Abstract
In mammals, the neuronal pathways by which rod and cone photoreceptors mediate vision have been well documented. The roles that classical photoreceptors play in photoentrainment, however, have been less clear. In mammals, intrinsically photosensitive retinal ganglion cells (ipRGCs) that express the photopigment melanopsin project directly to the suprachiasmatic nucleus (SCN) of the hypothalamus, the site of the circadian clock, and thereby contribute to non-image forming responses to light. Classical photoreceptors are not necessary for photoentrainment since loss of rods and cones does not eliminate light entrainment. Conflicting evidence arose, however, when attenuated phase-shifting responses were observed in the retinal degenerate CBA/J mouse. In this study, we examined the time course of retinal degeneration in CBA/J mice and used these animals to determine if maturation of the outer retina regulates the morphology, number and distribution of ipRGCs. We also examined whether degeneration during the early development of the outer retina can alter the function of the adult circadian system. We report that dendritic stratification and distribution of ipRGCs was unaltered in mice with early retinal degeneration, suggesting that normal development of the outer retina was not necessary for these processes. We found, however, that adult CBA/J mice have greater numbers of ipRGCs than controls, implicating a role for the outer retinal photoreceptors in regulating developmental cell death of ipRGCs.
Keywords: circadian rhythms, photoreceptor cells, SCN, photoentrainment, dendritic stratification
Introduction
In mammals, the photoreceptive mechanisms underlying vision have been known for some time. However, the pathways mediating non-image forming responses to light, such as circadian photoentrainment, are not as well understood. It was traditionally thought that all photoreception in the eye was mediated by rod and cone photoreceptors. However, discovery of melanopsin-containing retinal ganglion cells (RGCs) that exhibit light responses independent of rod and cone signaling (Berson et al, 2002; Hattar et al, 2002; Warren et al, 2003) led to an understanding that light reception is not exclusively mediated by rods and cones.
Studies have attempted to elucidate the respective roles of the ocular photoreceptors in photoentrainment. Mice and rats in which rods and cones degenerate during adulthood retain the ability to phase-shift in response to light (Foster et al, 1991; Semo et al, 2003; Tosini et al, 2007), though some differences are seen at high light intensities (Freedman et al, 1999). Conversely, melanopsin knockout mice (Opn4 −/−) show attenuated phase-shifting (Panda et al, 2002), suggesting an important role of these melanopsin-containing RGCs in circadian light responses. In addition, loss of both melanopsin and rods and cones eliminates all non-visual light responses (Hattar et al, 2003). A similar phenotype is seen in mice in which the ipRGCs are genetically ablated (Guler et al, 2008). These data suggest that rods and cones are not necessary for photoentrainment, but do play a role.
Although photoentrainment is possible in the absence of rods and cones, CBA/J mice which are homozygous for the retinal degeneration allele Pde6brd1 display attenuated phase-shifting responses to light (Yoshimura et al, 1994b). Most of the models used to isolate the role of photoreceptors in photoentrainment display outer retinal degeneration during adulthood, after the retina has fully matured. However, CBA/J mice display degeneration that is complete before the first month of life. During this time, ipRGCs in wild-type mice undergo changes in cell number, morphology and physiology (Sekaran et al, 2005; Tu et al, 2005). Whether photoreceptor degeneration influences these events is unclear.
During normal development, endogenous activity within the retina, which is likely mediated by the release of glutamate from bipolar cells, plays a role in directing the stratification of RGCs involved in vision (Chalupa and Gunhan 2004). In addition, RGCs undergo developmental cell death that is completed around the time rods and cones have matured (Tian and Copenhagen 2003). These findings led to the question: “Do classical photoreceptors play a role in the normal development of ipRGCs and, as a result, the circadian system as a whole?”
We set out to determine whether the differences in photoentrainment seen in CBA/J mice are a result of photoreceptor loss during development. If the photoreceptors play a role in regulating the number, distribution and/or dendritic stratification patterns of ipRGCs during early postnatal development it would be expected that the photosensitivity of the adult circadian system would be altered. We used immunohistochemistry and wheel running experiments to address the hypothesis that perturbation of the outer retina disrupts the proper development of ipRGCs and alters optimal circadian function.
Materials and Methods
Animals
All procedures were carried out in compliance with the guidelines of the National Institutes of Health. All protocols were approved in advance by the Institutional Animal Care and Use Committee of Oregon Health & Science University. Male CBA/J mice, which carry the Pde6brd1 mutation and exhibit blindness by weaning age (Jackson Laboratory, Bar Harbor, ME, USA), were used to examine the effects of early retinal degeneration on the development of ipRGCs. For controls, we used male CBA/N (National Cancer Institute, Frederick, MD, USA) mice, which have the same genetic background as the CBA/J mice, but lack the Pde6brd1 mutation and are, therefore, visually intact.
The mice were housed in facilities that permit the maintenance of a 12-hour light-dark cycle. Animals used in the behavioral studies were kept in separate chambers under the environmental conditions described below. Studies were restricted to male mice to avoid potential effects of fluctuations in female reproductive hormones on circadian behaviors. All mice used for entrainment studies were between 3 months and 7 months of age, to avoid variability in their abilities to entrain (Pittendrigh and Daan 1976).
Wheel-running experiments
Mice used in the behavioral experiments were housed in an Intellus Control System chamber (Percival Scientific, Perry, IA) that allows for constant lighting and temperature (24°C) conditions. Within the chamber animals were kept in Nalgene cages equipped with running wheels and magnetic switches (Mini Mitter, Bend, Oregon, USA) that allow for recording of wheel revolutions. Wheel-running data was collected continuously by VitalView (Mini Mitter) acquisition software. Actograms depicting activity and rest cycles during entrainment and free-running assays were generated using ClockLab (Actimetrics Software, Wilmette, IL, USA).
The ability of CBA/J and CBA/N mice to entrain to a photoperiod of 12 hours of light (1000 lux) followed by 12 hours of darkness (0 lux) (LD 12:12) was observed by measuring their wheel-running activity. Once entrained, the mice were kept in constant darkness (DD) to measure the period length of their free-running rhythms. To examine the ability of the mice to phase shift in response to light, a 15 min white light pulse administered by a 32-W fluorescent bulb (300 lux) was given at circadian time 15 during the free-running activity. Phase-shifts and period lengths were calculated using ClockLab.
Tissue preparation
Animals were deeply anesthetized with isoflurane and sacrificed by cervical dislocation. Eyes were removed, placed in a Petri dish containing 0.1 M phosphate buffer (PB) (pH 7.4), and quickly hemisected. Using a Leica GZ4 dissection microscope, the retinas were carefully removed from the eyecup and fixed in 4% paraformaldehyde (PFA) (pH 7.4) overnight at 4°C.
Characterizing retinal degeneration
To characterize the outer retina degeneration, retinas were obtained from mice at 4, 8, 10, 15, and 21 days of postnatal development (P4, P8, P10, P15, P21) and from adults. Following fixation, the retinas were cryoprotected by successive immersion in phosphate-buffered solutions containing 10% and 30% sucrose at 4°C overnight. The tissue was embedded in Thermo Shandon Cryochome (Thermo Scientific, Pittsburgh, PA, USA), fast-frozen over dry-ice mixed with 100% ethanol for 3–5 minutes and stored at −80°C.
Vertical cross-sections (15 µm) of embedded retinas were cut on a Leica 1720 digital cryostat and thaw-mounted onto glass slides. Sections were rinsed with 0.1 M PB (pH 7.4) and incubated in a Modified Giemsa Stain (Sigma-Aldrich, St. Louis, MO, USA) (diluted 1:5 in dH20) for 10 min at room temperature (RT; 20–22°C). Sections were rinsed with 0.1 M PB for 5 min and then dehydrated in 20%, 80%, and 100% ethanol. Tissue was mounted in Entellan New (Electron Microscopy Sciences, Hatfield, PA, USA) and imaged by brightfield microscopy at 20× using a Zeiss Axioscope 2TM. Degeneration was quantified using AxioVision software (Carl Zeiss Microimaging, Germany) to measure the thickness of the ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL) and outer nuclear layer (ONL) at each stage of development.
Dendritic stratification
To observe the dendritic stratification patterns of ipRGCs during development, retinas were processed as described above. To visualize ipRGC bodies and dendrites, sections were rinsed in 0.1 M PB with 0.3% Triton-X for 15 min, placed in blocking solution (1% BSA + 0.3% Triton-X) for 1 hour at RT and stained with an affinity-purified polyclonal antibody generated in rabbit and directed against the amino-terminal peptide of mouse melanopsin O/N at 4°C (Warren et al, 2006; Walker et al, 2008). Next, the tissue was incubated with a fluorophore-conjugated secondary antibody (Alexa-488 labeled goat anti-rabbit IgG; Molecular Probes, Eugene, OR) diluted in blocking solution (1:2000) for 2 hours at RT in the dark. The retinas were then rinsed with 0.1 M PB, counterstained with the nuclear stain, DAPI (80 ng/ml) for 1–3 min at RT and mounted on a glass slide in Aqua Mount (Fisher Scientific, Pittsburgh, PA, USA). Immunostained tissue was imaged by fluorescence microscopy at 20X using a Zeiss Axioscope 2TM.
Cell numbers and distribution
To assess ipRGC number and distribution, whole mount retinas from CBA/J and CBA/N mice of the same developmental ages were fixed immediately after dissection for 30 min at RT. They were then rinsed in 0.1 M PB with 0.3% Triton-X and PFA (1:1 v/v) for 30 min and placed in blocking solution for 1 hour at RT. The retinas were then incubated with the α-melanopsin antibody in blocking solution (0.5 µg/ml) for 2 days at 4°C and rinsed with 0.1 M PB. Next, the tissue was incubated with a fluorophore-conjugated secondary antibody and DAPI and mounted as described above. Fluorescence microscopy was used to image melanopsin-positive cells with a 20X objective. Paintshop Pro (Corel Corporation, Fremont, CA, USA) was used to create a composite image of the entire whole mount. The number of melanopsin-positive cell bodies and their coordinates were calculated using ImageJ software (http://rsb.info.nih.gov/ij/).
To determine ipRGC distribution, the distance between melanopsin-positive cell bodies used in the cell count experiments was calculated and normalized to the maximum distance between cells. A smoothed non-parametric kernel density estimate was computed for each set of normalized distances. Density estimates were averaged for each strain at different stages of development.
Pupillometry
The pupillary light response was measured in both CBA/J and CBA/N adults. Mice were dark adapted for 1 hour prior to testing the PLR and experiments were conducted between 2 and 6 hours prior to lights off (12:12 LD) (Lucas et al, 2001). One eye of each animal was exposed to bright light for 30 seconds and was video taped under infrared light using a CCD camera. The light intensity was controlled by different combinations of neutral density filters placed in the light path. Pupil area was measured and normalized to area prior to light exposure.
Statistics
Averages are reported as the mean ± SD. Significance was determined using the Student’s t-Test.
Results
Light entrainment is altered in CBA/J mice
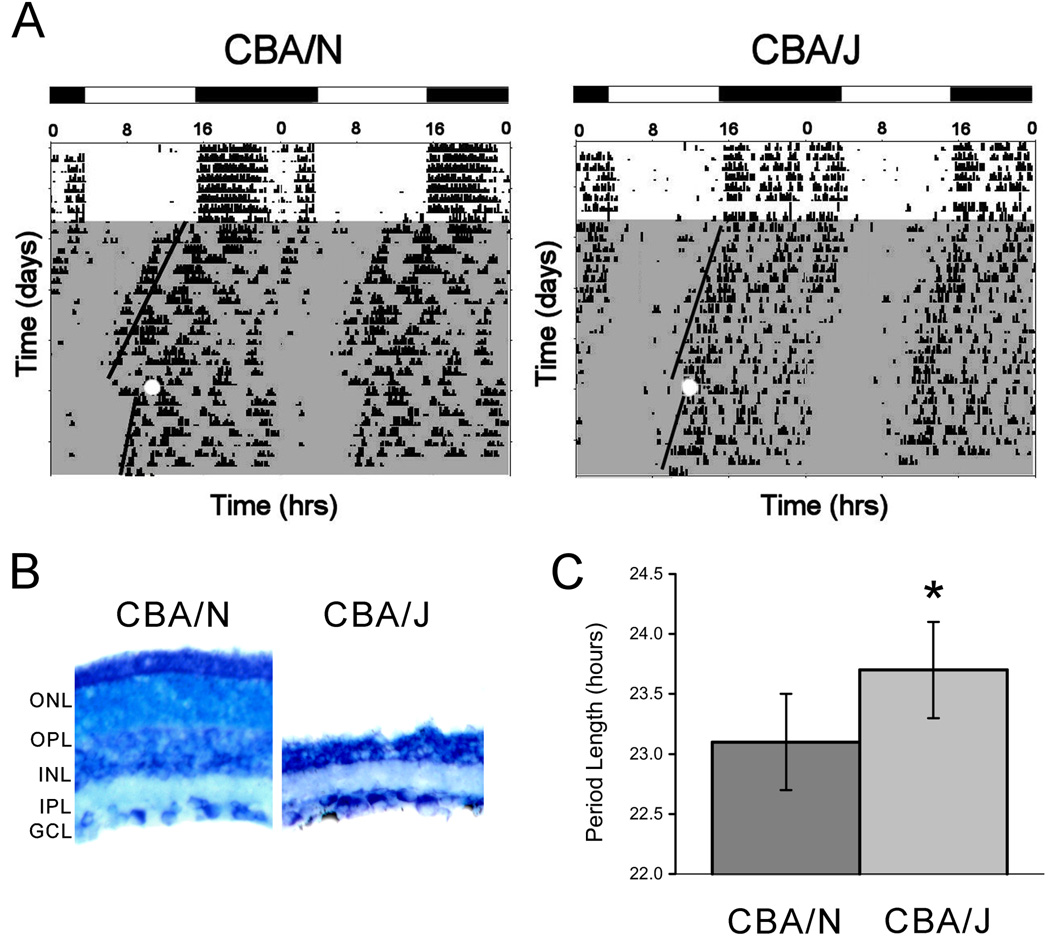
To examine the potential role of outer retina maturation on the photosensitivity of the adult circadian system during development, wheel-running experiments were conducted with adult CBA/J (n= 6) and CBA/N mice (n = 6). The actograms in Figure 1A show that both strains can entrain to a photoperiod of 12 hours of light (1000 lux) followed by 12 hours of darkness (LD 12:12). When put into constant darkness (DD) (0 lux) for 17 days, the CBA/J mice show free-running rhythms. The period lengths of the CBA/J and CBA/N mice are 23.7 ± 0.4 and 23.1 ± 0.4 hrs, respectively, and are significantly different (p = 4 × 10−3, t10 = 5.1) (Figure 1B).
Figure 1. Light entrainment, free-running rhythms and phase-shifting in CBA/J and CBA/N mice.
A. Actograms depicting wheel running behavior in CBA/J and CBA/N mice under 12:12 (LD). Activity and rest were double-plotted. White bars indicate lights on and black bars indicate lights off. The shaded region depicts constant darkness (12:12 (DD)) in which the animals exhibit free-running rhythms. The white circle indicates a light pulse given at CT 15 to cause a phase-shift. Both strains of mice exhibit a phase shift, though the response in CBA/J animals was slightly attenuated. The actograms and values reported are representative of data from 6 mice of each strain. B. Period lengths in CBA/J and CBA/N mice are significantly different ( * = p = 4 × 10−3, t10 = 5.1). White bars = period length in CBA/N mice; dark grey bars = period length in CBA/J mice. C. Giemsa-stained retinal cross-sections taken from mice used in the wheel-running experiments confirm that CBA/J mice lack ONL and OPL. Scale bar = 50 µm.
To examine the ability of light to shift the circadian phase of CBA/J (n = 3) and CBA/N (n = 3) mice, a pulse of light (300 lux) was administered at circadian time 15 during the period of free running activity. The animals were then maintained in DD for an additional 8 days. The degree to which the animals shifted their activity was measured. While both groups were able to phase shift in response to light, the shift seen in CBA/J mice was attenuated compared to the controls (CBA/N = 3 ± 0.6 hrs; CBA/J = 1.67 ± 0.23 hrs; p = 0.03, t4 = 3.2). These data are in agreement with those previously reported (Yoshimura et al, 1994b).
To determine the degree of degeneration in CBA/J animals that were used in the wheel running experiments, retinal cross-sections were stained with Giemsa (Figure 1C). The retinas from the CBA/J mice lack all outer cellular layers, indicating complete outer retinal degeneration.
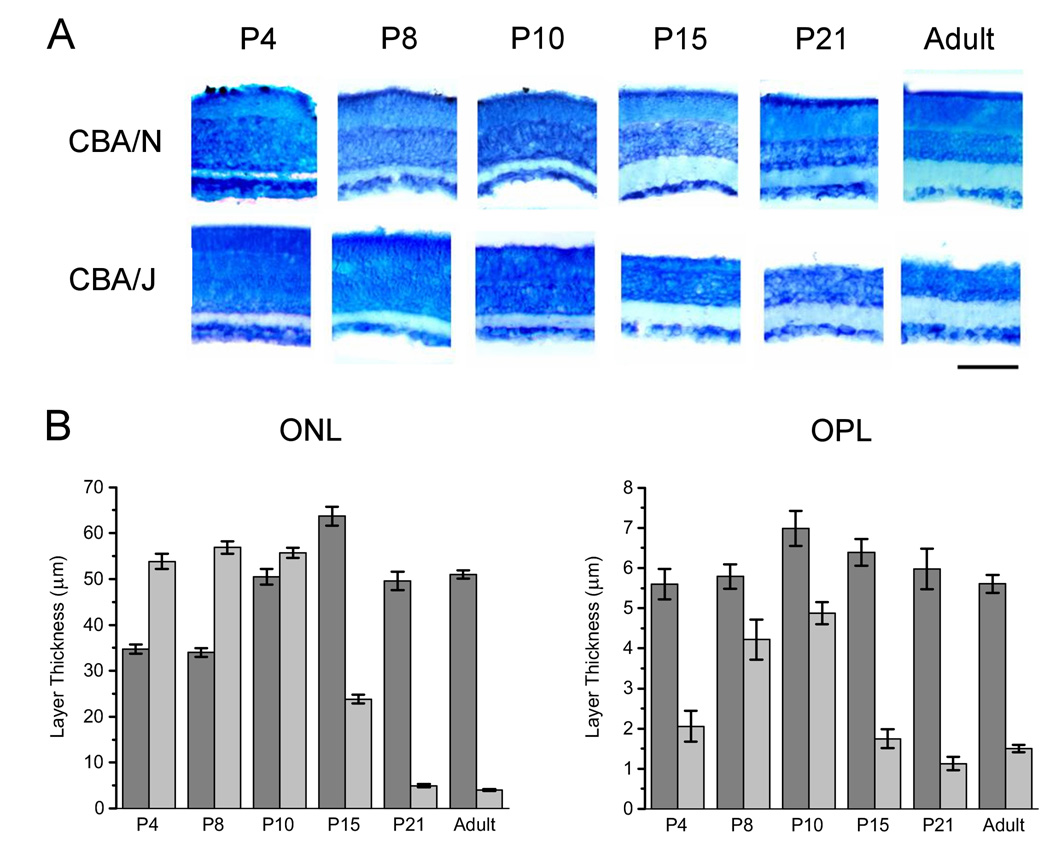
Time course of retinal degeneration in CBA/J mice
To characterize the time course of degeneration during development, retinas were taken from mice at P4, P8, P10, P15, P21 and adults (3+ months). For CBA/J’s, n = 3 – 5, and for CBA/N’s, n = 3 –6. Figure 2A shows cross-sections of representative retinas stained with Giemsa. From these images it is clear that in CBA/J mice, the ONL and OPL, the layers where the rod and cone photoreceptors reside, are present and intact until P10. By P15 these layers show signs of degeneration, which continues rapidly and is nearly complete by P21, when both layers are almost undetectable. This degeneration was quantified by using AxioVision software to measure the thickness of each retinal layer. By P15, The OPL (1.75 ± 2.0 µm, 6.39 ± 2.2 µm) and ONL (23.82 ± 8.1 µm, 63.72 ± 13.5 µm) are significantly reduced in CBA/J compared to CBA/N (p = 2.7 × 10−21, t8 = 11.7 for OPL and p = 1.5 × 10−39, t8 = 20.1 for ONL), and by P21 they are almost nonexistent (Figure 2B).
Figure 2. Time course of outer retinal degeneration during development.
A. Retinal cross-sections taken from CBA/J and CBA/N mice at various stages of postnatal development show that the CBA/J mice display retinal degeneration that begins around P10 and is completed by P21. Scale bar = 100 µm. B. Quantification of the degeneration shows that by P21 the ONL and OPL are significantly reduced in CBA/J mice. Black bars = layer thickness in CBA/J mice; white bars = layer thickness in CBA/N mice.
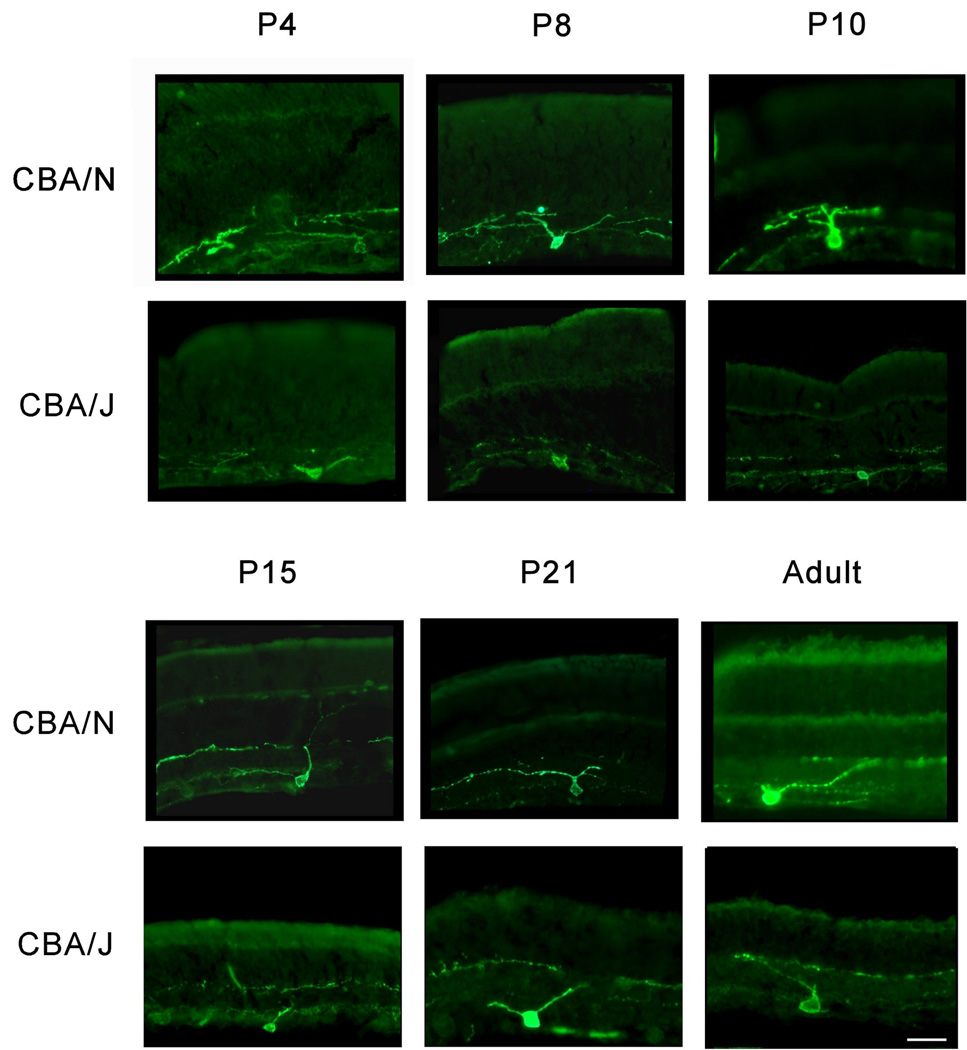
Dendritic stratification of ipRGCs is unaffected by loss of photoreceptors
During early stages of postnatal development (P4), the dendrites of ipRGCs are unstratified. Over time, however, they begin to stratify into distinct regions in the IPL. To determine whether the outer retinal degeneration affects the ability of ipRGC dendrites to properly stratify, retinal cross-sections were taken from mice at P4, P8, P10, P15, P21 and adults and immunostained for melanopsin (for CBA/J’s, n = 3 – 5, and for CBA/N’s, n = 3 – 5). Data from these studies show that in both strains the ipRGC dendrites are unstratified at P4. As the retina develops, the dendrites stratify into distinct regions in the IPL within the same time frame for both CBA/J and CBA/N mice. The dendrites of the ipRGCs show no discernible differences in their ability to stratify compared to CBA/N controls at all time points analyzed (Figure 3). As a result, we conclude that despite the massive outer retinal degeneration in the CBA/J mice, the melanopsin-expressing RGCs can still extend their dendritic processes correctly, suggesting that photoreceptors are not necessary for this process.
Figure 3. Dendritic stratification of melanopsin-positive RGCs during development.
Retinal cross sections from CBA/N and CBA/J mice stained with an anti-melanopsin antibody. Scale bar = 50 µm.
Potential control of ipRGC number and distribution by photoreceptors
The results from the stratification studies described above demonstrate that photoreceptor loss during development does not affect stratification of ipRGCs. We conclude that the differences in the animals’ behavioral responses to light are not the result of aberrant morphological development of melanopsin-containing RGCs. An additional possibility to consider is that outer retina maturation might control ipRGC development by regulating cell number and/or distribution. To examine this, we immunostained retinal whole mounts from CBA/J and CBA/N mice at P4, P8, P10, P15 and P21 for melanopsin (for CBA/J’s, n = 4 – 8 and for CBA/N’s, n = 4 – 5). The tissue was imaged by fluorescence microscopy, and the resulting images were stitched together to reconstruct the entire retina (Figure 4A). Data from these studies show that at P4, CBA/J and CBA/N mice have the same number of melanopsin-positive cells (CBA/J = 54.4 ± 29.7 cells/mm2; CBA/N = 51.8 ± 17.2 cells/mm2). However, the number of melanopsin-positive cells seen at P21, is greater in the CBA/J animals compared to the controls (CBA/J = 44.4 ± 9.0 cells/mm2; CBA/N = 27.4 ± 6.6 cells/mm2; p = 7 × 10 −3, t9 = 3.5) (Figure 4B), suggesting that the ipRGCs do not experience the same degree of cell death in the CBA/J mice compared to CBA/N’s. This suggests that the cells in the outer retina may play a role in regulating ipRGC number.
Figure 4. Cell number and distribution of melanopsin-positive RGCs in CBA/N and CBA/J mice.
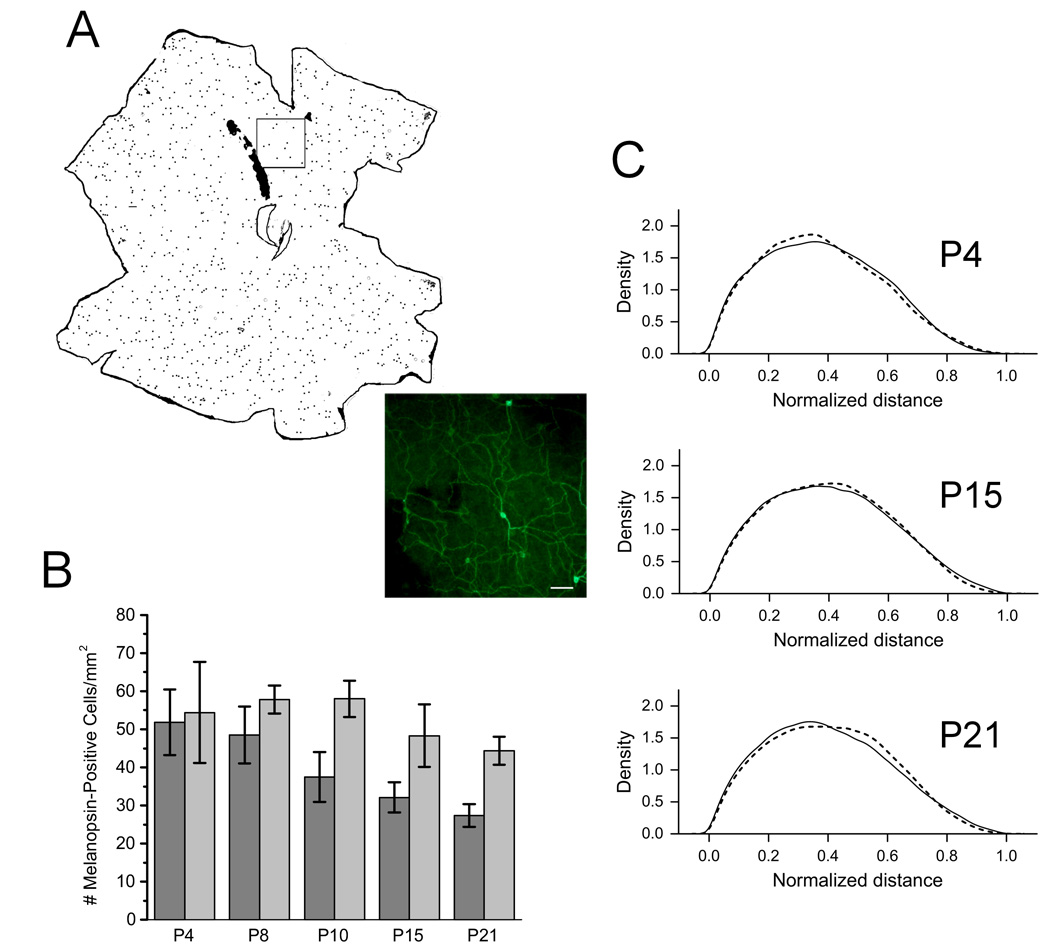
A. Whole mount retinas were stained with an anti-melanopsin antibody and imaged by fluorescence microscopy, and the location and number of cell bodies were mapped using ImageJ. Inset shows the actual staining of cells (20×). Scale bar = 100 µm. B. Quantification of melanopsin-positive cell number in developing mice. The y-axis represents the number of cells per unit area (µm). Black bars = CBA/J mice; Gray bars = CBA/N mice. C. Distribution of melanopsin-positive cells in P4, P15 and P21 mice. Dotted lines depict distributon of cells in CBA/J mice and solid lines represent distribution in CBA/N mice.
To examine the distribution of RGCs across the retina, ImageJ was used to calculate x, y-coordinates for each melanopsin-positive cell body. Normalized distance between cell bodies was calculated and used to determine differences in the distribution of cells. No significant differences in the distribution of cells in CBA/J mice were observed at any time points compared to CBA/N mice (Figure 4C). Moreover, within strains, the melanopsin-expressing cells remains evenly distributed across the retina throughout development. In addition, there are no differences found at any developmental time point. We conclude that although ipRGC number is changing, the relative distribution remains the same and is unaffected by photoreceptor loss.
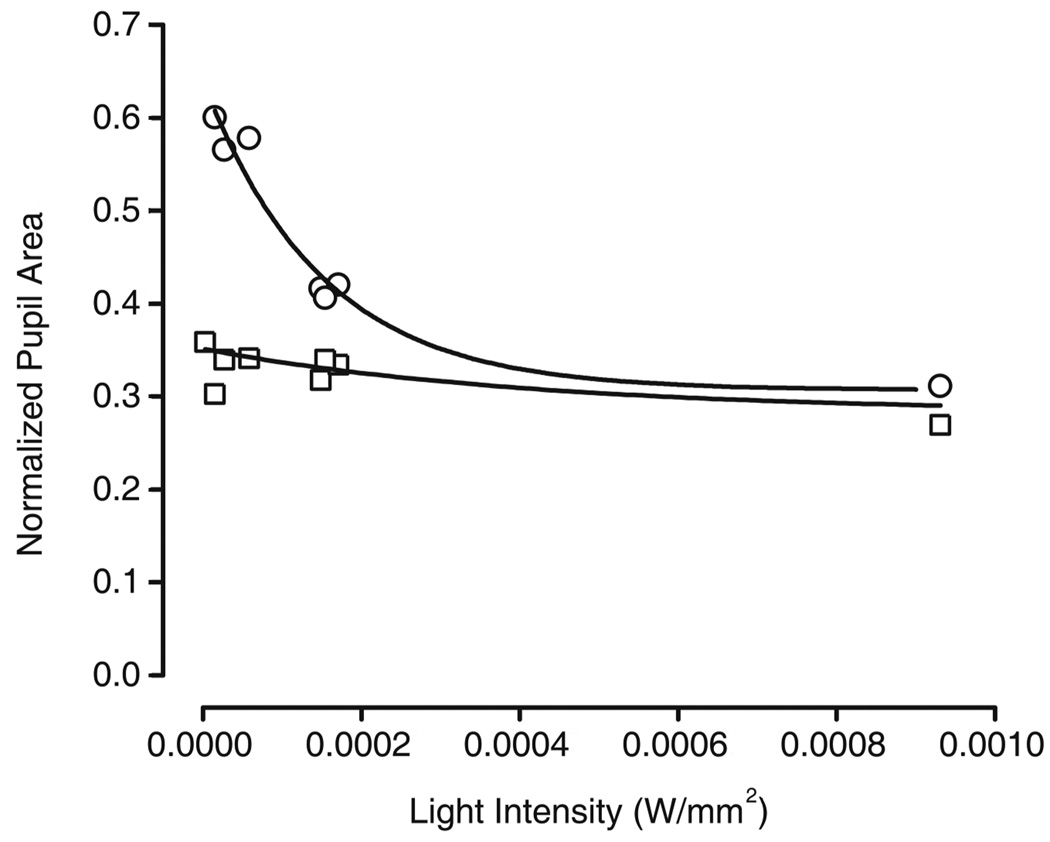
PLR is intact at high light intensities in CBA/J mice
We examined the PLR in adult CBA/J mice and found that the response to bright light was attenuated at low light intensities. This is to be expected of animals lacking rods and cones (Lucas et al, 2003). However, at high light intensities, the CBA/J mice did not differ in their PLR from the controls, suggesting that the melanopsin pathway is functioning properly at the level of the retina (Figure 5). We conclude that developmental retinal degeneration does not affect the melanopsin-based contribution to the PLR and that the differences seen in behavior are not due to functional changes within the melanopsin-containing cells, at least at the level of the retina.
Figure 5. Pupillary light reflex in CBA/J and CBA/N mice.
The y-axis depicts area of pupil normalized to pupil size at the start of the experiment. The x-axis shows increasing light intensities. Solid squares = CBA/J; Open circles = CBA/N.
Discussion
A number of animal models have been used to determine the roles of rods and cones in the process of photoentrainment. Phase-shifting studies in 80-day old C57BL/6J mice homozygous for the rd allele showed that these animals have light-induced circadian responses that are identical to control animals (rd/+ and +/+) (Foster et al, 1991; Tosini et al, 2007). These mice exhibit an extreme loss of rod photoreceptors early in adult life, leaving a small number of cones present in adults. A similar study showed that rdta mice, which express the gene for an attenuated diphtheria toxin under the control of a portion of the rhodopsin promoter and exhibit photoreceptor loss, also show light-induced phase-shifting responses (Lupi et al, 1999). In addition, mice in which both the rods and cones are rendered nonfunctional but remain intact are also able to phase-shift in response to light (Hattar et al, 2003). Conversely, melanopsin knockout mice have attenuated phase-shifting behaviors, implicating their role in entrainment (Panda et al, 2002). In addition, genetic ablation of melanopsin-containing RGCs lead to a significant phase-shift reduction, suggesting that these cells serve as conduits for the rods and cones to the circadian clock (Guler et al, 2008; Hatori et al, 2008). Together these data suggest that the melanopsin-containing cells are responsible for light entrainment.
Despite work suggesting that normal phase shifting can occur in the absence of rods and cones, a study done by Yoshimura et al. on CBA/J mice led us to a different hypothesis. These mice display outer retinal degeneration as a result of a mutation in the B subunit of the phosophodiesterase gene that mediates rod transduction. This leads to the complete loss of both the outer plexiform layer (OPL) and outer nuclear layer (ONL). However, unlike the animals in the experiments discussed above, these retinal degenerate mice show attenuated phase-shifting responses to light (Yoshimura et al, 1994a). The difference between these mice and the other models is the time course of retinal degeneration. Our studies agree with the phase-shifting data reported by this group, and we characterize the time course of retinal degeneration and show that the ONL and OPL deteriorate during early postnatal development (P10–P21).
We have hypothesized that maturation of the outer retina is involved in regulating the proper development of ipRGCs and thereby dictates the photosensitivity of the adult circadian system. If true, this would explain the differences seen in the light-induced circadian behaviors of the CBA/J mice compared to the other models. There is significant anatomical and physiological evidence to suggest that photoreceptors regulate ipRGCs (Belenky et al, 2003; Sakamoto et al, 2004; Perez-Leon et al, 2006; Wong et al, 2007). Electron micrographs and immunohistolgical staining show that ipRGCs are contacted by amacrine and bipolar cell terminals (Belenky et al, 2003; Ostergaard et al, 2007) suggesting that these cells receive input from photoreceptor-driven pathways. In addition, in adult rats, photoreceptor degeneration leads to a decrease in overall melanopsin levels and an elimination of its circadian expression (Sakamoto et al, 2004; Ostergaard et al, 2007).
Because of the radical changes RGCs undergo during the maturation of the vertebrate retina, there are many potential points of regulation. During development, the dendritic stratification of RGCs experience substantial remodeling. Early on these neurons are unstratified, but as the animal matures, RGC dendrites stratify into specific retinal layers. Glutamate released from bipolar cells plays a role in determining the stratification of RGCs involved in vision (Bodnarenko and Chalupa 93). We reasoned that if glutamate played a similar role in ipRGC stratification, the loss of photoreceptors in CBA/J mice might impede this process. We find, however, that despite the massive degeneration of the outer retina, the dendrites of ipRGCs can still stratify as well as the controls, suggesting that photoreceptors are not required for this process. These data are consistent with an intrinsic mechanism being responsible for defining stratification. Additional possibilities would include signals from other cell types, such as amacrine and/or bipolar cells, as well as those from ipRGC targets.
RGCs also undergo changes in number. At birth there is an overproduction of RGCs that is followed by cell death as the animal matures (Young 1984). Studies have shown that melanopsin-containing RGCs also undergo a similar reduction in number during early postnatal stages (Sekaran et al, 2005). By P14, a time that coincides with the maturation of photosensitivity in rods and cones (Tian and Copenhagen 03), the number of these cells is similar to that seen in the adult (Sekaran et al, 2005). We find that the number of ipRGCs is the same in both CBA/J and CBA/N mice at P4. However, the cell number does not change during the development of CBA/J mice. While the controls exhibit the expected decrease in number with increasing age, the number of melanopsin-containing RGCs seen in CBA/J mice at P21 is the same as it is as P4, suggesting that these cells do not experience the pruning seen in normal mice. While the mechanisms of control of ipRGC developmental cell death are unclear, in vitro studies suggest RGCs require a number of survival factors during development (Mey and Thanos 1993; Meyer-Franke et al, 1995). Photoreceptor regulation might make sense if the rods and cones were responsible for regulating the availability of essential factors. This is an interesting finding, given that a greater number of ipRGCs would suggest that the circadian system is more sensitive to light. However, CBA/J mice have attenuated phase-shifting. Future work might look at changes in central processing to more closely look at the differences in behavior.
We have shown that the distribution of ipRGCs was not altered by loss of photoreceptors. We see that throughout development, the relative distribution of ipRGCs remains constant. This suggests that the loss of photoreceptors does not result in clustering or in any significant change in cell distribution. We also observe this in the CBA/N mice, suggesting that although cell number changes in development, the relative distribution of cells is the same.
Studies have shown that input from all three photoreceptor cells contribute to the PLR in mice. The rods and cones contribute to the PLR at both low and high light intensities (Lucas et al, 2003), while loss of rods and cones results in an attenuation of the PLR at low, but not high, light intensities (Lucas et al, 2001). These results suggest a role for melanopsin-containing RGCs at higher light intensities. Because the PLR is mediated by retinal input to the olivary pretectal nucleus, it can be used to test the function of the melanopsin pathway independent of the SCN. As expected, CBA/J mice show attenuated PLR’s at low intensities, because they lack rods and cones. At high light intensities, the PLR is the same as that in CBA/N, suggesting that the melanopsin pathway is intact, at least at the level of the retina. As a result, the attenuation in behavior seen in CBA/J mice does not appear to be due to functional changes in the retina.
Further research on central processing may provide more information on how development of photoreceptive pathways within the retina influences circadian function. Changes in the degree of retinal innervation of the SCN might account for differences seen in the behaviors of the CBA/J mice. In addition, altered neural networks of the SCN might also play a role. Together these provide areas for future research in understanding what dictates the proper functioning of the circadian system.
Acknowledgements
This work was supported by the NIMH grant R01MH067094 to RLB, R01MH70922 to CNA, and an OHSU Medical Research Foundation Grant to DWR. We would like to thank Mike Lasarev for assistance with statistical analysis, Dr. Robert Kayton for advice on histological techniques and Benjamin Cottam for help with wheel-running data collection.
Abbreviations
- DD
constant darkness
- GCL
ganglion cell layer
- INL
inner nuclear layer
- IPL
inner plexiform layer
- ipRGCs
intrinsically photosensitive retinal ganglion cells
- LD
light/dark
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- SCN
suprachiasmatic nucleus
References
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J.Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Gunhan E. Development of On and Off retinal pathways and retinogeniculate projections. Prog.Retin.Eye Res. 2004;23:31–51. doi: 10.1016/j.preteyeres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J.Comp Physiol [A] 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat.Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS.ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat.Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lupi D, Cooper HM, Froehlich A, Standford L, McCall MA, Foster RG. Transgenic ablation of rod photoreceptors alters the circadian phenotype of mice. Neuroscience. 1999;89:363–374. doi: 10.1016/s0306-4522(98)00353-4. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Ostergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol.Vis.Sci. 2007;48:3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Lane BR. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur.J.Neurosci. 2006;24:1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A Functional Analysis of Circadian Pacemakers in Nocturnal Rodents. J.Comp Physiol [A] 1976;106:291–331. [Google Scholar]
- Sakamoto K, Liu C, Tosini G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J.Neurosci. 2004;24:9693–9697. doi: 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr.Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo M, Lupi D, Peirson SN, Butler JN, Foster RG. Light-induced c-fos in melanopsin retinal ganglion cells of young and aged rodless/coneless (rd/rd cl) mice. Eur.J.Neurosci. 2003;18:3007–3017. doi: 10.1111/j.1460-9568.2003.03061.x. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Tosini G, Aguzzi J, Bullock NM, Liu C, Kasamatsu M. Effect of photoreceptor degeneration on circadian photoreception and free-running period in the Royal College of Surgeons rat. Brain Res. 2007;1148:76–82. doi: 10.1016/j.brainres.2007.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Walker MT, Brown RL, Cronin TW, Robinson PR. Photochemistry of retinal chromophore in mouse melanopsin. Proc.Natl.Acad.Sci.U.S.A. 2008;105:8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur.J.Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J.Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Nishio M, Goto M, Ebihara S. Differences in circadian photosensitivity between retinally degenerate CBA/J mice (rd/rd) and normal CBA/N mice (+/+) J.Biol.Rhythms. 1994b;9:51–60. doi: 10.1177/074873049400900105. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Nishio M, Goto M, Ebihara S. Differences in circadian photosensitivity between retinally degenerate CBA/J mice (rd/rd) and normal CBA/N mice (+/+) J.Biol.Rhythms. 1994a;9:51–60. doi: 10.1177/074873049400900105. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell death during differentiation of the retina in the mouse. J.Comp Neurol. 1984;229:362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]