Abstract
The oligo-acyl-lysyl, C12(ω7)K-β12, is comprised of only three Lys residues. Despite its small size, it exhibits potent bacteriostatic activity against Gram-positive bacteria, but it is ∼10-fold less potent against Gram-negative bacteria. We followed the interactions of C12(ω7)K-β12 from its initial contact with the bacterial surface across the cell wall down to the cytoplasmic membrane. Binding to anionic lipids, as well as to negatively charged LPS and LTA, occurs with very high affinity. The C12(ω7)K-β12 does not cross the outer membrane of Gram-negative bacteria; rather, it achieves its action by depositing on the LPS layer, promoting surface adhesion and blocking passage of solutes. In Gram-positive bacteria, the thick peptidoglycan layer containing LTA allows passage of C12(ω7)K-β12 and promotes its accumulation in the small periplasm. From that location it is then driven to the membrane by strong electrostatic interactions. Despite its high potency against Gram-positive bacteria, this agent is not capable of efficiently breaking down the permeability barrier of the cytoplasmic membrane or of reaching an intracellular target, as suggested by the fact that it does not interact with DNA.
Abbreviations: ANTS, 8-aminonaphthalene-1,3,6-trisulfonic acid; CAC, critical aggregation concentration; CFU, colony-forming unit; CL, cardiolipin; DOPG, dioleoyl phosphatidylglycerol; DPX, p-xylene-bis-pyridinium bromide; DSC, differential scanning calorimetry; ITC, isothermal titration calorimetry; LB, Luria-Bertani broth; LPS, lipopolysaccharide; LTA, lipoteichoic acid; LUV, large unilamellar vesicle; MIC, minimal inhibitory concentration; OAK, oligo-acyl-lysyl; ONP, ortho-nitrophenyl; ONPG, ortho-nitrophenyl-β-D-galactoside; PBS, phosphate-buffered saline; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; POPE, 1-palmitoyl-2-oleoylphosphatidylethanolamine; SPR, surface plasmon resonance; TOCL, tetraoleoyl cardiolipin; TSB, tryptic soy broth
Introduction
A novel approach to the design of antibacterial agents has led to the development of agents that are inexpensive to produce, resistant to proteolytic degradation, water-soluble, and chemically stable. These compounds have been termed OAKs, reflecting the fact that they are oligomers of acyl-lysyl (1). There are many variations in the structure of OAKs, including differences in the size and charge of the oligomer as well the nature of the linkage between subunits. In one study, Thennarasu et al. (2) used NMR to study the structure of a lipopeptide designed to generate nonlytic short antimicrobial peptides (in this case containing the nonstandard amino acid ornithine).
We recently showed that one form of OAK, the octamer C12K-7α8, promotes clustering of anionic lipids away from zwitterionic ones (3). As a consequence of this mechanism of action, C12K-7α8 is more toxic against species of bacteria whose membranes contain both anionic and zwitterionic lipids. Thus, generally they have lower MICs for Gram-negative bacteria. In contrast, another form of OAK, C12(ω7)K-β12, is more specific for Gram-positive bacteria. This OAK has a particularly short and simple structure (Fig. 1), having only two acyl chains and three positive charges. In this work, we systematically studied the molecular interactions that determine the access of C12(ω7)K-β12 to the cytoplasmic membrane for both Gram-positive and Gram-negative bacteria. The results allowed us to better understand the reasons for the difference in bacteriostatic activity that C12(ω7)K-β12 exhibits against Gram-positive and Gram-negative bacteria. The schematics of the architecture of bacteria is given in Fig. 2.
Figure 1.
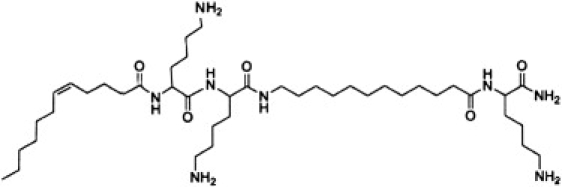
Structure of C12(ω7)K-β12.
Figure 2.
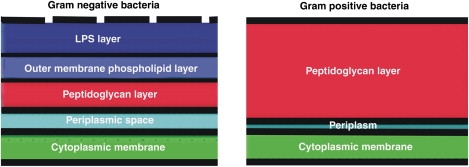
Schematic diagram of the architecture of the cell wall of Gram-negative (left) and Gram-positive (right) bacteria. Blue: outer membrane LPS layer; light blue: outer membrane phospholipids layer; red: peptidoglycan layer; cyan: periplasmic space in Gram-negative bacteria and periplasm in Gram-positive bacteria; and green: cytoplasmic membrane.
Materials and Methods
Materials
The phospholipids POPE, DOPG, and TOCL were purchased from Avanti Polar Lipids (Alabaster, AL). The OAK and control peptide used were synthesized in-house as previously described (4).
Minimal inhibitory concentration
MIC values were determined by means of a microdilution assay performed in sterilized 96-well plates in a final volume of 200 μL, as follows: Bacteria were grown overnight and diluted 10,000-fold in growth medium (LB: 10 g/L trypton, 5 g/L yeast extract, 5 g/L NaCl, pH 7.4). Then 100 μL of LB containing bacteria (5 × 105 CFU/mL) were added to 100 μL of culture medium containing the OAK (0–50 μM in serial twofold dilutions). Inhibition of proliferation was determined by optical density measurements (620 nm) after incubation overnight at 37°C.
Kinetics of cytotoxic action
To determine the time-kill curves, bacterial suspensions of Escherichia coli ATCC 35218 were cultured (37°C under shaking) in the presence of 25 or 50 μM OAK. Bacteria were then sampled at various time intervals, subjected to serial 10-fold dilutions, and plated on LB agar for CFU counts after overnight incubation at 37°C. Statistical data for each experiment were obtained from at least two independent assays performed in duplicate.
Outer and inner membrane permeabilization with E. coli ML-35p
The E. coli bacterial strain ML-35p was engineered specifically to monitor permeation of both the inner and outer membranes of the same bacterial strain (5). The mutant E. coli ML35p is constitutive for cytoplasmic β-galactosidase, lacks lac permease, and expresses a plasmid-encoded periplasmic β-lactamase. Two chromogenic reporter molecules are used to monitor permeabilization of the outer and inner membranes in a single assay (5–7). Nitrocefin, a chromogenic cephalosporin, cannot cross the outer membrane and is excluded from the periplasmic space. However, permeabilization of the outer membrane allows nitrocefin to enter the periplasm, where its cleavage by a β-lactamase produces a color change that can be monitored spectrophotometrically at 486 nm. Since there is no lactose permease in this mutant E. coli ML-35p, ONPG cannot traverse its inner membrane to be cleaved by cytoplasmic β-galactosidase to ONP unless permeabilization of the inner membrane occurs. ONPG cleavage produces a color change that can be measured spectrophotometrically at 420 nm.
The assay was performed in sterilized 96-well plates in a final volume of 200 μL as follows: Bacteria grown overnight in LB were washed three times in PBS and diluted to 106 CFU/mL in PBS containing 3% LB. Aliquots of this suspension (100 μL) were added to 100 μL of PBS containing a test compound (at the specified concentration) and either ONPG (2.5 mM) or nitrocefin (unknown concentration). Nitrocefin was obtained by extraction overnight of a commercial disk (Fluka Analytical, Buchs, Switzerland) with 1 mL PBS; 45 μL of this extract were included in each well. Hydrolysis of ONPG and nitrocefin was monitored by measuring absorbance at 420 or 490 nm, respectively, at various time intervals, with shaking at 37°C (BioTek Instruments, Winooski, VT).
Binding to LPS or LTA by displacement of dansyl-polymyxin
C12(ω7)K-β12 binding to LPS (from E. coli O111:B4) and LTA (from Staphylococcus aureus) was studied by displacement of bound dansyl-polymyxin, as described previously (8,9).
Isothermal titration calorimetry
Titrations were performed in a VP-ITC instrument (MicroCal, GE Healthcare, Piscataway, NJ). The OAK was placed in the syringe at a concentration of 400 μM, and 10 μL injections were made into the cell compartment. LPS from E. coli O111:B4 or LTA from S. aureus was placed in the cell compartment (volume: 1.476 mL) at a concentration of 125 μg/mL. The buffers were 10 mM HEPES, 140 mM NaCl, pH 7.4, or 10 mM HEPES, 140 mM NaCl, 1 mM EDTA, pH 7, and they were always matched between the syringe and the cell compartment. Titrations were performed at 30°C. The heat of dilution of OAK into the buffer was subtracted from all the other curves. Data were analyzed with the program Origin 7.0. Because of the heterogeneity of the LTA and LPS preparations, a detailed thermodynamic analysis could not be carried out.
Leakage of aqueous contents from liposomes
Leakage of aqueous contents was studied with ANTS/DPX as previously described (10).
Fluorescence assay for membrane depolarization
S. aureus (ATCC 29213) in mid-logarithmic phase were washed and resuspended in 5 mM HEPES buffer. This cell suspension was incubated with diSC3 (5) (0.4 mM) and quenching was allowed to occur at room temperature for 60 min. KCl (100 mM) was added to equilibrate the cytoplasmic and external K+ concentrations. OAK (four multiples of the MIC value) was added to the bacterial suspensions, and changes in fluorescence were continuously recorded (excitation and emission wavelengths at 622 nm and 670 nm, respectively).
Light scattering
Light-scattering measurements of the OAK solution (200 μM) were carried out in the presence of S. aureus ATCC 29213 and E. coli ATCC 35218 (105 CFU/mL) by holding both the excitation and the emission at 400 nm (slit width: 1 nm) using a Jobin-Yvon Horiba Fluorolog-3 system (Longjumeau, France) with FluorEssence.
Light microscopy
Microscopy studies were performed using a Zeiss LSM Microsystems microscope (model 210) with a CoolSnap H2 photometrics camera and an oil immersion objective. Bacteria were grown in LB for 18 h. An aliquot containing 106/mL bacterial cells in LB was imaged with and without 200 μM OAK in PBS.
Results
Evidence for interaction of C12(ω7)K-β12 with the LPS layer in Gram-negative bacteria
In Gram-negative bacteria, unlike in Gram-positive bacteria, antimicrobial potency and kinetics are improved in the presence of EDTA. Antimicrobial activity as represented by the MIC indicates that this OAK is more efficient at inhibiting the growth of the Gram-positive bacterium S. aureus (Table 1). In several strains of the Gram-negative bacterium E. coli, the antibacterial activity is enhanced by EDTA (Table 1). EDTA is a well-known permeabilizer of the outer wall. This result suggests that the OAK's access to the cytoplasmic membrane is inhibited by interaction with the LPS layer.
Table 1.
MIC for c12(ω7)k-β12 against Gram-negative versus Gram-positive bacteria
| Effects of EDTA on the MIC for Gram-negative bacteria | ||
|---|---|---|
| Gram-negative bacteria | No EDTA | With EDTA |
| E. coli strain | ||
| ATCC 354218 | >50 μM | 25 μM |
| C.I. 16328† | >50 μM | 12.5–25 μM |
| C.I. 16323 | >50 μM | 50 μM |
| Gram-positive bacteria | ||
| S. aureus | 3–6 μM | na∗ |
na, not applicable.
C.I., clinical isolate.
The role of EDTA in facilitating permeation by C12(ω7)K-β12 is supported by the kinetics of the bactericidal action against E. coli ATCC 35218. This occurs only in the presence of EDTA (Fig. 3), whereas in its absence the Gram-negative bacteria show only a bacteriostatic effect.
Figure 3.
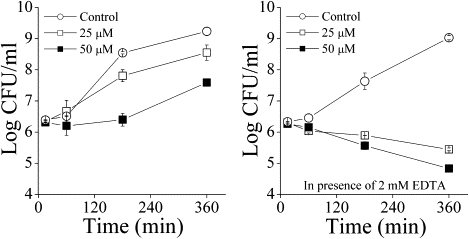
Kinetics of cytotoxicity of E. coli ATCC 35218 by C12(ω7)K-β12 in the absence and presence of EDTA at 0, 25, and 50 μM OAK.
Interaction of C12(ω7)K-β12 with LPS prevents access to the cytoplasmic membrane of E. coli
The E. coli ML35p mutant strain was used to simultaneously test the passage of the chromogenic substrate, nitrocefin, across the outer membrane, and of ONPG across the inner membrane. The OAK was tested in comparison with a dermaseptin derivative as a positive control. The C12(ω7)K-β12 was ineffective in permeabilizing either the outer membrane or the inner membrane of E. coli ML35p (Fig. 4). A small amount of OAK appears to penetrate the outer wall at longer times (>40 min). However, the same is observed in the absence of OAK. This assay is normally not carried further than 30 min since at longer times a small amount of nitrocefin hydrolyzes due to traces of β-lactamase appearing in the medium.
Figure 4.
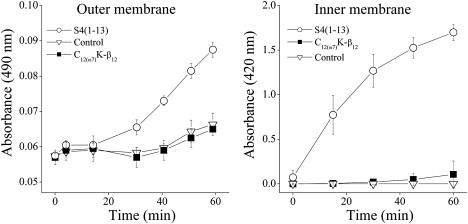
Time dependence of permeabilization of the inner (ONPG) and outer (nitrocefin) membranes of E. coli ML35p by C12(ω7)K-β12 (50 μM) using PBS as the untreated control and dermaseptin S4(1-13) as the positive control (25 μM).
Unlike binding to LPS, binding to LTA is unaffected by the presence of EDTA
No difference was observed in the displacement of dansyl-polymyxin by OAK from the LPS of E. coli O111:B4 or from the LTA of S. aureus in the absence of EDTA (Fig. 5). In the presence of EDTA, displacement of dansyl-polymyxin from LTA remained the same. However, EDTA allowed more dansyl-polymyxin to bind to LPS, and therefore a higher amount of OAK was required to displace it (Fig. 5).
Figure 5.
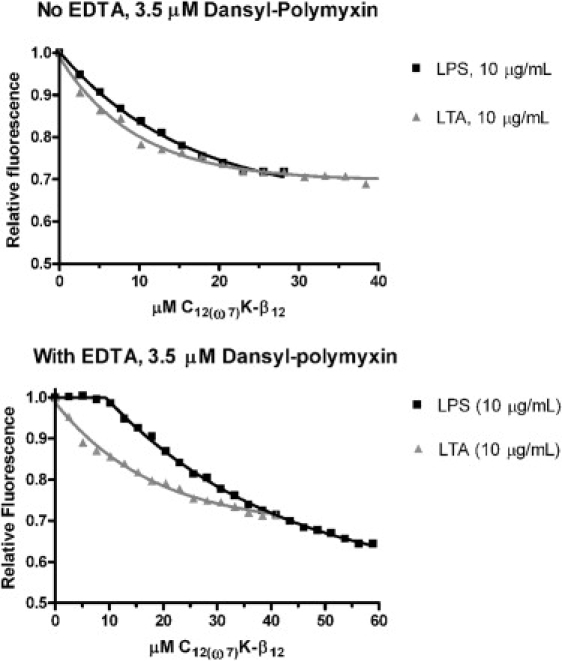
Displacement of 3.5 μM dansyl polymyxin binding to LPS (squares) and LTA (triangles) with increasing amounts of C12(ω7)K-β12. Top panel: no EDTA; bottom panel: 1 mM EDTA.
Binding to LPS from E. coli O111:B4 and to LTA from S. aureus by ITC
The OAK is initially present in the syringe at a high concentration of 400 μM, and is aggregated at this concentration. Since the OAK is diluted over 100-fold upon injection into the cell compartment, it must undergo a disaggregation process, in addition to its interaction with the macromolecule. The enthalpy of this disaggregation process was separately measured by determining the heat of dilution of C12(ω7)K-β12 into buffer and found to be small compared with the heats of reaction described below.
Binding to LPS from E. coli O111:B4 in the absence of EDTA is a complex process in which two types of reaction (one exothermic and one endothermic) take place simultaneously (Fig. 6). In the presence of EDTA, the initial binding is generally indicative of a single process largely based on electrostatic interaction. When nearing saturation of binding (∼30 μM OAK for the 125 μg/mL macromolecule in the cell), a new phenomenon takes place both with and without EDTA, which could entail a new type of association with the macromolecule. This is more evident in the binding to LTA. The binding kinetics with LPS is very slow at every step in both the presence and absence of EDTA.
Figure 6.
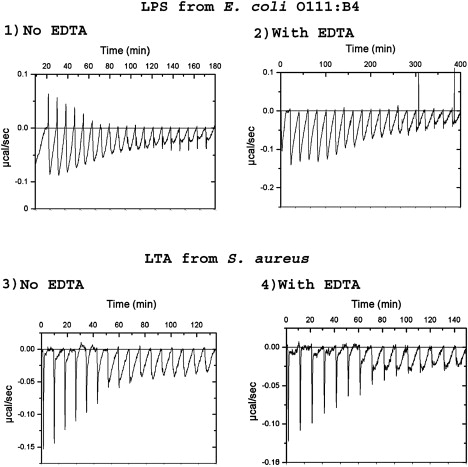
ITC of the reaction of C12(ω7)K-β12 with LPS (top) and LTA (bottom) at 30°C, and in the absence of EDTA (left) or presence of 1 mM EDTA (right).
Initial binding to LTA is fast and has an electrostatic nature, but after the seventh injection (at ∼20 μM OAK for the 125 μg/mL macromolecule in the cell), when an apparent near-equivalancy is achieved, a very slow process appears, as in the case of binding to LPS. The titration in LTA is unaffected by EDTA, as expected.
The slow process seen after electrostatic interaction is similar in both LPS and LTA, and it is unusual in that it is associated with a very large enthalpy. This process would involve the OAK binding to an electrically neutralized particle, producing a large exothermic reaction and suggesting a large change in intermolecular associations.
Can C12(ω7)K-β12 allow passage of small molecules from model membranes mimicking the membrane composition of Gram-positive bacteria?
Although the cytoplasmic membrane of E. coli bacteria contains a large amount of zwitterionic PE, the cytoplasmic membrane of S. aureus is composed largely of anionic lipids, such as PG and CL, at a molar ratio of 58:42. The much higher affinity for PG:CL means that the OAK fraction that manages to bypass the large peptidoglycan layer would also be subjected to a higher attraction exerted by the plasma membrane of S. aureus.
Up to 40 μM OAK was tested in 50 μM LUVs of DOPG:TOCL 6:4, and no significant permeabilization was observed (Fig. 7). Therefore, despite the fact that this OAK binds very strongly to anionic membranes, it is unable to allow passage of small molecules (∼400 Da in size). The small amount of leakage that was detected over a period of 180 min for 40 μM peptide (Fig. 7) is not very significant, as it could be caused by a small osmotic imbalance after peptide addition.
Figure 7.
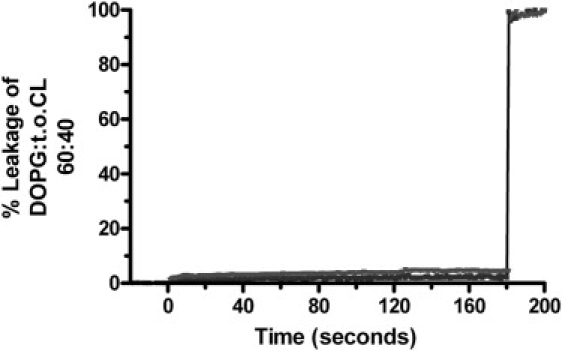
C12(ω7)K-β12 at 4 μM (lower curve) and 40 μM (upper curve) does not cause leakage of fluorescent dyes from liposomes composed of DOPG:TOCL (60:40). At t = 180 s, Luberol LX was added (0.2% in the cuvette) to disrupt the vesicles.
In addition, there was essentially no depolarization of the cytoplasmic membrane of S. aureus (Fig. 8). Only slow and partial depolarization was observed in comparison with a controlled peptide (dermaseptin S4 (1–13)) that shows typical large and instantaneous depolarization (Fig. 8). This confirms that the toxicity of C12(ω7)K-β12 in Gram-positive bacteria is not primarily the result of it breaking down the permeability barrier of the cytoplasmic membrane. In addition, it is not likely that there is an intracellular target for C12(ω7)K-β12, since there was also no interaction with DNA (H. Sarig, L. Livne, V. Held-Kuznetsov, F. Zaknoon, A. Ivankin, D. Gidalevitz, and A. Mor, unpublished results). Nevertheless, concentrations of C12(ω7)K-β12 higher than the MIC may cause some damage to the plasma membrane of Gram-positive bacteria after long incubation times. For example, at C12(ω7)K-β12 concentrations four times higher than the MIC, a very small and slow depolarization of the membrane occurs as a function of time (Fig. 8).
Figure 8.
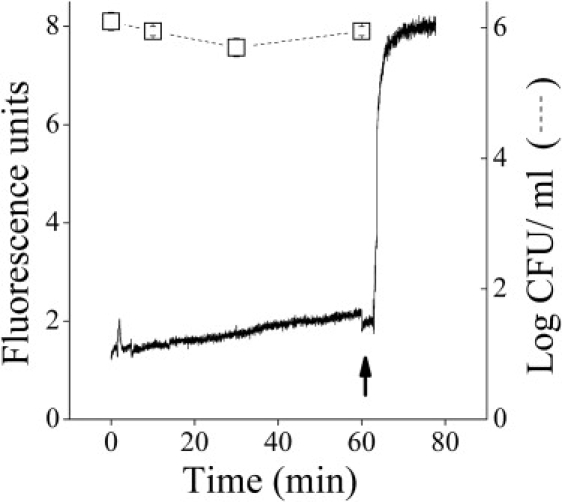
Bactericidal kinetics and membrane depolarization of S. aureus (ATCC 29213) (DiSC3(5) assay) induced by C12(ω7)K-β12 at 25 μM (4× MIC). Arrow points to time of addition of the control peptide (dermaseptin S4(1-13) at 25 μM).
OAK disaggregates more slowly in the presence of E. coli than in the presence of S. aureus
OAK disaggregation is shown in Fig. 9. In this experiment, it was first confirmed that the light scattering observed is a property of OAK and not of bacteria. It takes ∼15 min for the light scattering to reach a minimum in S. aureus, but >1 h in E. coli. An explanation for this difference was obtained with light microscopy.
Figure 9.
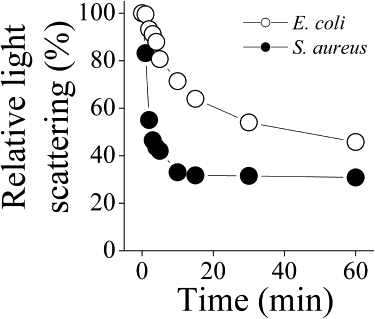
Disaggregation of the OAK shown by light scattering obtained with 200 μM OAK in the presence of S. aureus (•) or E. coli (○).
Light microscopy reveals bacterial surface adhesion in E. coli, but no morphological changes in S. aureus, in the presence of OAK
Light microscopy images show that ∼1 h after addition of OAK at concentrations above the CAC, the Gram-negative bacterium E. coli undergoes an adhesion process, forming clumps of individual cells, probably with OAK bound on the surface. The Gram-positive bacterium S. aureus, on the other hand, remains as single, intact bacteria that overall show no apparent morphological change (Fig. 10, a and b).
Figure 10.
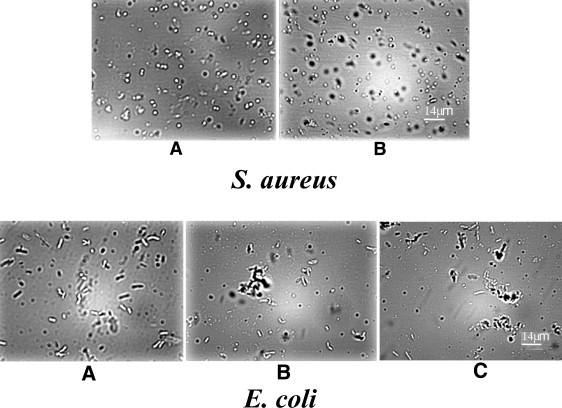
Top panel: Light microscopy obtained with S. aureus in the absence (A) or presence of 200 μM OAK (B) after incubation for ∼1 h. White bar indicates 14 μm. Lower panel: Light microscopy obtained with E. coli in the absence (A) or presence of 200 μM OAK (B and C) after incubation for ∼1 h. White bar indicates 14 μm.
Discussion
The OAK C12(ω7)K-β12 is of particular interest because of its potent and broad bacteriostatic activity against Gram-positive bacteria, including antibiotic resistant strains (H. Sarig, L. Livne, V. Held-Kuznetsov, F. Zaknoon, A. Ivankin, D. Gidalevitz, and A. Mor, unpublished results). The C12(ω7)K-β12 has high affinity for anionic amphiphiles. The binding to lipids representing those of the Gram-positive S. aureus is ∼10,000-fold greater than it is to a lipid mixture with PE approximating that of the Gram-negative bacteria E. coli (H. Sarig, L. Livne, V. Held-Kuznetsov, F. Zaknoon, A. Ivankin, D. Gidalevitz, and A. Mor, unpublished results). In addition, C12(ω7)K-β12 binds somewhat more strongly to the anionic LTA than to LPS (Fig. 5). LPS is found only in Gram-negative bacteria, whereas a much thicker peptidoglycan layer containing LTA is found in Gram-positive bacteria (Fig. 2). The roles of LPS in bacterial toxicity, from the “self-promoted uptake mechanism” (12) to its endotoxic effects (13), have been studied widely.
We find that C12(ω7)K-β12 cannot abruptly permeabilize liposomes of anionic lipids mimicking the cytoplasmic membrane of Gram-positive bacteria (Fig. 7); therefore, even if some C12(ω7)K-β12 were able to access the cytoplasmic membrane in S. aureus, it would not be able to form a stable pore. This is confirmed by the fact that this OAK cannot access the DNA of S. aureus (H. Sarig, L. Livne, V. Held-Kuznetsov, F. Zaknoon, A. Ivankin, D. Gidalevitz, and A. Mor, unpublished results). By what mechanism, then, can C12(ω7)K-β12 be bacteriostatic to S. aureus? Rapid disaggregation of OAK occurs in the presence of S. aureus (Fig. 9), and in addition, the overall morphology of the bacteria after 1 h incubation at high concentrations does not change (Figs. 9 and 10 A), in contrast to what occurs in Gram-negative bacteria. Because of the rapid electrostatic interactions between the C12(ω7)K-β12 and LTA (Fig. 6), it is likely that C12(ω7)K-β12 reaches the inner membrane but is not able to permeabilize or depolarize it. This would cause accumulation of OAK in the very small volume of the periplasm of Gram-positive bacteria (Fig. 2), resulting in the presence of higher concentrations of OAK at the surface of the inner membrane. The lack of a sudden burst in cytoplasmic membrane depolarization with Gram-positive bacteria, as shown here with C12(ω7)K-β12 (Fig. 8), was previously observed with the peptides buforin 2 and penetratin (14). However, despite their weak depolarizing action, both buforin 2 and penetratin are able to translocate through the membrane and kill the bacteria by interacting with the DNA, causing rapid cell death. For buforin 2, a mechanism was proposed by which a pore with an extremely short lifetime forms that allows it to cross the membrane without permeabilizing it (15). Another example is a cationic polymer that is cytotoxic without disrupting the bacterial membrane (16). The lack of interaction of this OAK with DNA argues against this explanation as a mechanism. An alternative possible mechanism is that a strong inhibition of a metabolically relevant enzyme occurs at low bulk concentrations of OAK, causing rapid inhibition of growth. Many cell surface proteins are anchored in the cell wall, and the cytoplasmic membrane contains a myriad of transporter and signaling proteins. It has been suggested that this OAK inserts into the cytoplasmic membrane, but only down to the headgroup region (H. Sarig, L. Livne, V. Held-Kuznetsov, F. Zaknoon, A. Ivankin, D. Gidalevitz, and A. Mor, unpublished results), which in Gram-positive bacteria is almost exclusively anionic, containing 58% PG and 42% CL. When this happens, many neighboring proteins can be affected because the lateral packing of the headgroups and the surface charge of the membrane will change, affecting protein insertion and even their structure, as well as competing with the interactions between anionic lipids and membrane proteins. These effects may be exaggerated because of the higher concentration of OAK in the small volume of the periplasmic space.
The MIC for C12(ω7)K-β12 is much higher against Gram-negative bacteria than against Gram-positive bacteria. We suggest that different mechanisms are operating for the two kinds of bacteria. The binding of C12(ω7)K-β12 to LPS appears to block the flux of solutes into and out of the bacteria. This is supported by our experiments showing that entry of substrates is inhibited by C12(ω7)K-β12 across both the inner and outer membranes of E. coli ML35p (Fig. 4). C12(ω7)K-β12 binds to LPS (Figs. 5 and 6) and exhibits bacteriostatic activity, but the addition of EDTA makes the OAK bactericidal, suggesting that removal of divalent cations from LPS loosens its structure and allows more facile entry of the C12(ω7)K-β12 (Fig. 3), resulting in cell death. We suggest that C12(ω7)K-β12 blocks passage of solutes across the outer membrane of E. coli in a manner similar to what we previously observed with a cationic sequence-random copolymer (10). C12(ω7)K-β12 requires higher concentrations, close to the CAC, for bacteriostatic action in Gram-negative bacteria. Both light scattering and light microscopy indicate that a slow bacterial surface aggregation process occurs above the CAC, with the formation of clumps of cells (Figs. 9 and 10, B and C). Therefore, the OAK remains bound on the surface, facilitating surface adhesion of cells. A recent NMR study with an antimicrobial lipopeptide, MSI-594, in LPS micelles provided some insights into the structural nature of the interactions that occur between antimicrobial agents and LPS (17).
The mechanism of slowing passage across the cell wall is more important for LPS (Gram-negative bacteria), where the MIC is higher (Table 1), than for LTA (Gram-positive bacteria), which is generally more permeable. However, although C12(ω7)K-β12 reacts rapidly with LTA at low concentrations, at higher concentrations of OAK, the ITC experiments indicate that another process is occurring after neutralization of charge (Fig. 6). This process could result in a decreased flux through the peptidoglycan layer, particularly in view of the large thickness of this layer.
It is clear, then, that several alternative mechanisms, including lateral phase separation in Gram-negative bacteria or action of C12(ω7)K-β12 on an intracellular target, are not likely. The OAK C12(ω7)K-β12 was unable to induce clear lateral phase separation in membranes mimicking the cytoplasmic membrane of Gram-negative bacteria (75% PE and 25% CL; data not shown). The difference between C12K-7α8, which induces lateral phase separation (10), and C12(ω7)K-β12 is that C12(ω7)K-β12 has only three positive charges, whereas C12K-7α8 has eight cationic groups and is therefore more potent in clustering anionic lipids (results not shown).
In conclusion, we suggest that the action of C12(ω7)K-β12 against Gram-negative and Gram-positive bacteria results from different outer wall interactions. C12(ω7)K-β12 exerts bacteriostatic activity against Gram-negative bacteria only at high concentrations (above the CAC), where it may coat the cell by aggregating on the rigid outer wall through electrostatic and perhaps hydrophobic interactions, thus preventing passage of solutes. With Gram-positive bacteria, the toxicity is high. At low concentrations, when C12(ω7)K-β12 is not aggregated, the OAK is probably able to traverse the thick peptidoglycan lattice and reach the cytoplasmic membrane. Several alternative mechanisms can be eliminated, suggesting that inhibition of growth is not the result of either an intracellular target or a consequence of damage to the plasma membrane.
Acknowledgments
We thank Marnie Tedeske, Arpita Gantayet, and Januvi Jegatheswaran for their technical assistance.
This research was supported by the Israel Science Foundation (grant 283/08) and the Canadian Institutes of Health Research (MOP 86608).
Footnotes
Raquel F. Epand and Hadar Sarig contributed equally to this work.
References
- 1.Radzishevsky I.S., Rotem S., Bourdetsky D., Navon-Venezia S., Carmeli Y. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat. Biotechnol. 2007;25:657–659. doi: 10.1038/nbt1309. [DOI] [PubMed] [Google Scholar]
- 2.Thennarasu S., Lee D.K., Tan A., Prasad K.U., Ramamoorthy A. Antimicrobial activity and membrane selective interactions of a synthetic lipopeptide MSI-843. Biochim. Biophys. Acta. 2005;1711:49–58. doi: 10.1016/j.bbamem.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epand R.M., Rotem S., Mor A., Berno B., Epand R.F. Bacterial membranes as predictors of antimicrobial potency. J. Am. Chem. Soc. 2008;130:14346–14352. doi: 10.1021/ja8062327. [DOI] [PubMed] [Google Scholar]
- 4.Radzishevsky I., Krugliak M., Ginsburg H., Mor A. Antiplasmodial activity of lauryl-lysine oligomers. Antimicrob. Agents Chemother. 2007;51:1753–1759. doi: 10.1128/AAC.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehrer R.I., Barton A., Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J. Immunol. Methods. 1988;108:153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- 6.Ericksen B., Wu Z., Lu W., Lehrer R.I. Antibacterial activity and specificity of the six human {α}-defensins. Antimicrob. Agents Chemother. 2005;49:269–275. doi: 10.1128/AAC.49.1.269-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrer R.I., Barton A., Daher K.A., Harwig S.S., Ganz T. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore R.A., Bates N.C., Hancock R.E. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 1986;29:496–500. doi: 10.1128/aac.29.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epand R.F., Schmitt M.A., Gellman S.H., Sen A., Auger M. Bacterial species selective toxicity of two isomeric α/β-peptides: role of membrane lipids. Mol. Membr. Biol. 2005;22:457–469. doi: 10.1080/09687860500370562. [DOI] [PubMed] [Google Scholar]
- 10.Epand R.F., Mowery B.P., Lee S.E., Stahl S.S., Lehrer R.I. Dual mechanism of bacterial lethality for a cationic sequence-random copolymer that mimics host-defense antimicrobial peptides. J. Mol. Biol. 2008;379:38–50. doi: 10.1016/j.jmb.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 11.Reference deleted in proof.
- 12.Falla T.J., Karunaratne D.N., Hancock R.E. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 13.Mangoni M.L., Epand R.F., Rosenfeld Y., Peleg A., Barra D. Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. J. Biol. Chem. 2008;283:22907–22917. doi: 10.1074/jbc.M800495200. [DOI] [PubMed] [Google Scholar]
- 14.Zhu W.L., Shin S.Y. Antimicrobial and cytolytic activities and plausible mode of bactericidal action of the cell penetrating peptide penetratin and its lys-linked two-stranded peptide. Chem. Biol. Drug Des. 2009;73:209–215. doi: 10.1111/j.1747-0285.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S., Chikushi A., Tougu S., Imura Y., Nishida M. Membrane translocation mechanism of the antimicrobial peptide buforin 2. Biochemistry. 2004;43:15610–15616. doi: 10.1021/bi048206q. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel G.J., Madkour A.E., Dabkowski J.M., Nelson C.F., Nusslein K. Synthetic mimic of antimicrobial peptide with nonmembrane-disrupting antibacterial properties. Biomacromolecules. 2008;9:2980–2983. doi: 10.1021/bm800855t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhunia A., Ramamoorthy A., Bhattacharjya S. Helical hairpin structure of a potent antimicrobial peptide MSI-594 in lipopolysaccharide micelles by NMR spectroscopy. Chemistry (Easton) 2009;15:2036–2040. doi: 10.1002/chem.200802635. [DOI] [PubMed] [Google Scholar]


