Abstract

A two-step procedure for preparing 2-alkyl-1,3-butadienes is described. Cuprate addition to commercially available 1,4-dibromo-2-butene yields 3-alkyl-4-bromo-1-butene, a product of SN2′ substitution. Dehydrohalogenation gives 2-alkyl-1,3-butadienes.
One of the fundamental building blocks of organic chemistry is the 1,3-diene.1 Butadienes substituted at C-2 are a pivotal component of our new method for constructing optically active 3-alkyl-4-silyl-1-butenes, but few 2-alkyl-1,3-butadienes are commercially available.2 A broad set of methods have been described for preparation of substituted 1,3-dienes;3,4 however, a review of these procedures with a goal of generating simple 2-substituted derivatives in multigram quantities found them to be neither simple nor amenable to scaleup, and this led us to consider alternative procedures that would allow routine synthesis of these compounds. We report here our investigation of a two-step conversion of 1,4-dibromo-2-butene 1, Scheme 1.
Scheme 1. Two-Step Diene Synthesis.

Bromination of 1,3-butadiene and distillation of the product yields the crystalline trans-1,4-dibromo-3-butene 1,5 a product that is also commercially available. This bis-allylic bromide 1 was reported to react with alkyl Grignard reagents, forming almost exclusively SN2′ product 3.6 This reaction was also reported for benzyl Grignard in the presence of catalytic copper(I) iodide.7,8 We noted that dehydrohalogenation of 3 would then yield a direct route to 2-substituted 1,3-dienes 4.
Treatment of a THF solution of dibromide 1 with commercial benzylmagnesium bromide in the presence of CuI was found to yield a mixture of both SN2 and SN2′ products. In contrast, selectivity for SN2′ was was high in diethyl ether, which was used in all subsequent reactions, Table 1. Previous studies utilized ether6 or mixtures of ether and THF.7 For primary and secondary alkyl Grignard reagents, 3–5% CuI was often found to be adequate, but with the benzyl Grignard reagent 20% CuI was required to achieve a good yield, and the isopropyl Grignard reagent required 50% CuI for the preparative procedure.
Table 1. Cuprate Addition to 1,4-Dibromo-2-butene 1, Dehydrohalogenation of 3, and Isolation of 4 as Diels–Alder Adduct 5a.
 | |||
|---|---|---|---|
| R= | 3 (%) | 4 (%) | 5 (%) |
| R =PhCH2 | 3a (70)c | 5a (62) | |
| n-C6H13 | 3b (74) | 5b (51) | |
| i-Bu | 3c (75) | 4c (52) | 5c (67) |
| i-Pr | 3d (79) | 4d (30–77)d | 5d (72) |
| c-C6H11 | 3e (72) | 5e (71) | |
Isolated yields.
reactions utilized 1.3 equiv of Grignard reagent in diethyl ether at 0 °C; 3–50% CuI relative to the amount of Grignard reagent used.
Product contaminated with dibenzyl; ratio determined by 1H NMR.
See Table 2 for details.
Dehydrohalogenation of 3 with DBU in dichloromethane at reflux gave complete consumption of the starting bromide and formation of 1,3-diene product in good yield.9 To ascertain the yield of the volatile 1,3-diene product 4, it was trapped as the N-phenylmaleimide (NPM) Diels–Alder adduct 5.10 In all cases, the yield of this two-step process was good (51–72%).
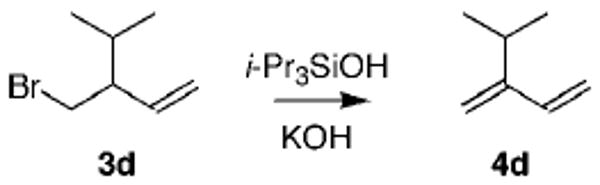
In addition to the DBU conditions, we also evaluated Soderquist's triisopropylsilanol-catalyzed KOH method with 2-isopropyl-1,3-butadiene as the product.11 In this case, the reactions were run on a larger scale (ca. 25 mmol) and the diene was distilled. To enhance purification of the product (bp = 85–87 °C4a,b), higher boiling solvents were used, and the results are shown in Table 2. Consistent with the findings of Soderquist, use of DMF as solvent gave a good isolated yield of the diene. We observed traces of a contaminant, however, tentatively identified as dimethylamine. In an attempt to avoid this byproduct, other solvents were evaluated, but none were as effective as DMF. THF as solvent gave the desired product 4d, but this could not be separated from the THF.
Table 2. Triisopropylsilanol-Catalyzed Dehydrohalogenation.
 | |
|---|---|
| solvent | distilled yield (%) |
| DMF | 77 |
| DMPU | 68 |
| xylene | 60 |
| diglyme | 50 |
| THF | a |
| DBU | 30 |
Product could not be separated from solvent.
Overall, this two-step process is easily conducted on a multigram scale. Beginning with the commercially available 1,4-dibromo-2-butene 1, alkyl Grignard reagents readily yield the SN2′ product 3. Dehydrohalogenation of the primary halide can be effectively conducted with DBU or Soderquist's reagent. When low molecular weight diene products require isolation, Soderquist's reagent is preferred. Where isolation of the diene is not required or the product is less volatile, the DBU method is simple and effective.
Experimental Section
3-Bromomethyl-4-methylpent-1-ene (3d)6,12
To a −10 °C mixture of CuI (3.31 g, 17.5 mmol) and 1,4-dibromo-2-butene (4.8 g, 23 mmol) in ether (35 mL) was added dropwise a freshly prepared solution of isopropylmagnesium bromide in ether (2.5 M, 14 mL, 35 mmol). The reaction was followed by TLC (hexanes), and after consumption of 1 the mixture was diluted with saturated NH4Cl solution. The aqueous phase was extracted with ether (2 × 20 mL), and the combined organics were washed with brine (20 mL), dried over MgSO4, and concentrated. Kugelrohr distillation (100–120 °C/7 mmHg) gave 3d as a colorless oil (3.2 g, 79%). 1H NMR (400 MHz, CDCl3): δ 5.57 (m, 1H), 5.05 (m, 2H), 3.40 (m, 2H), 2.11 (m, 1H), 1.86(m, 1H) 0.83 (d, J =6.9 Hz, 3H), 0.76 (d, J =6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 138.9, 118.2, 52.2, 37.4, 29.9, 21.1, 18.9. IR (neat): 2960, 1670, 1486, 905, 790 cm −1.
3-(Bromomethyl)non-1-ene (3b)
The procedure for 3d using 1-hexylmagnesium bromide and 1.0 g of 1 gave 3b as a colorless oil (0.76 g, 74%). 1H NMR (300 MHz, CDCl3): δ 5.67 (m, 1H), 5.19 (d, 1H, J = 7.2 Hz), 5.16 (d, 1H, J = 13.5 Hz), 3.44 (m, 2H), 2.40 (m, 1H), 1.63 - 1.26 (m, 10H), 0.93 (t, 3H, J = 6 Hz). 13C NMR (75 MHz, CDCl3): δ 139.7, 116.6, 45.6, 38.9, 32.9, 31.7, 29.7, 26.8, 22.7, 14.1. IR (neat): 2850, 2960, 1620, 910, 980, 675 cm −1. Exact mass (MH+): calcd for C10H20Br 221.0728, found 221.0720.
3-(Bromomethyl)-5-methylhex-1-ene (3c)7,8
The procedure for 3d using isobutylmagnesium chloride gave 3c as a colorless oil (3.3 g, 75%). 1H NMR (300 MHz, CDCl3): δ 5.45 (m, 1H), 4.98 (m, 2H), 3.22 (m, 2H), 2.31 (m, 1H), 1.46 (m, 1H), 1.20 (m, 2H), 0.75 (d, 3H, J = 6.6 Hz), 0.71(d, 3H, J = 6.6 Hz). 13C NMR (75 MHz, CDCl3): δ 140.1, 117.0, 43.9, 42.6, 39.8, 25.6, 23.7, 22.1. IR (neat): 3095, 2970, 1630, 1480, 1345, 1360, 1176 cm−1.
(1-Bromobut-3-en-2-yl)cyclohexane (3e)8
A 0 °C mixture of cyclohexylmagnesium chloride (2 M, 15 mL, 30 mmole) and CuI (0.16 g, 0.9 mmole) in ether (10 mL) was stirred under argon for 2 h and then transferred via cannula to a −78 °C solution of 1,4-dibromo-2-butene 1 (5.0 g, 23 mmol) in ether (75 mL). The resulting dark mixture was allowed to warm to rt over a period of 6 h. Aqueous ammonium chloride was added, and the aqueous phase was extracted with ether (3 × 50 mL). The combined organic phases were washed with brine (2 × 30 mL), dried over magnesium sulfate, concentrated, and distilled under vacuum (60–75 °C/10 mmHg) to give 3e as a colorless liquid (3.6 g, 73%). 1H NMR (300 MHz, CDCl3): δ 5.70 (m, 1H), 5.20 (d, 1H, J = 10.5 Hz), 5.14 (d, 1H, J = 16.5 Hz), 3.51 (m, 2H), 2.22 (m, 1H), 1.81–1.73 (m, 5H), 1.29–1.21 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 138.2, 117.4, 65.9, 51.4, 39.6, 36.8, 30.8, 26.3. IR (neat): 2850, 2932, 1605, 1445, 1234, 1164, 970 cm−1.
2-Isobutyl-1,3-butadiene (4c)
To a solution of 3-(bromomethyl)-5-methylhex-1-ene (5.0 g, 26 mmol) in CH2Cl2 (90 mL) was added DBU (5.4 g, 39 mmol), and the mixture was heated to reflux for 8 h. The mixture was cooled and washed with 10% HCl (20 mL), and the aqueous phase was extracted with CH2Cl2 (3 × 20 mL). The combined organics were dried over sodium sulfate and filtered. The solvent was removed by Kugelrohr distillation to give the diene as a colorless oil (1.5 g, 52%). 1H NMR (300 MHz, CDCl3): δ 6.30 (dd, 1H, J = 10.8, 17.7 Hz), 5.21–4.86 (m, 4H), 2.01 (d, 2H, J = 7.2 Hz), 1.73 (m, 1H), 0.80 (d, 6H, J = 6.6 Hz). 13C NMR (75 MHz, CDCl3): δ 145.4, 139.1, 116.7, 113.3, 41.3, 26.7, 22.7. IR (neat): 3190, 2879, 1280 cm−1.
Dehydrohalogenation with TIPSOH: 2-Isopropyl-1,3-butadiene (4d)4b,c,e
To a solution of KOH (3.14 g, 48 mmol) in DMF (70 mL) was added triisopropylsilanol (39 mg, 0.2 mmol). After the mixture was stirred at rt for 1 h, 3-bromomethyl-4-methylpent-1-ene (5 g, 28 mmol) was added dropwise. After being stirred for an additional 12 h, the reaction was judged complete by TLC and the product was distilled directly from the reaction mixture using a Kugelrohr apparatus at 70 °C to give 3d as a colorless oil (2.08 g, 77%). 1H NMR (300 MHz, CDCl3): δ 6.24 (dd, 1H, J = 10.8, 17.7 Hz), 5.15 (d, 1H, J = 17.4 Hz), 5.03 (m, 3H), 2.64 (septet, 1H, J = 6.9 Hz), 0.99 (d, 6H, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3): δ 153.4, 139.5, 112.9, 112.3, 29.5, 22.1. IR (neat): 3221, 3073, 2926, 2852, 1618, 1441, 1312 cm−1.
Dehydrohalogenation and Diels–Alder Reaction: 5-Cyclohexyl-3a,4,7,7a-tetrahydro-2-phenyl-2H-isoindole-1,3-dione (5e)
To a solution of 3e (1.0 g, 4.6 mmol) in CH2Cl2 (18 mL) was added DBU (1.1 g, 7.2 mmol) and the solution was heated to reflux for 8 h. The mixture was cooled, washed with 10% HCl (4 mL), dried over sodium sulfate, and filtered.
To this filtrate was added N-phenylmaleimide (1.6 g, 9.2 mmol), and the resulting solution was stirred at rt for 24 h. Saturated ammonium chloride was added, and the aqueous phase was extracted with CH2Cl2 (10 × 3 mL). The combined organic extracts were dried over sodium sulfate and concentrated. Purification by flash chromatography (1:4 ethyl acetate/hexanes) gave 5e as a colorless solid (1.01 g, 71%, for two steps). Rf = 0.25 (1:4 ethyl acetate/hexanes). 1H NMR (300 MHz, CDCl3): δ 7.39–7.11 (m, 5H), 5.50 (m, 1H), 3.16 (m, 2H), 2.62(m, 2H), 2.17 (m, 2H), 1.88 (m, 1H), 1.64–1.59 (m, 5H), 1.15–1.05 (m, 5H). 13C NMR (75 MHz, CDCl3) δ 179.6, 179.4, 146.0, 127.9, 129.2, 128.6, 126.4, 118.1, 55.2, 40.0, 39.8, 32.0, 27.7, 27.5, 26.8, 23.4. IR (neat): 2940, 1705, 1690, 1410, 1230, 760 cm−1. Exact mass (MH+): calcd for C20H24NO2 310.1802, found 310.1806.
5-Benzyl-3a,4,7,7a-tetrahydro-2-phenyl-2H-isoindole-1,3-dione (5a)4n
Following the procedure for 5e, 2-(bromomethyl)-3-buten-1-yl-benzene 3a (1.0 g, 4.4 mmol) gave 5a (0.86 g, 62%). Rf = 0.24 (1:4 ethyl acetate/hexanes). 1H NMR (300 MHz, CDCl3): δ 7.56–7.02 (m, 10H), 5.58 (m, 1H), 3.27 (s, 2H), 3.15 (m, 2H), 2.62–2.49 (m, 2H), 2.22 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 178.1, 177.7, 138.9, 130.9, 128.0, 127.9, 127.6, 127.4, 125.3, 120.5, 42.7, 38.8, 38.6, 26.6, 23.3. IR (neat): 3028, 2938, 2842, 1710, 1700, 1456, 1186, 1070 cm −1.
5-Hexyl-3a,4,7,7a-tetrahydro-2-phenyl-2H-isoindole-1,3-dione (5b)
Following the procedure for 5e, compound 3b (1.0 g, 4.6 mmol) gave 5e (0.72 g, 51%). Rf = 0.27 (1:4 ethyl acetate/hexanes). 1H NMR (300 MHz, CDCl3): δ 7.27–7.13 (m, 5H), 5.53 (m, 1H), 3.17, (m, 2H), 2.60 (m, 2H), 2.21 (m, 2H), 1.95 (t, 2H, J = 7.5 Hz), 1.31-1.16, (m, 8H), 0.78 (t, 3H, J = 6.3 Hz). 13C NMR (75 MHz, CDCl3): δ 178.4, 139.9, 131.0, 128.0, 127.4, 125.3, 118.6, 38.7, 38.4, 36.1, 30.5, 28.1, 26.7, 26.3, 23.3, 21.5, 13.0. IR (neat): 2940, 1700, 1410, 1230, 760 cm −1. Exact mass (MH+): calcd for C20H26NO2 312.1964, found 312.1974.
3a,4,7,7a-Tetrahydro-5-isobutyl-2-phenyl-2H-isoindole-1,3-dione (5c)
Following the procedure for 5e, compound 3c (1.0 g, 5.2 mmol) gave 5c (0.98 g, 67%). Rf = 0.23 (1:4 ethyl acetate/hexanes). 1H NMR (300 MHz, CDCl3): δ 7.14–7.40 (m, 5H), 5.54 (m, 1H), 3.17 (m, 2H), 2.51–2.56 (m, 2H), 2.23 (m, 2H), 1.82 (d, 2H, J = 6.9 Hz), 1.63 (m, 1H), 0.75 (d, 3H, J = 6.3 Hz), 0.72 (d, 3H, J = 6.6 Hz). 13C NMR (75 MHz, CDCl3): δ 179.2, 139.8, 129.1, 128.5, 126.38, 126.3, 121.2, 47.0, 39.8, 39.4, 27.7, 25.7, 24.4, 22.5. IR (neat): 2945, 1710, 1690, 1405, 1230 cm−1. Exact mass (MH+): calcd for C18H22NO2 284.1645, found 284.1650.
3a,4,7,7a-Tetrahydro-5-isopropyl-2-phenyl-2H-isoindole-1,3-dione (5d)4n
Following the procedure for 5e, compound 3d (1.0 g, 4.4 mmol) gave 5d (1.1 g, 72%). Rf = 0.25 (1:4 ethyl acetate/hexanes). 1H NMR (300 MHz, CDCl3): δ 7.38–7.12 (m, 5H), 5.56 (m, 1H), 3.19 (m, 2H), 2.64 (m, 2H), 2.20 (m, 3H), 0.90 (d, 6H, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3): δ 179.5, 179.2, 146.6, 132.1, 129.1, 128.6, 126.4, 117.1, 40.0, 39.8, 34.6, 26.6, 24.3, 20.9, 20.8. IR (neat): 1710, 1685, 1495, 1350 cm−1. Exact mass (MH+): calcd for C17H20NO2 270.1496, found 270.1479.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIH (5R01GM076471).
Footnotes
Supporting Information Available: Proton and carbon NMR spectra of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Fringuelli F, Taticchi A. Dienes in the Diels–Alder Reaction. Wiley-Interscience; New York: 1990. [Google Scholar]; (b) Mehta G, Rao HSP. In: The Chemistry of Dienes and Polyenes. Rappoport Z, editor. Vol. 1. John Wiley & Sons; New York: 1997. pp. 359–480. [Google Scholar]
- 2.Sen S, Purushotham M, Qi Y, Sieburth S McN. Org Lett. 2007;9:4963–4965. doi: 10.1021/ol7021559. [DOI] [PubMed] [Google Scholar]
- 3.Review: Mehta G, Rao HSP. In: The Chemistry of Dienes and Polyenes. Rappoport Z, editor. Vol. 1. John Wiley & Sons; New York: 1997. pp. 359–480.
- 4.For examples, see: Braun Jv, Keller W. Chem Ber. 1931;64B:2617–2621.Marvel CS, Myers RL, Saunders JH. J Am Chem Soc. 1948;70:1694–1699. doi: 10.1021/ja01185a006.Overberger CG, Fischman A, Roberts CW, Arond LH, Lal J Am Chem Soc. 1951;73:2540–2543.Aufdermarsh CA. J Org Chem. 1964;29:1994–1996.Kaempf B, Kieffer R. Bull Soc Chim Fr. 1967:3062–3063.Nunomoto S, Yamashita Y. J Org Chem. 1979;44:4788–4791.Brown PA, Jenkin PR. Tetrahedron Lett. 1982;23:3733–3734.Sahlberg C, Quader A, Claesson A. Tetrahedron Lett. 1983;24:5137–5138.Djahanbini D, Cazes B, Gore J. Tetrahedron. 1984;40:3645–3655.Wada E, Kanemasa S, Fujiwara I, Tsuge O. Bull. 1985;58:1942–1945.Block E, Aslam M, Eswarakrishnan V, Gebreyes K, Hutchinson J, Iyer R, Laffitte JA, Wall A. J Am Chem Soc. 1986;108:4568–4580.Arenz T, Vostell M, Frauenrath H. Synlett. 1991:23–24.Brown PA, Bonnert RV, Jenkins PR, Lawrence NJ, Selim MR. J Chem Soc Perkin Trans 1. 1991:1893–1900.Katritzky AR, Serdyuk L, Toader D, Wang X. J Org Chem. 1999;64:1888–1892. doi: 10.1021/jo9818881.Trost BM, Pinkerton AB, Seidel M. J Am Chem Soc. 2001;123:12466–12476. doi: 10.1021/ja011428g.Tonogaki K, Mori M. Tetrahedron Lett. 2002;43:2235–2238.Diver ST, Giessert AJ. Synthesis. 2003:466–471.
- 5.(a) Shantz EM. J Am. 1946;68:2553–2557. doi: 10.1021/ja01216a038. [DOI] [PubMed] [Google Scholar]; (b) Hatch LF, Gardner PD, Gilbert RE. J Am Chem Soc. 1959;81:5943–5946. [Google Scholar]
- 6.Mesnard D, Miginiac L. Compt Rend. 1973;277:567–560. [Google Scholar]
- 7.Jennings-White C, Almquist RG. Tetrahedron Lett. 1982;23:2533–2534. [Google Scholar]
- 8.Recently copper thiophene carboxylate has been found to provide very high SN2′ chemoselectivity: Falciola CA, Tissot-Croset K, Alexakis A. Angew Chem Int Ed. 2006;45:5995–5998. doi: 10.1002/anie.200601855.Falciola CA, Alexakis A. Angew Chem Int Ed. 2007;46:2619–2622. doi: 10.1002/anie.200604963.
- 9.(a) Oediger H, Möller F, Eiter K. Synthesis. 1972;59:1–598. [Google Scholar]; (b) Baidya M, Mayr H. Chem Commun. 2008:1792–1794. doi: 10.1039/b801811a. [DOI] [PubMed] [Google Scholar]
- 10.(a) Micalizio GC, Schreiber SL. Angew Chem Int Ed. 2002;41:152–154. doi: 10.1002/1521-3773(20020104)41:1<152::aid-anie152>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; (b) Caballero E, Alonso D, Pela′ez R, Álvarez CAA, Puebla P, Sanz F, Medarde M, Tome′ F. Tetrahedron. 2005;61:6871–6878. [Google Scholar]
- 11.Soderquist JA, Vaquer J, Diaz MJ, Rane AM, Bordwell FG, Zhang S. Tetrahedron Lett. 1996;37:2561–2564. [Google Scholar]
- 12.DeGraw JI, Almquist RG, Hiebert CK, Colwell WT, Crase J, Hayano T, Judd AK, Dousman L, Smith RL, Waud WR, Uchida I. J Med Chem. 1997;40:2386–2397. doi: 10.1021/jm950803a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


