Abstract
Giant honeybees (Apis dorsata) nest in the open and have therefore evolved a variety of defence strategies. Against predatory wasps, they produce highly coordinated Mexican wavelike cascades termed ‘shimmering’, whereby hundreds of bees flip their abdomens upwards. Although it is well known that shimmering commences at distinct spots on the nest surface, it is still unclear how shimmering is generated. In this study, colonies were exposed to living tethered wasps that were moved in front of the experimental nest. Temporal and spatial patterns of shimmering were investigated in and after the presence of the wasp. The numbers and locations of bees that participated in the shimmering were assessed, and those bees that triggered the waves were identified. The findings reveal that the position of identified trigger cohorts did not reflect the experimental path of the tethered wasp. Instead, the trigger centres were primarily arranged in the close periphery of the mouth zone of the nest, around those parts where the main locomotory activity occurs. This favours the ‘special-agents’ hypothesis that suggest that groups of specialized bees initiate the shimmering.
Electronic supplementary material
The online version of this article (doi:10.1007/s00114-009-0605-y) contains supplementary material, which is available to authorized users.
Keywords: Apis dorsata, Giant honeybee, Shimmering, Defence waving, Trigger cohorts, ‘Special-agents’ hypothesis
Introduction
Giant honeybees are the second oldest honeybee species after the dwarf honeybees Apis florae (Ruttner 1988). Giant and dwarf honeybees build their nests predominantly in the open and are directly exposed to a variety of predators, particularly to birds (Kastberger and Sharma 2000) and wasps (Kastberger et al. 2001, 2008a). These predatory pressures have probably evoked the evolution of a series of defence strategies, which are characteristic of honeybees in general and of giant honeybees in particular (Butler 1954; Koeniger and Fuchs 1975; Morse and Laigo 1969; Seeley et al. 1982; Breed et al. 2004).
One of the most striking lines of defence in giant honeybees is shimmering (Roepke 1930; Lindauer 1956; Sakagami 1960; Seeley et al. 1982; for summary, see Oldroyd and Wongsiri 2006; Kastberger et al. 2008a). It is evoked by visual stimuli mainly by predators, in particular, by wasps hovering in close vicinity of the nest (Kastberger et al. 2008a). Generated at distinct spots of giant honeybee nests, shimmering spreads over the whole nest within a fraction of a second, forming Mexican wavelike pattern (Farkas et al. 2002) on the surface of the nest. Shimmering may unite hundreds of colony members to flip their abdomens, thus distributing ‘information’ on predatory pressure to two addressees: first, colony-intrinsically to the nest mates, stimulating them to participate, possibly arousing or alarming them through mechanoceptive and pheromonal pathways (Kastberger et al. 1998); second, externally to predatory wasps and mammals by providing dynamic visual cues that may confuse, misguide, or repel approaching enemies (Kastberger et al. 2008a).
While specifically found in honeybees, shimmering has relevance for a series of important topics regarding research in the biology of social systems in general. Shimmering bees display a remarkable capacity of fast communication within a society, unique in the animal kingdom, and together with its antipredatory goal, shimmering alludes to the domains of ‘social communication’ (Pasteels et al. 1987; Ratnieks and Anderson 1999a) and ‘social defence’ (Breed et al. 2004; Hepburn and Radloff 1998; Kastberger et al. 2008a, b), ‘collective decision-making’ (Reynolds 1987; Seeley 1995; Seeley and Buhrman 2001) and ‘task partitioning’ (Winston and Fergusson 1986; Robinson et al. 1992; Ratnieks and Anderson 1999a, b).
Shimmering is also a compelling example of ‘self-organisation’ (Pasteels et al. 1987; Camazine and Sneyd 1991; Camazine et al. 2003). However, the phenomenon of ‘information transfer’ is still unexplored. Central questions about how shimmering is generated, in particular the principles of its initiation, are still open. It is known that shimmering is started by one single bee or by a small group of bees and leads to a wavelike process in which hundreds or thousands of surface bees participate. Thus, shimmering activity is established by synchronising and cascading abdominal flipping of worker bees, performed on the surface of the threatened side of the nest. Production and scenting of Nasonov pheromone ensures that the bees stay together for further defence action (Kastberger et al. 1998), preventing the surface bees from adopting the role of flying guards (Breed et al. 2004; Kastberger et al. 2008b) that would fight the predator on an individual basis and at a higher risk. Shimmering is exclusively evoked by visual cues, typically by wasps that hover in front of the bee nests, and can also be released by approaching moths, birds, or even by colony members themselves (Kastberger et al. 2008a). Shimmering has been proven as an efficient strategy to keep wasps away from the nest and could therefore be synonymously addressed as ‘defence waving’ (Kastberger et al. 1997, 2001, 2008a).
By visual inspection using the naked eye, it seems that shimmering is triggered at random spots of the nest at the same chance. This would conform to the ‘everywhere hypothesis’ that the bees closest to the threatening source would initiate the shimmering. However, preliminary observations (Kastberger et al. 1997, 2001) led to the assumption that defence waving is not triggered uniformly over the nest surface. If this assumption was correct, centres of trigger bees would comprise groups of bees specialised by either age criteria (Lindauer 1952; Breed et al. 1990; Beshers et al. 2001; Kastberger et al. 2008b) or by experience so that they can respond quicker to threatening cues than other colony members. This ‘special agents’ hypothesis predicts that the initiation of shimmering will not necessarily happen close to the threatening source.
In this paper, we exposed giant honeybee nests to living tethered wasps to provoke shimmering under controlled experimental conditions and to assess the positions of the groups of honeybees that had triggered the waves. Automated image analysis of video recordings in conjunction with manual inspection was used to identify groups of bees that generated shimmering responses. Using this experimental approach, we were able to show that shimmering in giant honeybees is triggered by ‘special agents’ rather than randomly selected surface bees positioned close to the provoking visual cue.
Materials and methods
Species and study site
The experiments were conducted in preliminary trials with five giant honeybee (Apis dorsata) colonies in Hajo, near Guwahati, Assam, India, in November 1998. At this time, free-flying wasps were observed in the vicinity of the honeybee nests. For the main quantitative investigation, one test colony was used, which was 55 × 45 cm in size and attached to a ledge inside bare brickwork. This experimental site was selected to enable a convenient approach to the nest, placing the devices for video documentation and applying stimulations. At the time of the experiment, the ‘mouth’ zone (the active zone of the nest where the foragers depart, arrive and dance, Morse and Laigo 1969) comprised 30% the size of the nest and was located at the left bottom side in the experimental view (Movie S1). The complimentary ‘quiescent area’ above and to the right of the ‘mouth zone’ comprised about 600 surface bees.
Stimulation
Prior to each stimulation, the colony was observed for its arousal state; it was ensured that the bees were calm and undisturbed without a tendency for shimmering when the experimenter approached slowly. This pre-stimulation session was regarded as the reference state for the movement activity at the surface of the bee nest. Subsequently, the test colony was exposed to seven stimulation sessions to provoke shimmering. For the stimulation, a living wasp (Vespa sp.) was used. An elastic wire was wrapped around the waist of the wasp between thorax and abdomen and attached to a rod. The resilience of the wire allowed small wiggly oscillations on the tethered wasp, which increased the responsiveness of the bee colony for releasing the shimmering behaviour. This kind of manipulation with a tethered wasp was chosen to mimic free-flying wasps, which frequently hover in front of Giant honeybee nests. The tethered wasp was manoeuvred in front of the Giant honeybee nest, horizontally from the left side to the right side, and immediately back, from the right to the left side, slightly beneath the horizontal median of the nest (Movie S1). In a single go, the tethered wasp was moved for about 5 s with constant velocity and 15 cm distance from the nest. The rod to which the wire with the wasp was attached was positioned outside the range of the video image in order not to disturb subsequent image analysis.
The presence of the tethered wasp in front of the nest strongly aroused the giant honeybee colony causing shimmering activities, which also continued after the wasp had disappeared from the nest. The seven experimental sessions resulted in a total of 118 shimmering waves that exceeded the threshold of four active bees per frame. The phases between the stimulations were about 50 s, during which the colony recovered from its higher arousal. Therefore, each experiment had three phases in which the arousal state was quantified by the number and strength of shimmering waves: (a) the pre-presentation (preP) phase, in which hardly any shimmering activity was observed, and (b) the presentation (P) phase, in which the colony was exposed to the tethered wasp. This visual cue generated a series of shimmering waves. Finally, (c) in the subsequent post-presentation (postP) phase, which started after the disappearance of the tethered wasp, shimmering activity still continued but declined within minutes. A subsequent presentation of the tethered wasp was only started after the colony has returned to a low arousal level, which was assessed by the fact that the colony had ceased to produce shimmering waves without any external cues.
Video recording and image analysis
For videotaping, the video camera was placed 2 m in front of the test colony. This focal length provided undistorted views of the whole nest and kept the colony at a low state of arousal. The video film was transferred into tif images at the PAL rate of 25 frames per second, using Avid X-press-Pro as full-featured editing software for real-time video. Image analysis was processed with macros based on the software Image-Pro (Flir). In total, we analysed video sequences of 10,700 images, corresponding to an experimental time of 426 s.
Assessment of shimmering activity
The videos were analysed frame by frame by a movement detector procedure programmed with Image-Pro. This allowed us to spot changes in grey pixel (luminance) values between subsequent frames. The luminance changes displayed from the surface of the honeybee nest were visualised in ‘differential’ images (Fig. 1, Movie S2). Shimmering activity contrasts here well against the general “noise” of differential luminance caused by the basic locomotory and non-locomotory moving activity on the nest surface. The differential images predominantly tracked the abdomen-thrusting surface bees (Fig. 1) and allowed to sum up the luminance changes as waving strength (W value) per image. This measure was calibrated by the number of surface bees that actively moved their abdomens, as a change in their posture and essentially without locomotion.
Fig. 1.
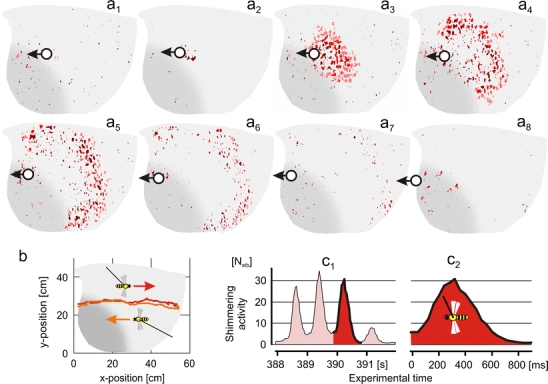
Example of a shimmering wave produced by the giant honeybee test colony in the presence of a tethered wasp. a 1 –a 8 Twenty-two sequential images disclose abdominal movements of nest bees, assembled and superimposed in eight charts: a 1 three images recorded prior to the onset of the shimmering wave; a 2 image showing the group of bees that had started the wave (dark red spots); a 3 –a 7 six charts with three images each displaying the subsequent spreading of the wave over the nest surface; a 8 three images after the wave had ceased. Successive frames were superimposed and coloured from dark to bright red. Grey areas on the left bottom side of the charts signify the ‘mouth zone’; complimentary bright grey areas signify the ‘quiescent zone’ of the nest. The positions of the tethered wasp are given by full white circles; the direction of its movement is marked by black arrows. b Single stimulation in which the tethered wasp was drawn from the left to the right side (red symbols) of the experimental nest and afterwards back to the left (orange symbols); during this experiment, the vertical position of the wasp and its distance from the nest (15 cm) were kept within narrow limits. c 1, c 2 Time plots of waving strength of the sample waves; ordinate shows the number of abdomen-thrusting bees per frame (N atb); red (c 1,c 2) refers to the sample wave displayed in (a 1 –a 8); abscissa, the experimental time in seconds (c 1) and the time course of the sample wave in millisecond (c 2)
The peak in shimmering activity was detected per wave by the following automated searching rules: (1) a shimmering wave was traced if the W values exceeded the threshold waving strength (thW) of four bees per frame, which corresponded to approximately 9% of the overall maximum W values of shimmering in the whole observation session (cf. Fig. 2a). (2) The peak of a shimmering wave was detected at the time t i (at the frame i) if the respective value of W i was higher than three frames (that is 120 ms) before (W i − 3) and after (W i + 3) the reference time t i. The respective differential values ΔW ref_before = [W i − W i − 3], respectively, ΔW ref_after = [W i - W i + 3] should be higher than the value of four bees per frame, which corresponded nominally to the absolute threshold thW. Consequently, the starting time of the respective wave was identified at the minimum in waving strength observed three to ten frames prior to the peak. The period of up to 400 ms proposed for the automated analysis was taken because it should correspond with the average time after which shimmering (Figs. 1 and 2) reaches its peak waving strength (Kastberger et al. 2008a).
Fig. 2.
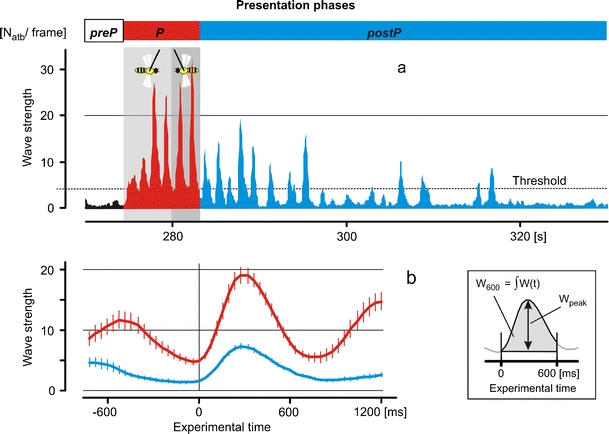
Temporal pattern of shimmering in the presence of a tethered wasp. a The three phases of the experiments are marked by the horizontal bars at the top: white bar pre-presentation phase (prep); red bar presentation phase (P); blue bar post-presentation phase (postP). The sequence of shimmering waves was recorded from 270 to 330 s of the experiment, while the tethered wasp was presented (red curves for the P phase of experiments), when it was moved from the left to the right (bright grey background) and from the right to the left (darker grey background) and after its disappearance (blue curves for the postP phase). For pooling and testing, only waves above the threshold value were used (for definition see “Materials and methods”). b Means and SEs (vertical bars) of shimmering responses of P phase (red lines) and postP phase (blue lines) state, as pooled at time zero as the start of shimmering. The ordinate gives the waving strength as the number of bees per frame (N atb) that thrust their abdomens upwards. Shimmering waves during the presence of a tethered wasp exhibit higher waving peaks (for definition of W peak, see inset), with more participating surface bees in 600 ms (W 600, see inset) and higher repetition rates than waves after the disappearance of the wasp
However, the W value tracks movement of bees on the nest surface in general and is therefore not necessarily associated with ‘shaking’ or ‘lifting’ of the abdomens, respectively. Therefore, manual inspection was necessary to identify the luminance changes, identified by the differential images as wavelike processes, to distinguish them reliably from other movement activities of nest bees, such as walking, dancing or flying. In the identified waves, the parameter W was then applied to the time course of shimmering in two aspects: (1) by W peak, the intensity of shimmering at its peak activity, and (2) by W 600 = ∫W(t), the number of bees that participated in the first 600 ms of the wave.
Assessment of the position of the tethered wasp and the spatial distribution of trigger centres of shimmering
In the experiments, the video camera was directed horizontally and frontally towards the nest. The real-world coordinates of the tethered wasp were approximated as its two-dimensional projection of its position onto the surface of the honeybee nest. For that, we marked the image-based horizontal (left–right) x values and vertical (up–down) y values of the thorax positions of the tethered wasp interactively frame by frame.
The locations of those bee cohorts, which triggered the waves on the nest surface, were detected by manual decision. For that, we subdivided the nest into 11 sectors of 8.75° between the horizontal (0°) and vertical (90°) deflection and into nine segmental stripes of 4.23 cm width around the mouth zone. Sectors and segments were related to the reference position (x = 0; y = 0) on the left bottom side of the nest, and consequently, the positions of the trigger centres were classified in terms of sector and segment numbers.
Statistics
The mean time course of shimmering waves was assessed by pooling the waves and averaging the W values obtained in consecutive frames relative to the onset times of the waves using basic statistics (means ± SE). Data sequences were compared by parametric tests (t test) or, if the normality test failed, by nonparametric tests (Wilcoxon signed-rank test). W values and repetition rates of shimmering were used to assess the arousal state of the colony in the P and postP phases of the experiment. Correlations were characterised generally by the regression functions of the original data values and of the means of the respective response classes. The regressions were fitted by optimising their coefficients of determination (R 2) and tested by Spearman rank order correlation test. One- and two-way analysis of variance (ANOVA) was used to compare more than two components within one test, e.g. to prove the sequence of shimmering waves for progressiveness in their time courses or to prove whether the trigger centres distributed themselves in batches or arbitrarily over the nest.
Furthermore, we proved whether and how much the spatial distributions of the trigger centres deviated between the arousal states of the colony in the P- and postP phases. For that, we used two independent methods: First, we normalised the distributions of the data of the sector and segment analysis (see above) regarding their maximal values (not shown in Fig. 5). In another step, we progressively shifted the distribution of trigger centres under the P phase in steps of sector unit angles and of segment unit distances, respectively. The sums of deviation squares between the shifted test distribution regarding the P phase and the fixed reference under postP phase were then correlated by a second-order polynomial. The significance level of the polynomial regression was used to test the bias between the distributions of both experimental states using SigmaPlot. The bias between both distributions was addressed by the shifting position at which the polynomial function had developed its minimum.
Fig. 5.
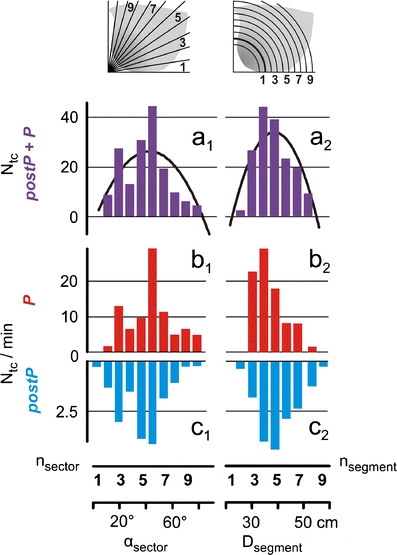
Distribution of ‘trigger centres’ on the nest of the test colony of Apis dorsata. a Ordinate, number of trigger centres (N tc) during the whole observation session in the P and postP phases (coded in violet) as distributed in 11 ‘sectors’ (a 1) and nine ‘segments’ (a 2) of the nest area; for definition of sectors and segments, see charts at the top and abscissas at the bottom, which give the numbers (n sector) and the mean angles (α sector) of the sectors and, respectively, the numbers (n segment) and the mean segment distances (D segment). Curves show the polynomial regressions with (N tc/min = −2.637 + 1.399 × n sector − 0.0169 × n 2sector with R 2 = 0.614; n = 11; p = 0.036; and N tc/min = −158.475 + 9.923 × n segment − 0.128 × n 2segment with R 2 = 0.7960; n = 9; p = 0.014; polynomial regression test, SigmaPlot). b, c Distributions of trigger centres in the P phase (red columns) and in the postP phase (blue columns) regarding the divisions by sectors (b 1,c 1) and segments (b 2,c 2). Ordinates give the number of trigger centres per minute (N tc/min)
Second, we subtracted the absolute values of the P phase from those of the postP phase and checked (t test) the resulting differential distribution for asymmetry between its left and right portion. Asymmetry between both distributions verified, similarly to the first test, that the arousal state of the colony was biased regarding the topology of the trigger centres for shimmering.
Results
A sample shimmering wave as evoked by a tethered wasp
A typical shimmering wave lasts less than 1 s, irrespective of whether the colony has been provoked by a wasp predator or by other visual cues (Kastberger et al. 2008a, b). This allowed us to document a wave by a sequence of at least 20 PAL-formatted frames in steps of 40 ms. Figure 1 shows one characteristic shimmering wave of the tethered wasp experiment as documented by a series of ‘differential’ images superimposed into a sequence of charts (a 1–a 8). The blotches displayed differences in pixel luminance between two subsequent frames (see “Materials and methods”), which predominantly referred to groups of surface bees that lifted their abdomens during the shimmering activity.
The red dots in Fig. 1 indicate the projected position of the tethered wasp and the red arrows, the direction of its movement. Chart a 1 displays the situation prior to the onset of the wave with the background activity of the nest, in particular that of the ‘mouth’ zone, where ‘movement’ activities refer primarily to forager traffic and dancing. Chart a 2 refers to the initial frame of the wave, when a small group of bees, located some centimetres to the left of the projected position of the tethered wasp, had started to thrust their abdomens upwards. The subsequent charts (a 3–a 7) document the sample activity as a wavelike process that spreads over the whole nest surface. However, the wave did not spread into the ‘mouth’ zone and became weaker the more it approached the rims of the nest. The three frames on chart a 8 refer to the situation after the sample wave had ceased; only five frames later (not shown in Fig. 1), the consecutive wave started.
Temporal structure, strength and repetition rate of shimmering in tethered wasp experiments
The characteristic trait of shimmering activity in a single tethered wasp experiment has been exemplified in Fig. 2. It concerned three stimulation phases (see “Materials and methods”): In the ‘pre-presentation’ phase (preP phase), the colony showed normal flight traffic without any shimmering activity. In the ‘presentation’ phase (P phase), the wasp was presented in front of the honeybee nest, which evoked repetitive shimmering waves of progressive strength. Lastly, in the ‘post- presentation’ phase (postP phase), after the disappearance of the wasp, the shimmering activity decayed in strength and repetitiveness. The time of the onset of the shimmering waves and the locations of the groups of surface bees that initialised the waves were identified by both automated detection and manual inspection (see “Materials and Methods”). The shimmering waves were quantified by the parameters W peak and W 600 for the waving strength (for definition, see “Materials and methods” and inset of Fig. 2) and by S rep for the repetition rate (Figs. 2 and 3). The W peak and W 600 values strongly correlated linearly (Fig. 3a).
Fig. 3.
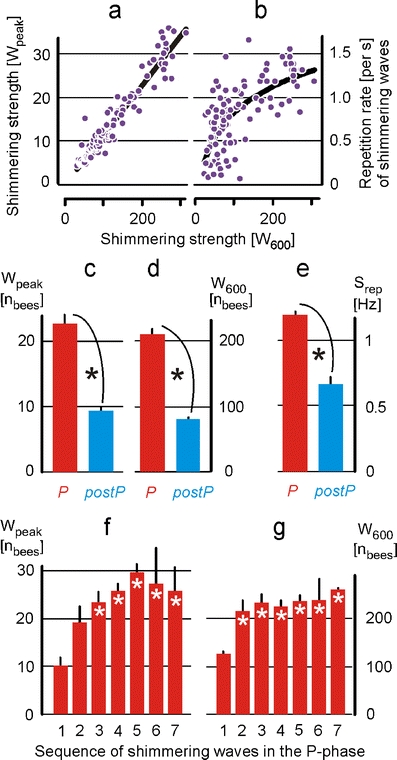
Strength and repetition rate of shimmering. Shimmering was assessed by W peak and W 600 (a, c, d), and by the repetition rate S rep (b, e) from the P phase (red) and the postP phase (blue) of the experiment with the test colony of Apis dorsata. Violet closed circles in a, b refer to shimmering data of both P phase and postP phase and give the regression of W 600 with a W peak [W peak = 0.1128 × W 600 − 0.022 (p < 0.001; n = 118, r = 0.964); Pearson product moment correlation] and with b the repetition rate of shimmering waves with the shimmering strength W 600 [S rep = 0.4561 × ln(W 600) − 1.3077 (n = 102; R 2 = 0.5; p < 0.001)]. c–e Shimmering waves in the P phase (red columns, n = 38) have greater waving strengths and higher repetition rates than those in the postP phase (blue columns) with *p < 0.001 (n = 80; Mann–Whitney rank sum test). f, g Provide waving strengths assessed in the P phase (red columns), averaging W peak and W 600 values of the initial waves (abscissa value, 1), of the second and any further waves (value 2) in a series of waves, irrespective of the direction in which the tethered wasp had been moved; stars indicate that the initial waves of an arousal session differ from the sequential ones (*p < 0.001; n = 7 sessions; one-way ANOVA, Holm–Sidak method). Note that the progressive escalation of shimmering power is more expressed under W peak (f) than under W 600 (g); columns and vertical bars signify means ± SE
We then compared shimmering activity during both states of arousal (Figs. 2 and 3c–e) and found that shimmering activity was stronger when the colony was exposed to the tethered wasp (P phase), compared to the state of the colony in the postP phase after the disappearance of the tethered wasp. In the presence of a tethered wasp, the test colony showed shimmering waves with a strength of W peak = 22.65 ± 4.00 bees per frame at the peak time (mean ± SE; n = 38; Fig. 3c) resulting in W 600 = 210.33 ± 26.06 bees participating in an average wave during 600 ms (n = 38; Fig. 3d). The repetition rate converged (Fig. 3b) to a maximum of S rep = 1.20 ± 0.04 waves per second (n = 33; Fig. 3e) at which the shimmering activity was also strongest.
After the disappearance of the wasp, the waves died away over 30–60 s and had much lower waving strengths (W peak = 8.11 ± 1.48; W 600 = 68.8 ± 10.44) and lower repetition rates (S rep = 0.67 ± 0.06; n = 71). These data prove a higher (p < 0.001, t test) level of arousal in the P phase than in the postP phase.
To investigate the time course of the waving strength of shimmering waves in repetitive series, we averaged the W values of the initial, the second, the third and any further waves of the arousal experiments. The averaged waving strengths increased gradually (Fig. 3f) in their ascending order, which proves that the giant honeybee colony became sensitised over time regarding their readiness for shimmering. The strength parameters W peak and W 600 were, however, disproportionate in tracing the obviously ascending responsiveness to consecutive expositions to wasps. W peak values revealed here a rather steady progression in response strength, which escalated in the course of five successive waves (Fig. 3f), while W 600 displayed only a single increase after the initial wave (Fig. 3g).
Spatial distribution of trigger zones
We backtracked each waving episode by retrograde frame-by-frame inspection, starting from its peak expression back to its initial phase, to detect the location of its first appearance. We assessed the nest coordinates of the initial group of bees, which had started the wave. The positional data of these ‘trigger bees’ was plotted into the evaluation grid (Fig. 4) to assess how many waves were initialised per grid unit area in the course of the tethered wasp experiments. Figure 4 summarises this distribution of trigger processes. The ‘trigger’ centres were distributed closely around the ‘mouth’ zone and did not occur in the mouth zone itself or the far distal site in the periphery of the nest. Furthermore, the positions of the trigger centres of the waves in the P phase (Fig. 4a) were not congruent to that in the postP phase (Fig. 4b). That means that some groups of bees, which were not identical with those that had triggered the waves in the presence of the wasp, had a residual tendency to start waves on their own without visual cues after the preceding arousal.
Fig. 4.
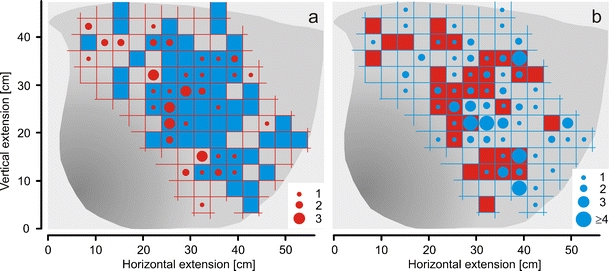
The starting locations of the shimmering waves in the P and postP phases. The spatial representation of the ‘trigger centres’ were plotted into a grid of real-world coordinates with 3.41 cm unit square length. a Closed red circles show ‘trigger centres’ per grid unit area during the arousal state in the P phase; the circle size corresponds to the respective rate level (see inset with red circles); full blue squares in the background signify that respective trigger centres were traced in the postP phase in the same areas. b Blue closed circles show trigger centres per grid unit area in the postP phase in four rate classes (see inset with blue circles); the closed red squares in the background signify that trigger centres were also detected in the P phase in the same area
A more detailed analysis of the trigger centre topology is given in Fig. 5 and is based on the sectoral and segmental partitioning of the nest (see “Materials and methods”). In Fig. 5 (a 1, a 2), all waves of both arousal states (of the P and postP phases) were cumulated; it was found that the waves were mainly initialised at the centre of the nest (sector analysis, Fig. 5, a 1) and mainly in a region adjacent to the mouth zone (segment analysis, Fig. 5, a 2). Figure 5 (b 1, b 2, c 1 and c 2) considers both arousal phases of wave history separately.
For testing the lags between the occurrence of shimmering activity on the nest in terms of degrees (sector analysis) and length (segment analysis), we established the best fit of both distributions in Fig. 5 (b 1 versus c 1 and b 2 versus c 2, respectively) if the data of the P phases were shifted compared to that of the postP phases. The test achieved best results for a shifting of 2.13° (p = 0.033; n = 11, for test, see “Materials and methods”) to the bottom of the nest (sector analysis) and 3.27 cm (p = 0.007; n = 9) to the periphery (segment analysis). In other words, the distributions differ slightly but significantly: In the P phase (b 1, b 2), the waves were triggered by bee groups located in a sector angle on average 2.13° degree ‘higher’ in the nest and in a segment 3.27 cm closer to the mouth zone than compared (c 1, c 2) to the waves released in the postP phase.
The findings deliver reasonable justification to accept the ‘special agents’ hypothesis, which favours the surmise that specific bee groups initialise shimmering waves rather than the alternative assumption (‘everybody’ hypothesis) that all surface bees were able to initialise shimmering waves. The ‘special agents’ hypothesis is supported by three facts. (1) The trigger centres were not uniformly distributed in the test colony but arranged in the centre of the nest rather than in the periphery or in the ‘mouth’ zone. (2) The topology of trigger centres differs between both arousal states. The strongest evidence (3) that validates the ‘special agent’ hypothesis, however, was provided by the following analysis by which the threatening signals were compared with the trigger centres of the respective defence waves regarding their locations (Fig. 6).
Table 1.
Equations of the regression functions of the means shown in Fig. 6
| Equation | Reference chart | a 0 | a 1 | a 2 | r | n | p value |
|---|---|---|---|---|---|---|---|
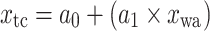
|
c | 5.99 | 0.53 | 0.89 | 10 | <0.001 | |
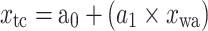
|
d | 46.20 | −0.48 | −0.89 | 10 | <0.001 | |
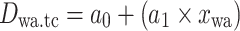
|
e | 6.19 | 0.32 | 0.77 | 11 | 0.006 | |
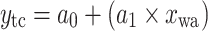
|
f | 15.63 | 0.43 | 0.84 | 7 | 0.02 | |
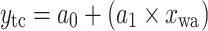
|
g | 11.70 | 0.40. | 0.53 | 5 | 0.36 | |
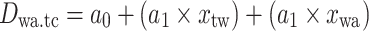
|
h | 14.11 | −0.56 | 0.01 | 0.79 | 7 | 0.127 |
r regression coefficient, n number of mean values considered for the regression, p significance of the regression function
Fig. 6.
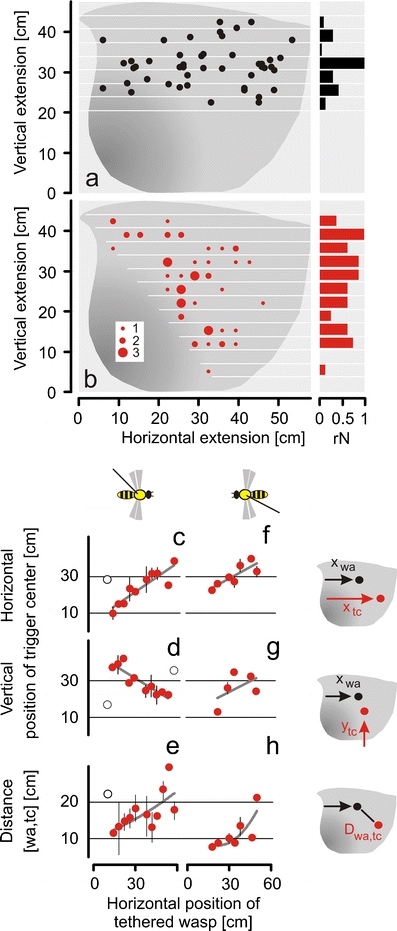
Effect of the position of a tethered wasp and its movement direction on the topology of the trigger centres of shimmering. a Positions of the tethered wasp at the onset of shimmering 15 cm in front of the experimental nest. Black horizontal bars on the right show the relative distribution of the positions of the tethered wasp regarding the vertical grid steps. b Waves initialised at the respective grid square in the P phase (corresponding to the positions of the tethered wasp as displayed in chart a), different sizes of closed red circles summarised in three rate classes (see inset of red closed circles). Red horizontal bars on the right give the distribution of trigger centres regarding the vertical grid steps (cf. Fig. 4); the data (rN) were weighted in relation to (from top to bottom) the progressively smaller region of ‘quiescence’ (indicated by the white grid lines on the nest) and by progressively larger mouth zones (dark grey area of the nest). c–h Correlations of the horizontal (x tc,, c, f) coordinates and of the vertical (y tc d, g) coordinates of the trigger centres and the distances between the tethered wasp and the trigger centre D (wa, tc) between the positions of the wasp (x wa, y wa) and the positions of the trigger centre (x tc, y tc) of the waves (e, h) with the respective horizontal position of the tethered wasp (x wa) at the time of the onset of wave; correlations refer to the shifting of the tethered wasp from the left to the right (c–e) and vice versa (f–h). For regression functions and test statistics, see text and Table 1
Correlating the wasp’s positions with the locations of trigger centres
During the experiment, the tethered wasp was repeatedly moved from the left to the right side of the nest and back again, in constant height and in constant distance to the nest. The locations of the tethered wasp at the onset of the respective waves were assessed as their projections at their shortest distances to the nest (Fig. 6, a), and the locations of the respective trigger centres as displayed in Fig. 4a were plotted into the evaluation grid (Fig. 6, b)
The accessory plots (Fig. 6, c–h) reveal the correlations between the positions of the wasp and the trigger centres at the onset of shimmering. Both wasp and trigger centre positions correlated positively with their horizontal coordinates, in both directions of the wasp’s moving (Fig. 6, c, f), but the colony responded in a more complex way regarding the vertical coordinates of the trigger centres. The more the wasp was shifted to the right side, the lower the trigger centres were positioned. This was unambiguously found during the moving of the tethered wasp from the left to the right side (Fig. 6, d) but not on its way backwards (Fig. 6, g).
Finally, the distances between the projected positions of the wasp correlated with the horizontal coordinates of the trigger centres in both directions of the tethered wasp (Fig. 6, e, h). While the wasp was moving from the left to the right side of the nest, the respective trigger centres were positioned further away than in the second part of the movement of the wasp. This finding was verified by means of horizontal grid classes with selected paired data (p < 0.001; n fLtR = 8/n fRtL = 8); the comparison of unrestricted single data only achieved sub-significant proof (p = 0.06, n fLtR = 28/n fRtL = 26; two-way ANOVA).
Discussion
Giant honeybees produce shimmering waves at the surface of their nests during which groups of surface bees display wavelike patterns that spread from the centre to the peripheral parts of the rim and attachment zones of the bee nest, even across its ‘mouth’ zone (Morse and Laigo 1969; Kastberger et al. 2008a). Shimmering is not only a single-wave event but mostly produced repetitively. The spatial and temporal patterns of waving display varying strength levels and repetition rates and are adaptive in shaping visual cues for external addressees. It has been shown that shimmering reduces the chance of predation by wasps (Kastberger et al. 2008a), and possibly, it deters big game such as rhinos or elephants, in order to protect the nest from molestation. Against birds, the giant honeybees have evolved defence lines with flying guards (Kastberger and Sharma 2000).
Shimmering behaviour is an example of synchronized rhythmic activities that can be observed in the animal kingdom, such as the synchronized swaying in male fiddler crabs with their specific pincers to attract females (Koga et al. 1998) or the synchronized alarm drumming in Camponotus ants (Hölldobler and Wilson 1990; for a further list, see Camazine et al. 2003). Wavelike behaviours that also belong to the group of synchronized rhythmic activities are much rarer. Only three processes have been reported in literature so far that can be viewed as an epiphenomenon of self-organisation (Camazine et al. 2003): First, the body spiral waves of the slime mould Dictyostelium discoideum as the result of cAMP initiation during feeding activities (Bonner 1983, for summary see Camazine et al. 2003); second, the synchronized flashing of fireflies (Buck and Buck 1980, Buck et al. 1981), which have reproductive goals; and lastly, the Mexican waves in human aggregations (Farkas et al. 2002). The slime moulds coordinate the wavelike processes by chemical principles; the fireflies (Buck and Buck 1980; Buck et al. 1981) and the humans, e.g. in football stadiums, do this solely visually after predefined logistic rules (Farkas et al. 2002).
Shimmering in giant honeybees is supposed to be an analogue of a ‘La-Ola’ or “Mexican-wave” process and can be added to the prominent wavelike examples of self-organisation in biological systems (Camazine et al. 2003). These ‘defence waves’ (Kastberger et al. 2008a) are triggered visually, but the spreading process is supposed to be mainly under mechanoceptive control. It is supposed that shimmering also follows simple rules, which scale up individual traits to swarm intelligence (Camazine et al. 2003). The conventional view, which would meet the principles of self-organization, is that a shimmering wave should be released by bees nearest to the disturbing source; this surmise has been addressed in this paper by the ‘everywhere’ hypothesis. Shimmering would then be formed as a sophisticated collective behaviour of all colony members by uniform control of their internal parameters, such as sensory thresholds, and capacities for decision making, memorisation of past disturbances and sensory and refractory fittings.
So far, we have only scarce knowledge about the generation and spreading of shimmering, and it is unclear which kinds of proximate processes are involved. We knew from preliminary observations (Kastberger et al. 2001) that curtain bees contribute in different ways to shimmering. Two aspects can be noted: First, different workloads may strongly modulate the individual participation in shimmering; curtain members of diverse nest regions (such as the mouth zone, quiescent zone, attachment and rim zones, lower and upper regions, as well as surface and subsurface layers) share the generation and spreading of the shimmering wave differently by ‘deflecting’, strengthening or lowering it. Second, the threshold for responding by shimmering to stimulatory cues varies throughout daytime; in particular, it is harder or even impossible to provoke shimmering in the morning or during the periodic mass flight activities (Kastberger et al. 1996).
This paper investigates one of these aspects of the generation of shimmering waves. We designed a tethered-wasp experiment and identified the groups of surface bees that flipped their abdomens first in response to a threatening cue. The advantage of this method is that a tethered wasp can initiate shimmering waves, while the stimulation conditions can be controlled in many respects, such as the relative position of the tethered wasp and the direction and velocity of its passive moving in front of the bee nest. However, this method also has the disadvantage of only allowing the investigation of the bee colony as prey responding to an experimental threatening cue, while it does not permit any response of the experimental wasp to the shimmering reaction of the colony that would feed back to the colony for a subsequent shimmering response. Therefore, the interpretation of the findings is restricted, in particular in the sense that the responses of the colony cannot be taken as fully ‘natural’ (Kastberger et al. 2008a). Any ‘natural’ response can only be expected within the time interval of less than hundred milliseconds—that is, the time lag by which a free-flying wasp would have responded to shimmering. Under ‘natural’ conditions, predatory wasps are attracted by the energy-rich resources of the honeybee nest to approach and hover around the bee nest and show avoidance response to shimmering. In turn, the honeybee colony would have produced further buzzes of shimmering if the wasp would not have disappeared from the nest (Kastberger et al. 2008a). We have previously observed shimmering activities in hundreds of giant honeybee nests (Kastberger et al. 1997, 1998, 2001, 2008a; Kastberger 1999) and have also provoked shimmering experimentally by predatory and non-predatory cues. We know from these qualitative naked eye observations that a shimmering wave may spread over all parts of the nest, but its initiation is restricted to areas outside the mouth zone (Movie S1).
The tethered wasp experiments described in this paper inquire the validity of both the ‘trigger’ hypotheses (‘everywhere’ and ‘special agents’) concerning the initiation of shimmering. The experiments provide the basis to suggest a dynamic topology of trigger centre arrangement as a species-specific feature of giant honeybee nest organisation in general. The cohorts that had initialised the shimmering waves were found to be arranged concerning three characteristic aspects: (1) The locations of the trigger centres varied throughout the tethered wasp experiment and depended on stimulation conditions, such as the direction of visual cue movement and on the arousal state of the colony, i.e. whether the colony had been affected by the presence of the tethered wasp to produce shimmering waves or when the tendency for non-elicited shimmering ceased after the disappearance of the tethered wasp. (2) Shimmering was predominantly initiated in the centre of the nest, preferably along a circle adjacent and peripheral to the ‘mouth’ zone. (3) There was some tendency that shimmering waves followed the tethered wasp on its way across the nest. However, the locations of trigger centres did not reveal the experimental path of the tethered wasp at all because there was a discrepancy in their vertical alignment to the projected trajectory of the experimental wasp. In other words, the bees that triggered shimmering were not necessarily arranged just nearest to the threatening cue. In conclusion, this paper strongly suggests that, in A. dorsata nests, cohorts with specialised abilities are tasked with the initiating of shimmering waves. It is reasonable to suggest that the wave is started by those specialised workers that are located nearest to the threatening source. This also means that the majority of surface bees remain followers in the cascading chain of shimmering. They may simply forward the signal received from their neighbours that had initiated the wave due to their capacity of responding faster to threatening signals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
The video shows the test colony of Apis dorsata with a nest size of 55 × 45 cm (width × height) during a typical stimulation session. The ‘mouth’ zone where forager bees depart, arrive and dance is on the bottom left, representing approximately 30% of the nest surface. The complimentary part of the nest, the ‘quiescent’ area above and to the right of the ‘mouth’ zone, comprises some 600 surface bees. A living wasp was tethered with an elastic wire, and a rod was used to arouse the test colony, provoking shimmering waves whereby hundreds of surface bees flipped their abdomens upwards. During the 7-s wasp’s presence, six shimmering waves occurred and a further three waves after the wasp had disappeared (QuickTime; 0.9 MB). (WMV 2185 kb)
Acknowledgements
The paper had been granted by the Austrian Science Fund (FWF Project P 20515-B16).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Beshers SN, Huang ZY, Oono Y, Robinson GE. Social inhibition and the regulation of temporal polyethism in honey bees. J Theor Biol. 2001;213:461–479. doi: 10.1006/jtbi.2001.2427. [DOI] [PubMed] [Google Scholar]
- Bonner JT. Chemical signals of social amoebae. Scientific American. 1983;248:114–120. [Google Scholar]
- Breed MD, Robinson GE, Page RE., Jr Division of labor during honey bee colony defense. Behav Ecol Sociobiol. 1990;27:395–402. doi: 10.1007/BF00164065. [DOI] [Google Scholar]
- Breed MD, Guzmán-Novoa E, Hunt GJ. Defensive behavior of honey bees: organisation, genetics, and comparison with other bees. Annu Rev Entomol. 2004;49:271–298. doi: 10.1146/annurev.ento.49.061802.123155. [DOI] [PubMed] [Google Scholar]
- Buck J, Buck E. Flash synchronization as tool and as enabler in firefly courtship competition. Am Nat. 1980;116:591–593. doi: 10.1086/283649. [DOI] [Google Scholar]
- Buck J, Buck E, Case JF, Hanson FE. Control of flashing in fireflies. V. Pacemaker synchronization in Pteroptyx cribellata. Flash synchronization as tool and as enabler in firefly courtship competition. J Comp Physiol A. 1981;144:287–298. doi: 10.1007/BF00612560. [DOI] [Google Scholar]
- Butler CG. The world of the honeybee. London: Collins; 1954. [Google Scholar]
- Camazine S, Sneyd J. A model of collective nectar source selection by honey bees: self-organization through simple rules. J Theor Biol. 1991;149:547–571. doi: 10.1016/S0022-5193(05)80098-0. [DOI] [Google Scholar]
- Camazine S, Deneubourg J-L, Franks NR, Sneyd J, Theraula G, Bonabeau E. Self-organization in biological systems. Princeton: Princeton University Press; 2003. [Google Scholar]
- Farkas I, Helbing D, Vicsek T. Social behaviour: Mexican waves in an excitable medium. Nature. 2002;419:131–132. doi: 10.1038/419131a. [DOI] [PubMed] [Google Scholar]
- Hepburn HR, Radloff SE. Honeybees of Africa. Berlin: Springer; 1998. [Google Scholar]
- Hölldobler B, Wilson EO. The ants. Cambridge: Harvard University Press; 1990. [Google Scholar]
- Kastberger G (1999) The magic trees of Assam—documentary film about the biology of the giant honeybee Apis dorsata. National Geographic, ZDF, ORF & epo-film Wien
- Kastberger G, Sharma DK. The predator-prey interaction between blue-bearded bee eaters (Nyctyonis athertoni) and Giant honeybees (Apis dorsata) Apidologie. 2000;31:727–736. doi: 10.1051/apido:2000157. [DOI] [Google Scholar]
- Kastberger G, Winder O, Hoetzl T, Raspotnig G. Behavioural features of a periodic form of massed flight activity in the Giant honeybee Apis dorsata. Apidologie. 1996;27:381–395. doi: 10.1051/apido:19960506. [DOI] [Google Scholar]
- Kastberger G, Biswas S, Habibulla M. Temporal and spatial patterns of colony alarming in Apis dorsata. Verh Dtsch Zool Ges. 1997;90:293. [Google Scholar]
- Kastberger G, Raspotnig G, Biswas S, Winder O. Evidence of Nasonov scenting in colony defence of the Giant honeybee Apis dorsata. Ethology. 1998;104:27–37. [Google Scholar]
- Kastberger G, Winder O, Steindl K (2001) Defence strategies in the Giant honeybee Apis dorsata. Proceedings of the Deutsche Zoologische Gesellschaft, Osnabrück 94.1:7
- Kastberger G, Schmelzer E, Kranner I. Social waves in Giant honeybees repel hornets. PLoS ONE. 2008;3(9):e3141. doi: 10.1371/journal.pone.0003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastberger G, Thenius R, Stabentheiner A, Hepburn R. Aggressive and docile colony defence patterns in Apis mellifera. A retreater-releaser concept. J Insect Behav. 2008 [Google Scholar]
- Koeniger N, Fuchs S. Zur Kolonieverteidigung der asiatischen Honigbienen. Z Tierpsychol. 1975;37:99–106. [PubMed] [Google Scholar]
- Koga T, Backwell PRY, Jennions MD, Christy JH. Elevated predation risk changes mating behaviour and courtship in a fiddler crab. Proc Biol Sci. 1998;265:1385–1390. doi: 10.1098/rspb.1998.0446. [DOI] [Google Scholar]
- Lindauer M. Ein Beitrag zur Frage der Arbeitsteilung im Bienenstaat. Z Vgl Physiol. 1952;36:299–345. doi: 10.1007/BF00298048. [DOI] [Google Scholar]
- Lindauer M. Über die Verständigung bei indischen Bienen. Z Vgl Physiol. 1956;38:521–557. doi: 10.1007/BF00341108. [DOI] [Google Scholar]
- Morse RA, Laigo FM. Apis dorsata in the Philippines. Philipp Assoc Entomol. 1969;1:1–96. [Google Scholar]
- Oldroyd BP, Wongsiri S. Asian honey bees. Cambridge: Harvard University Press; 2006. [Google Scholar]
- Pasteels JM, Deneubourg J-L, Goss S. Self-organization mechanisms in ant societies [I]: trail recruitment to newly discovered food sources. Experientia Suppl. 1987;54:155–175. [Google Scholar]
- Ratnieks FLW, Anderson C. Task partitioning in insect societies. Insect Soc. 1999;46:95–108. doi: 10.1007/s000400050119. [DOI] [Google Scholar]
- Ratnieks FLW, Anderson C. Task partitioning in insect societies II: use of queuing delay information in recruitment. Am Nat. 1999;154(5):536–548. doi: 10.1086/303256. [DOI] [PubMed] [Google Scholar]
- Reynolds CW. Flocks, herds, and schools: a distributed behavioural model. Comput Graph. 1987;21(4):25–34. doi: 10.1145/37402.37406. [DOI] [Google Scholar]
- Robinson GE, Page RE, Strambi C, Strambi A. Colony integration in honey bees: mechanisms of behavioral reversion. Ethology. 1992;90:336–348. [Google Scholar]
- Roepke W. Beobachtungen an indischen Honigbienen, insbesondere an Apis dorsata F. Meded Landbouwhogesch Wageningen. 1930;34:1–28. [Google Scholar]
- Ruttner F. Biogeography and taxonomy of honeybees. Berlin: Springer; 1988. [Google Scholar]
- Sakagami SF. Preliminary report on the specific difference of behaviour and other ecological characters between European and Japanese honeybees. Acta Hymenoptera. 1960;1:171–198. [Google Scholar]
- Seeley TD. The Wisdom of the hive. Cambridge: Harvard University Press; 1995. [Google Scholar]
- Seeley TD, Buhrman SC. Nest-site selection in honeybees: how well do swarms implement the "best-of-N" decision rule? Behav Ecol Sociobiol. 2001;49:416–427. doi: 10.1007/s002650000299. [DOI] [Google Scholar]
- Seeley TD, Seeley RH, Aratanakul P. Colony defence strategies of the honeybees in Thailand. Ecol Monogr. 1982;52:43–63. doi: 10.2307/2937344. [DOI] [Google Scholar]
- Winston ML, Fergusson LA. Influence of the amount of eggs and larvae in the honeybee colonies on temporal division of labour. J Apic Res. 1986;25(4):238–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video shows the test colony of Apis dorsata with a nest size of 55 × 45 cm (width × height) during a typical stimulation session. The ‘mouth’ zone where forager bees depart, arrive and dance is on the bottom left, representing approximately 30% of the nest surface. The complimentary part of the nest, the ‘quiescent’ area above and to the right of the ‘mouth’ zone, comprises some 600 surface bees. A living wasp was tethered with an elastic wire, and a rod was used to arouse the test colony, provoking shimmering waves whereby hundreds of surface bees flipped their abdomens upwards. During the 7-s wasp’s presence, six shimmering waves occurred and a further three waves after the wasp had disappeared (QuickTime; 0.9 MB). (WMV 2185 kb)


