The two outliers of the 20 variations assessed were the size of the tragus and antitragus. It is interesting that these were the only two components where the scoring and photographic illustrations were based upon a monograph and line drawings by Langer [1966]. As his population was German it is unlikely that the differences are ethnic, and if the current findings are reproduced it may be that the average size of the tragus and antitragus is smaller than it was thought to be by the EDS.
Although CdLS was not chosen for having characteristic changes in ear morphology, this study provides some preliminary evidence that the ears do differ from those of the general population and that many of those differences are highly statistically significant. Among the most characteristic (significant) differences, which perhaps best define the ear in CdLS, were posterior rotation, a shorter crus helix which lacks prominence, a sharper angle at the origin of the inferior antihelical crus, angulation of the antihelix and a greater width, especially of the upper margin, to the incisura. Large numbers are required to show statistical differences and the only possible comparisons using our data were between patients with a NIPBL mutation and those in whom no mutation was found. Given the number of tests performed, the only difference that can truly be accepted as different (p=0.0011) was a reduced definition of the superior crus in the NIPBL mutation-positive group. It would be of interest to compare adequate numbers of patients with different mutations.
This study provides an initial validation of the work of the EDS group in that it has shown that the ear can be examined systematically and that most of the definitions of average [Hunter et al., 2009] appear valid. It also shows that careful assessment of the specific components of the ear can reveal differences in the ears of patients with specific syndromes from the general population, even where the ear is not established significantly in the diagnosis or pathology of the syndrome. It seems axiomatic that this approach will serve to better define the anomalies of the ear, especially when it is more involved in a specific syndrome, and thus make the ear a more useful resource for diagnosis.
This study has significant limitations that compromise particularly the certainty of the control-patient comparisons. The most important is that the photographs were not taken for this specific purpose and the age distributions differed between patient and controls, although to date there are no data as to whether the morphological features examined change with age. Although the control photographs were limited to the ear and were taken with the head vertical and hair pushed aside, no measurement scale or vertical facial plane was applied. In the case of the patients, the ear generally had to be cropped from a profile and enlarged somewhat; in some cases the quality was poor and meant that data could not be easily assessed. In some cases where the original photograph was not a true profile it is possible that the angulation may have distorted some of the assessments. This may, at least in part, have contributed to a wider spread of results among patients. The control ears were from an older population than the patient group and this might have exaggerated some of the inter-group differences. In addition, the assessments were all carried out by a single individual who was not blinded as to patient versus control. Thus, scoring represents one person’s subjective view of scoring and raises potential bias as an explanation for the differences that were observed. However, given the number of significant differences between patients and controls, the fact that no analyses were performed until after all measurements were completed, and the lack of many differences between the NIPBL mutation-positive versus mutation-negative patients, such a bias seems unlikely to have been a major concern. While we think the study provides good evidence that the scales developed by the EDS are valid for the adult controls there is need to examine the ear systematically across childhood. The differences noted in CdLS require confirmation. Until consistency can be shown between different individuals in the assessment of controls, it will be important for researchers to employ their own control samples.
There are significant advantages in using standardized photographs that can be examined at leisure and compared to photographic scales. The careful placement of a measurement scale, close to the ear and parallel to the vertical axis of the skull, would allow for accurate measurement of various components of the ear and the development of more precise ranges for normal values. In addition, evaluation of a group of individuals by the same examiner(s) to compare photographic ear morphology and “in vivo” assessments of ear morphology would be interesting.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thanks all those who volunteered to have their ears photographed, and the patients and their families, without whom studies such as this would not be possible. This work was supported by the following NIH grants: NICHD PO1 HD052860 (IDK), NICHD K08 KHD055488A (MAD) and NICHD 5P30HD026979 (MAD). AH and JC were supported, in part, by grants from the South Carolina Department of Disabilities and Special Needs and the Genetics Endowment of South Carolina.”
REFERENCES
- Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodriguez C, Arnedo M, Loeys B, Kline AD, Wilson M, Lillquist K, Siu V, Ramos FJ, Musio A, Jackson LS, Dorsett D, Krantz ID. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of Cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–494. doi: 10.1086/511888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanson JE, Cunniff C, Hoyme HE, McGaughran J, Muenke M, Neri G. Defining morphology: standard terminology for the head and face. Am J Med Genet. 2009;149A:6–28. doi: 10.1002/ajmg.a.32612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker LG, Aase JM, Clericuzio C, Gurrieri F, Temple K, Toriello H. Defining morphology: standard terminology for the hands and feet. Am J Med Genet Part A. 2009;149A:93–127. doi: 10.1002/ajmg.a.32596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JC, Cohen MM, Jr, Curry C, Devriendt K, Holmes L, Verloes A. Defining morphology: standard terminology for the oral region. Am J Med Genet Part A. 2009;149A:77–92. doi: 10.1002/ajmg.a.32602. [DOI] [PubMed] [Google Scholar]
- Hall BD, Graham JM, Jr, Cassidy SB, Opitz JM. Defining morphology: standard terminology for the periorbital area. Am J Med Genet Part A. 2009;149A:29–39. doi: 10.1002/ajmg.a.32597. [DOI] [PubMed] [Google Scholar]
- Hennekam RC, Cormier-Daire V, Hall J, Mehes K, Patton M, Stevenson R. Defining morphology: standard terminology for the nose and philtrum. Am J Med Genet. 2009;149A:61–76. doi: 10.1002/ajmg.a.32600. [DOI] [PubMed] [Google Scholar]
- Hunter AG, Frias J, Gillessen-Kaesbach G, Hughes H, Jones K, Wilson L. Defining morphology: Ear. Am J Med Genet Part A. 2009;149A:40–60. doi: 10.1002/ajmg.a.32599. [DOI] [PubMed] [Google Scholar]
- Hunter AGW, Yotsuyanagi T. The external ear: more attention to detail may aid syndrome diagnosis and contribute answers to embryological questions. Am J Med Genet Part A. 2005;135A:237–250. doi: 10.1002/ajmg.a.30723. [DOI] [PubMed] [Google Scholar]
- Hunter AG. Defining Morphology of the Ear: Addendum; the Incisura. Am J Med Genet. 2009 in press. [Google Scholar]
- Jones KL. Smith’s Recognizable Patterns of Human Malformation. 6th edition Elsevier Saunders; Philadelphia: 2006. pp. p82–84. [Google Scholar]
- Lange G. Familieuntersuchingen uber die Erblichkeit metrischer und morphologischer Merkmale des ausserer Ohres. Z Morphol Anthropol. 1966;57:111–187. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.