Abstract
Background
We investigated effects of vaccination with AIDSVAX B/E HIV-1 candidate vaccine on blood and seminal plasma HIV-1 ribonucleic acid viral load (BVL and SVL, respectively) in vaccine recipients (VR) and placebo recipients (PR) who acquired infection.
Methods
Linear mixed models were fitted for repeated measurements of BVL. Generalized estimating equations were used to assess the difference in SVL detectability between VR and PR.
Results
A total of 196 participants became HIV-1 infected during the trial. Thirty-two (16%) became infected with HIV-1 subtype B and 164 (84%) with HIV-1 subtype CRF01_AE. Per protocol-specified analysis, there were no differences in BVL levels between VR and PR. When stratified by HIV-1-infecting subtype, vaccination with AIDSVAX B/E was initially associated with higher BVL among HIV-1 CRF01_AE-infected VR compared to HIV-1 CRF01_AE-infected PR, however, this difference did not persist over time. HIV-1 subtype B-infected VR had slightly higher BVL levels and were more likely to have detectable SVL during the follow-up period than HIV-1 subtype B-infected PR.
Conclusions
Subtle differences in BVL and SVL were detected between VR and PR. These results may help to further understand the dynamics between HIV-1 vaccination, HIV-1-infecting subtypes, and subsequent viral expression in different body compartments.
Keywords: HIV-1 vaccine, HIV-1 RNA viral load, Injecting drug users
INTRODUCTION
The optimal long-term approach to controlling the human immunodeficiency virus type 1 (HIV1) pandemic is an effective HIV-1 preventive vaccine that induces protective immunity against HIV-1 infection. At present, only two phase III HIV-1 vaccine trials have been completed; one in North America and Europe, and one in Thailand. Both candidate vaccines, AIDSVAX B/B and AIDSVAX B/E, are synthetic recombinant glycoproteins representing the specific HIV-1 subtypes circulating in the geographic regions of each trial. Unfortunately, neither AIDSVAX B/B nor AIDSVAX B/E showed protective efficacy against primary HIV-1 infection.1, 2
The lack of AIDSVAX B/B or AIDSVAX B/E efficacy and the absence of other candidate HIV1 vaccines for phase III trials make it important to explore whether recently tested HIV-1 vaccines have other potential effects.1, 2 For instance, as some candidate vaccines have reduced blood plasma HIV-1 ribonucleic acid (RNA) viral load level and delayed disease progression in animal models, viral load is used as a putative surrogate for secondary transmission and acquired immunodeficiency syndrome (AIDS) progression.3–7 We previously reported no significant differences in disease progression between AIDSVAX B/E recipients (VR) and placebo recipients (PR) who became HIV-1-infected, which was consistent with findings from the AIDSVAX B/B vaccine trial.1, 2 In this paper, we report results of our investigation of effects of vaccination with AIDSVAX B/E on BVL levels and seminal plasma HIV-1 RNA viral load (SVL) detectability among HIV-1-infected VR compared to HIV-1-infected PR, while controlling for HIV-1-infecting subtype and time since the estimated date of seroconversion (EDS).8
METHODS
Study population
A randomized, double-blind, placebo-controlled efficacy trial of phase III AIDSVAX B/E was initiated in Bangkok, Thailand, in March 1999. Details of the trial design and conduct are presented elsewhere.1, 9 Briefly, after giving voluntary informed consent, 2,546 HIV-1-uninfected injecting drug users (IDUs) were randomly assigned at a 1:1 ratio to receive seven doses of either AIDSVAX B/E or placebo. Based on positive enzyme linked immunoassay and confirmatory Western blot with two new bands other than glycoprotein (gp) 120 or gp160, 230 participants were identified as HIV-1-infected during the trial. Retrospective testing of sera with a highly sensitive nucleic acid-based amplification test found 19 participants HIV-1 infected at enrollment. Immunizations were discontinued from the time of detection of HIV-1 infection. HIV-1 seroconvertors were offered enrollment into a prospective follow-up study for a total duration of 36 months; 200 enrolled. Additional follow-up of 24 months (through September 2004) was offered to seroconvertors who completed the initial 36 months for extended immunologic and virologic evaluations. Study protocols were approved by the ethical review committees of collaborating institutes.
Data collection and laboratory methods
Standardized interviews collected demographic characteristics at enrollment and HIV-1 risk behaviors at every study visit. Thereafter, each participant received risk reduction counseling and was clinically evaluated by a study physician. The participants received medical care including ART for their HIV-1 infection.10, 11 Blood samples were collected at 2 weeks and at months 1, 2, 4, and every 4 months following first HIV-1 positive test. Samples were processed and tested according to the study protocol. Lymphocyte immunotyping was performed on fresh anti-coaggulated blood samples, using a FACScan flow cytometer (Becton Dickinson Immunocytometry System, U.S.A.).12 Blood plasma samples were tested to determine HIV-1 RNA levels, using the Amplicor HIV-1 Monitor Test, version 1.5 (Roche Molecular Systems, U.S.A.). Genetic characterization of HIV-1 subtypes was performed by molecular sequence analysis of the C2-V4 env region.1
Additionally, 95 HIV-1-infected male participants consented to provide semen samples scheduled at months 1, 2, 4, and every 4 months following EDS (median, three samples). Samples were checked for quality, and cells and sperm activity were microscopically evaluated. After separation of cells, HIV-1 RNA in seminal plasma was extracted using the NucliSen kit (Organon Teknika, The Netherlands) to remove an inhibitor of the Amplicor internal control and quantified using a modified Amplicor HIV-1 Monitor Test version 1.5.13, 14
Statistical analysis
The EDS was assumed to be the mid-point between the date of the last HIV-1 seronegative and the date of the first HIV-1 seropositive visit. For 122 BVL measurements beyond the assay’s reliable detection range (400–750,000 copies/mL), arbitrary levels of 200 and 750,000 copies/mL were assigned for values below and above the limit, respectively. BVL and detectable SVL levels were log-transformed to produce a normal distribution for the purpose of analysis.15–17
Demographic and behavioral characteristics and follow-up time of VR and PR were compared using the Chi-square test or the Fisher’s exact test for categorical variables and the Student’s t-test for continuous variables.18–20 Only individuals infected with HIV-1 subtype B or CRF01_AE were included; one PR infected with a non-B strain and three PR infected with a non-typable strain were excluded, resulting in 196 individuals for this analysis. To control for confounding effects of ART on BVL and SVL levels, only data collected prior to ART initiation were incorporated in these analyses (total samples=1,195 for BVL and 392 for SVL).
Because HIV-1 RNA viral load has been shown to vary according to stage of HIV-1 infection, serial HIV-1 RNA viral load levels were analyzed over time. Robust, locally weighted, nonparametric, smoothed regression was used to describe temporal trends of BVL and SVL.21 Linear mixed models, with random intercepts and slopes over time, were fitted for BVL data with exchangeable correlation structure to account for intra-individual correlations.22 All variables, except time since the EDS, were entered in the models as categorical variables. In this study, ART initiation guidelines were based on CD4+ T-lymphocyte (CD4) counts; hence, they predict data exclusion. However, given the similarity in proportions of VR and PR who started ART (p=0.36) and that CD4 was thought to be an intermediate factor between vaccination and viral load level, it was justified that approximately unbiased estimates of the vaccine efficacy on pre-ART viral load level could be obtained without CD4 level included as a covariate in the analysis. Two- and three-way interaction terms were generated as products of variables (study arm, HIV-1-infecting subtype, and time) and entered in the models.
The SVL levels were dichotomized into detectable or undetectable HIV-1 RNA viral load groups because HIV-1 RNA was unquantifiable in the majority of seminal plasma samples. Generalized estimating equation (GEE) models were fitted, with HIV-1-infecting subtype, study arm, sample collection time, and interaction terms as independent variables.23
For each infected participant, the average BVL and the average SVL were computed using all available pre-ART values from samples collected between 2 and 6 months and between 16 and 19 months following EDS. These windows were chosen because relatively large amounts of data were available, and they approximately reflect initial set-point viral load and a later value of viral load. BVL and SVL were compared using Wilcoxon rank-sum test.24
A 2-sided p-value of ≤0.05 was used to indicate statistical significance. Analyses were performed using Stata version 9.0 (StataCorp LP, TX, U.S.A.) and SAS version 9.1 (SAS Institute Inc., NC, U.S.A.). The following abbreviations were used: HIV-1 CRF01_AE-infected VR (VRHIV E+), HIV-1 CRF01_AE-infected PR (PRHIV E+), HIV-1 subtype B-infected VR (VRHIV B+), and HIV-1 subtype B-infected PR (PRHIV B+).
RESULTS
Demographic and other characteristics of study participants
Of the 196 participants, 97 (49%) were VR, and 99 (51%) were PR. All participants were Thai, and 186 (95%) were male. The median age at diagnosis of HIV-1 infection was 27 (interquartile range [IQR], 25–34) years; 189 (96%) had completed at least primary school. A lifetime history of incarceration was reported by 172 (88%), and the median number of incarceration times was 4 (IQR, 2–6). One hundred and ninety-five (99%) had received at least 3 doses of either vaccine or placebo (at 0, 1, and 6 months). Compared to VR, a higher proportion of PR reported currently cohabiting with a sexual partner (p=0.01) and reported less frequent sexual intercourse with a casual sex partner (p=0.03).
HIV-1-infecting subtypes and follow-up time
Of the 97 VR, 83 (86%) were infected with HIV-1 CRF01_AE and 14 (14%) with HIV-1 subtype B. Of the 99 PR, 81 (82%) were infected with HIV-1 CRF01_AE and 18 (18%) with HIV-1 subtype B. The relative hazard (vaccine vs. placebo) of HIV-1 CRF01_AE infection was similar to that of HIV-1 subtype B infection (discrete time Cox regression accounting for subtypes B and CRF_01 AE as competing risks, p=0.84).25 All HIV-1 subtype B strains clustered on phylogenetic analysis with strains known as “Thai B.”26, 27
Excluding post-ART visits, the median follow-up time was 25 (IQR, 18–32) months, corresponding to a median of 9 (IQR, 6–10) study visits. Follow-up times did not differ between VR and PR (p=0.79).
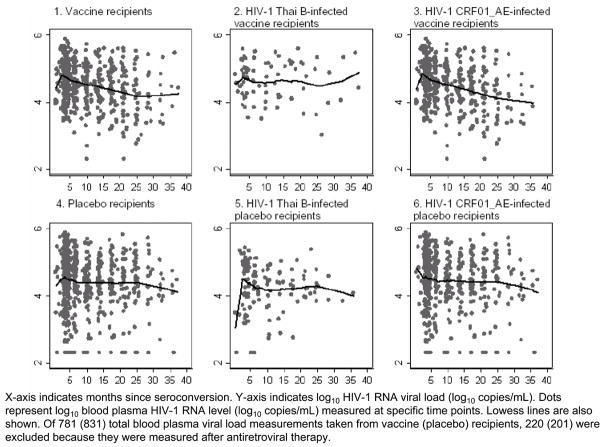
Blood plasma HIV-1 RNA viral load levels
Summaries of log10 BVL levels of pre-ART measurements are presented in Figure 1. The mean durations from the EDS to the first viral load determination were 2 (IQR, 1–3) months among VR and 2 months (IQR, 2–3) among PR (p=0.92). Of the 196 participants, none had a BVL level below or higher than the assay’s reliable detection limit at the first determination.
Figure 1.
Pre-antiretroviral therapy blood plasma HIV-1 RNA viral loads among injecting drug users who acquired HIV-1 infection while participating in AIDSVAX B/E efficacy trial, Bangkok, Thailand, 1999–2003
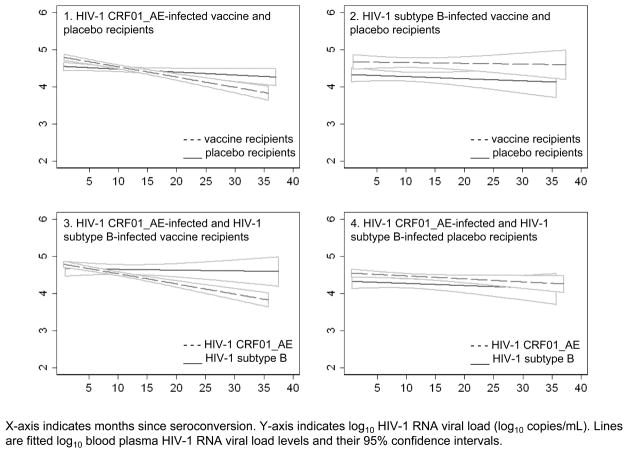
Vaccine recipients versus placebo recipients controlling for HIV-1-infecting subtype
The estimated difference in BVL levels between VRHIV E+ and PRHIV E+ at first month following EDS was 0.2 log10 (1.7-fold; VRHIV E+>PRHIV E+; p<0.01). However, this difference did not persist over time (Figure 2-1). BVL levels among VRHIV E+ were 0.2 log10 lower than those of PRHIV E+ at month 24 following EDS (1.7-fold; VRHIV E+<PRHIV E+; p<0.01). The estimated difference in BVL levels between VRHIV B+ and PRHIV B+ was 0.3 log10 through out follow-up time (1.8-fold; VRHIV B+>PRHIV B+; p<0.01; Figure 2-2).
Figure 2.
Fitted pre-antiretroviral therapy blood plasma HIV-1 RNA viral load levels and their corresponding 95% confidence intervals among injecting drug users who acquired HIV-1 infection while participating in AIDSVAX B/E efficacy trial, Bangkok, Thailand, 1999–2003
HIV-1 CRF01_AE-infected individuals versus HIV-1 subtype B-infected individuals controlling for study arm
The estimated difference in BVL levels at first month following the EDS between VRHIV E+ and VRHIV B+ was 0.1 log10 (1.4-fold; VRHIV E+>VRHIV B+; p=0.07). This difference decreased over time, but at approximately 12 months following EDS increased towards the end of follow-up (Figure 2-3). The estimated difference in BVL levels between PRHIV E+ and PRHIV B+ was 0.1 log10 (1.4-fold; PRHIV E+>PRHIV B+; p=0.03) throughout the follow-up period (Figure 2-4).
Seminal plasma HIV-1 RNA viral load
Detectability of seminal plasma HIV-1 RNA viral load
One hundred and twenty-nine (33%) samples from 95 participants had detectable SVL. Of these, 67 (52%) were collected from VR (47 from VRHIV E+ and 20 from VRHIV B+), and 62 (48%) were from PR (53 from PRHIV E+ and 9 from PRHIV B+). Fifty percent of samples were collected on the same day (IQR, 0–5) as blood. Figure 3 illustrates temporal trends of pre-ART log10 SVL levels over time by study arm and HIV-1-infecting subtype. Visually, SVL level of VRHIV E+ was similar to that of PRHIV E+ (Figures 3-3 and 3-6); SVL level of VRHIV B+ was higher than that of PRHIV B+ (Figures 3-2 and 3-5).
Figure 3.
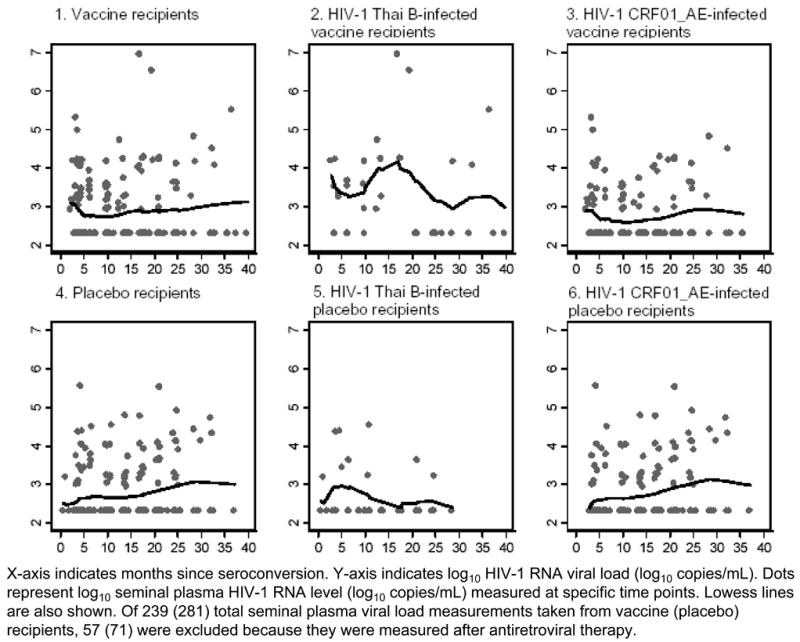
Pre-antiretroviral therapy seminal plasma HIV-1 RNA viral loads among 95 male injecting drug users who acquired HIV-1 infection while participating in AIDSVAX B/E efficacy trial, Bangkok, Thailand, 1999–2003
Vaccine recipients versus placebo recipients controlling for HIV-1-infecting subtype
PRHIV E+ and VRHIV E+ had no significant difference in SVL detectability (odds ratio [OR], 1.0; 95% confidence interval [CI], 0.5–2.2; p=0.98). VRHIV B+ were 7.4 times as likely as PRHIV B+ to have detectable SVL (OR, 7.4; CI, 1.7–32.5; p=0.01).
HIV-1 subtype B-infected individuals versus HIV-1 CRF01_AE-infected individuals controlling for study arm
VRHIV E+ were significantly less likely than VRHIV B+ to have detectable SVL (OR, 0.1; CI, 0.0–0.4; p=0.01). PRHIV E+ and PRHIV B+ had no significant difference in SVL detectability (OR, 0.4; CI, 0.1–2.3; p=0.28).
Association between blood and seminal plasma HIV-1 RNA viral load levels
Analysis of 374 paired samples showed that among VR the median SVL was 1.8 log10 copies/mL (IQR, 1.2–2.3) lower than BVL, and among PR, the median SVL was 1.9 log10 copies/mL (IQR, 1.2–2.4) lower. For the 2–6 month and 16–19 month windows, the average SVLs were significantly lower than the average BVLs based on paired data (p<0.01 for both VR and PR). Detectable BVL and undetectable SVL was present in 257 (66%) paired samples; undetectable BVL and detectable SVL was uncommon (one paired sample, 0.3%). Among participants with detectable average SVL, no correlation was observed between average BVL and average SVL for the 2–6 month window (Spearman’s r, 0.11; p=0.62), nor for the 16–19 month window (Spearman’s r, −0.35; p=0.20). The distribution of average 2–6 month BVL among those with detectable average 2–6 month SVL (median, 5.09 log10 copies/mL) differed (borderline) significantly from that of those with undetectable average 2–6 month SVL (median, 4.8 log10 copies/mL; p=0.06). The difference was larger at the 16–19 month window, with median average 16–19 month BVL 5.2 log10 copies/mL compared to 4.2 log10 copies/mL for those with detectable and undetectable SVL, respectively (p<0.01).
Twenty-four of 40 (60%) HIV-1 CRF01_AE-infected participants had undetectable SVL. Among the 16 detectable participants, the median 2–6 month average SVL was 3.1 log10 copies/mL (standard deviation [SD], 0.8). Two of 7 (29%) HIV-1 subtype B-infected participants had undetectable SVL. Among the 5 detectable participants, the median 2–6 month average SVL was 3.4 log10 copies/mL (SD, 0.6). For the 16–19 month window, 30 of 43 (70%) HIV-1 CRF01_AE-infected participants and 4 of 6 (67%) HIV-1 subtype B-infected participants had undetectable SVL. The median 16–19 month average SVL for the 13 HIV-1 subtype B-infected participants and the 2 HIV-1 CRF01_AE-infected participants with detectable SVL were 3.9 (SD, 0.5) and 3.4 log10 copies/mL (meaningful SD could not be calculated), respectively.
DISCUSSION
In the per protocol-specified analysis of this clinical trial, no differences in BVL levels were found between VR and PR.1 However, when taking into account interactions between study arm, HIV-1-infecting subtype, and time, we found that vaccination with AIDSVAX B/E was associated with slightly higher BVL levels among 83 VRHIV E+ compared to 81 PRHIV E+ during the first 12 months of the study. After that time, BVL levels among VRHIV E+ were lower than those of PRHIV E+. Fourteen VRHIV B+ had higher BVL levels compared to 18 PRHIV B+ throughout the follow-up period. VRHIV B+ were significantly more likely to have detectable SVL than PRHIV B+.
The natural history of HIV-1 infection among PR in this study is consistent with that of Hu et al. in that higher BVL levels were observed in PRHIV E+ compared to PRHIV B+ at the onset of HIV-1 infection.8, 28 The slightly higher BVL levels and higher odds of SVL detectability among VRHIV B+ is noteworthy, albeit the small number of HIV-1 subtype B-infected individuals. Additionally, the analyzed groups were selected by the post-randomization event of HIV-1 infection; thus, a causal interpretation of the findings may be limited by selection bias.29 The reported changes in BVL, while relatively slight, might be of concern, as these may potentially increase infectiousness. In a systematic review of studies assessing the association between changes in BVL and risk of HIV-1 transmission during heterosexual contact, Modjarrad et al. found evidence suggesting that an increment in BVL as small as 0.3 log10 could increase the likelihood of HIV-1 transmission by 20%.30 However, it should be noted that none of the included studies examined SVL, and the estimated 20% increase in transmission risk was abstracted from studies that varied by a number of factors including sample size, study population demographics, viral load assay type, and study design. In our study, BVL and SVL were concurrently evaluated and we found that despite the subtle increase in BVL, the SVL among the majority of our participants was still below the sexual transmission threshold of 3.2 log10 copies/mL, leading us to believe that transmission through this mode was unlikely.31 The small increase in individuals’ BVL, however, might have had some impact on HIV-1 transmission through other risk behavior, in particular through injection drug use.
Though the full understanding of the impact of vaccination on individuals’ infectiousness and clinical progression is limited, our findings emphasize the importance of inclusion of BVL and SVL as vaccine trial endpoints.32 In conjunction with virological endpoints, screening test of concept (STOC) trials may also be considered to accelerate the AIDS vaccine development and to better utilize limited resources available.33
Our study is the first to address the impact of gp120 candidate vaccines on HIV-1 RNA shedding in the male genital tract. Data from two recent animal studies suggested that gp120 may enhance viral loads when given with other vaccine components.34, 35 In both studies, vaccinated macaques showed less consistent control of post-challenge viremia with pathogenic simian immunodeficiency virus compared to controls receiving vaccine components without gp120. Statistically higher BVL levels were also observed in macaques receiving vaccine components with gp120 compared to controls, which suggests that pre-exposure to the Env component of the vaccine enhanced viral replication. Env 120 or 160 may raise enhancing antibodies to levels that were not evident by the in vitro assay, influence the activation of one T helper cell subset over another, or stimulate a cytokine response profile that drives overall viral expression differently than non-Env component.34 Expansion of previously primed-memory CD4 without a strong CD8+ T-lymphocyte (CD8) response, may lead to preferential infection of these lymphocytes and more rapid viral replication upon viral challenge than in controls.35 However, in another trial of the gp120 candidate vaccine (AIDSVAX B/B) in a population of predominantly men who have sex with men in North America and Europe, the pre-ART BVL was similar in the 225 VR and 122 PR who acquired HIV-1 subtype B infection; semen samples, though, were not collected.36
The findings may also be explained by a ‘sieve’ effect of the vaccine.37 The epidemic of HIV-1 subtype B among IDUs in Bangkok preceded the epidemic of HIV-1 CRF01_AE in this population.26, 27 Thus, HIV-1 subtype B-infected individuals may have had a more antigenetically diverse or more virulent virus than those with HIV-1 CRF01_AE infection, resulting in increased penetration of the vaccine barrier. Higher levels of nucleotide diversity and divergence have been shown to be associated with higher BVL levels.38 A sieve analysis may help determine if potential vaccine-induced protection depends on genotypic and phenotypic variations of the HIV-1 strain and could clarify if it was the HIV-1 strain, immune response, type of cells infected, or a combination of these factors that results in HIV-1-subtype specific BVL and SVL differences.
The findings of this study are of particular importance after the Merck adenovirus trial (“STEP trial”) was halted in late 2007.39 Our data and those of the STEP trial demonstrate that either humoral or cell-mediated immunity vaccine alone did not prevent HIV-1 infection or reduce the HIV-1 viral set-point.40 Our results cannot be directly compared to the STEP trial due to some aspects. First, AIDSVAX B/E was an HIV-1 Env vaccine, while the Merck vaccine was an HIV1 Gag/Pol/Nef vaccine. Second, the two vaccines elicited different immune responses (humoral vs cellular). Third, the AIDSVAX B/E trial was a phase III trial conducted based on promising safety and immunologic profiles derived from phase II studies while the STEP trial was a phase IIB test-of-concept study. To understand the complexity of viral-host interaction found in our study, additional genetic characterization of virus variation and studies of host such as CD4 and CD8 responses to gp120 in relation to virus control, CD4 phenotypes in terms of infection reservoir/enhancement, enhancing antibodies to HIV-1 CRF_01 AE and subtype B, and Fc receptor genotypes would have been necessary. However, these immunologic and viral studies were not conducted since the vaccine did not show efficacy and breakthrough infection did not occur. Note that in the ongoing prime-boost phase III efficacy trial in Thailand, AIDSVAX B/E is administered in combination with HIV-1 vCP1521 vaccine. The efficacy of this regimen in preventing HIV-1 infection remains to be seen.
In this study, we collected a large number of paired blood and semen samples to study BVL and SVL in the setting of an HIV-1 vaccine trial. This provided a unique opportunity to study interaction between HIV-1-infecting subtype, study arm, and viral load outcomes. Though our results are based on one study in a specific setting with limited sample size, they may help in formulating hypotheses for further HIV-1 research towards developing an effective HIV-1 vaccine.
Acknowledgments
Financial support: The phase III efficacy trial of AIDSVAX B/E was carried out with financial support from VaxGen, Inc., South San Francisco, CA, U.S.A. and the Centers for Disease Control and Prevention, Atlanta, GA, U.S.A., as part of a research collaboration with the Bangkok Metropolitan Administration, the Ministry of Public Health of Thailand, and Mahidol University, Bangkok, Thailand. Wanitchaya Kittikraisak was a doctoral trainee supported by the Fogarty AIDS International Training and Research Program grant number D43-TW00003 at the University of California, Berkeley, CA, U.S.A.
We are greatly indebted to all participants in our study. We thank the staff from the BMA, TUC, VaxGen, Inc., CDC, and Department of Microbiology, Faculty of Medicine, Siriraj Hospital, Mahidol University for administrative, clinical, laboratory, and data management support. Specifically, we would like to acknowledge: Thitima Cherdtrakulkiat and Supawadee Na-Pompet for study coordination; Philip Mock for statistical consultation; Nartlada Chantharojwong for data management; Wanna Leelawiwat, Punneeporn Wasinrapee, Nancy Young, and Thanyanan Chaowanachan for laboratory support; and Chonticha Kittinunvorakoon for laboratory consultation. Lastly, we sincerely thank: Robert Chen, Sal Butera, and John Brooks for input in the discussion section; and Arthur L. Reingold, Robert Linkins, and Peter Kilmarx for critical review of this manuscript.
Footnotes
Conflict of interest: None of the authors have a commercial or other financial interest associated with the information presented in this manuscript, except Dr Marc Gurwith who is employed by and owns stock in VaxGen, Inc.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of U.S. Centers for Disease Control and Prevention.
References
- 1.The Bangkok Vaccine Evaluation Group. Randomized, placebo-controlled efficacy trial of a bivalent rgp120 HIV-1 vaccine among injecting drug users in Bangkok, Thailand. J Infec Dis. 2006;194(12):1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 2.Flynn NM, Forthal DN, Harro CD, et al. Placebo-controlled phase 3 trial of recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191(5):654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 3.Barouch DH, Santra S, Schmitz JE, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290(5491):486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 4.Amara RR, Villinger F, Altman JD, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292(5514):69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 5.Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 6.Hu DJ, Vitek CR, Bartholow B, Mastro TD. Key issues for a potential human immunodeficiency virus vaccine. Clin Infect Dis. 2003;36(5):638–644. doi: 10.1086/367891. [DOI] [PubMed] [Google Scholar]
- 7.Buchacz K, Hu DJ, Vanichseni S, et al. Early markers of HIV-1 disease progression in a prospective cohort of seroconverters in Bangkok, Thailand: implications for vaccine trials. J Acquir Immune Defic Syndr. 2004;36(3):853–860. doi: 10.1097/00126334-200407010-00013. [DOI] [PubMed] [Google Scholar]
- 8.Hu DJ, Vanichseni S, Mastro TD, et al. Viral load differences in early infection with two HIV-1 subtypes. AIDS. 2001;15(6):683–691. doi: 10.1097/00002030-200104130-00003. [DOI] [PubMed] [Google Scholar]
- 9.Vanichseni S, Tappero JW, Pitisuttithum P, et al. Recruitment, screening and characteristics of injection drug users participating in the AIDSVAX B/E HIV vaccine trial, Bangkok, Thailand. AIDS. 2004;18(2):311–316. doi: 10.1097/00002030-200401230-00022. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Public Health. National guidelines for the clinical management of HIV infection in children and adults. 6. Nonthaburi: Division of AIDS, Department of Communicable Disease Control, Ministry of Public Health; 2000. [Google Scholar]
- 11.Ministry of Public Health. Guidelines for the care and treatment of HIV/AIDS in children and adults in Thailand. 7. Nonthaburi: Division of AIDS, Department of Communicable Disease Control, Ministry of Public Health; 2002. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Revised guidelines for performing CD4+ T-cell determinations in persons infected with human immunodeficiency virus (HIV) MMWR Recomm Rep 1997. 1997;46(RR2) [PubMed] [Google Scholar]
- 13.Coombs RW, Speck CE, Hughes JP, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177(2):320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 14.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyles RH, Lyles CM, Taylor DJ. Random regression models for human immunodeficiency virus ribonucleic acid data subject to left censoring and informative drop-outs. Appl Stat. 2000;49(4):485–497. [Google Scholar]
- 16.Smith SM, Holland B, Russo C, Dailey PJ, Marx PA, Connor RI. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res Hum Retroviruses. 1999;15(18):1691–1701. doi: 10.1089/088922299309739. [DOI] [PubMed] [Google Scholar]
- 17.Hudgens MG, Hoering A, Self SG. On the analysis of viral load endpoints in HIV vaccine trials. Stat Med. 2003;22(14):2281–2298. doi: 10.1002/sim.1394. [DOI] [PubMed] [Google Scholar]
- 18.Pearson ES. The choice of statistical test illustrated on the interpretation of data classed in a 2 × 2 table. Biometrika. 1947;34:139–167. doi: 10.1093/biomet/34.1-2.139. [DOI] [PubMed] [Google Scholar]
- 19.Upton GJG. Fisher’s exact test. J Roy Statist Soc A. 1992;155(3):395–402. [Google Scholar]
- 20.Gosset WS., Student The probable error of a mean. Biometrika. 1908;6(1):1–25. [Google Scholar]
- 21.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. [Google Scholar]
- 22.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 23.Liang KY, Zeger SL. Longitudinal data analysis using general linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 24.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1945;1(6):80–83. [Google Scholar]
- 25.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 26.Subbarao S, Limpakarnjanarat K, Mastro TD, et al. HIV type 1 in Thailand, 1994–1995: persistence of two subtypes with low genetic diversity. AIDS Res Hum Retroviruses. 1998;14(4):319–327. doi: 10.1089/aid.1998.14.319. [DOI] [PubMed] [Google Scholar]
- 27.Subbarao S, Vanichseni S, Hu DJ, et al. Genetic characterization of incident HIV type 1 subtype E and B strains from a prospective cohort of injecting drug users in Bangkok, Thailand. AIDS Res Hum Retroviruses. 2000;16(8):699–707. doi: 10.1089/088922200308693. [DOI] [PubMed] [Google Scholar]
- 28.Kivela PS, Krol A, Salminen MO, et al. High plasma HIV load in the CRF01-AE outbreak among injecting drug users in Finland. Scand J Infect Dis. 2005;37(4):276–283. [PubMed] [Google Scholar]
- 29.Gilbert PB, Bosch RJ, Hudgens MG. Sensitivity analysis for the assessment of causal vaccine effects on viral load in HIV vaccine trials. Biometrics. 2003;59(3):531–541. doi: 10.1111/1541-0420.00063. [DOI] [PubMed] [Google Scholar]
- 30.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. Aids. 2008 Oct 18;22(16):2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraborty H, Sen PK, Helms RW, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001 Mar 30;15(5):621–627. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- 32.Gupta SB, Jacobson LP, Margolick JB, et al. Estimating the benefit of an HIV-1 vaccine that reduces viral load set point. J Infect Dis. 2007;195(4):546–550. doi: 10.1086/510909. [DOI] [PubMed] [Google Scholar]
- 33.Excler JL, Rida W, Priddy F, Fast P, Koff W. A strategy for accelerating the development of preventive AIDS vaccines. Aids. 2007 Nov 12;21(17):2259–2263. doi: 10.1097/QAD.0b013e3282eee70c. [DOI] [PubMed] [Google Scholar]
- 34.Buge SL, Ma HL, Amara RR, et al. Gp120-alum boosting of a Gag-Pol-Env DNA/MVA AIDS vaccine: poorer control of a pathogenic viral challenge. AIDS Res Hum Retroviruses. 2003;19(10):891–900. doi: 10.1089/088922203322493067. [DOI] [PubMed] [Google Scholar]
- 35.Staprans SI, Barry AP, Silvestri G, et al. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci USA. 2004;101(35):13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert PB, Ackers ML, Berman PW, et al. HIV-1 virologic and immunologic progression and initiation of antiretroviral therapy among HIV-1 infected subjects in a trial of the efficacy of recombinant glycoprotein 120 vaccine. J Infect Dis. 2005;192:974–983. doi: 10.1086/432734. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert P, Self S, Rao M, Naficy A, Clemens J. Sieve analysis: methods for assessing from vaccine trial data how vaccine efficacy varies with genotypic and phenotypic pathogen variation. J Clin Epidemiol. 2001;54(1):68–85. doi: 10.1016/s0895-4356(00)00258-4. [DOI] [PubMed] [Google Scholar]
- 38.Mani I, Gilbert P, Sankale JL, Eisen G, Mboup S, Kanki PJ. Intrapatient diversity and its correlation with viral setpoint in human immunodeficiency virus type 1 CRF02_A/G-IbNG infection. J Virol. 2002;76(21):10745–10755. doi: 10.1128/JVI.76.21.10745-10755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. [Accessed December 07, 2007.];Statement from the WHO-UNAIDS HIV vaccine advisory committee (VAC) on NIH/HVTN/MERCK release of new data from the analysis of STEP trial results. http://data.unaids.org/pub/PressStatement/2007/20071112_who_unaids_step_trial_en.pdf.
- 40.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]