Abstract
The in vitro activity of ACHN-490, a novel aminoglycoside (“neoglycoside”), was evaluated against 102 multidrug-resistant (MDR) Klebsiella pneumoniae strains, including a subset of 25 strains producing the KPC carbapenemase. MIC50 values for gentamicin, tobramycin, and amikacin were 8 μg/ml, 32 μg/ml, and 2 μg/ml, respectively; MIC90 values for the same antimicrobials were ≥64 μg/ml, ≥64 μg/ml, and 32 μg/ml, respectively. ACHN-490 showed an MIC50 of 0.5 μg/ml and an MIC90 of 1 μg/ml, which are significantly lower than those of comparator aminoglycosides. ACHN-490 represents a promising aminoglycoside for the treatment of MDR K. pneumoniae isolates, including those producing KPC β-lactamase.
The spread of Klebsiella pneumoniae isolates producing extended-spectrum β-lactamases (ESBLs) represents a serious threat to our therapeutic armamentarium (21). These isolates are also frequently resistant to other classes of antibiotics, such as β-lactam/β-lactamase inhibitor combinations, quinolones, and aminoglycosides (8, 9), thereby limiting our choice to carbapenems for the treatment of serious infections (21).
Unfortunately, there is growing concern regarding the emergence of carbapenem-resistant K. pneumoniae isolates (20). In particular, K. pneumoniae isolates producing KPC carbapenemases (KPC-Kp) are spreading at an alarming rate in North and South America, the Caribbean, Europe, Israel, and Asia (6, 7, 15, 17, 18). Like ESBL producers, KPC-Kp are often resistant to quinolones and aminoglycosides (6). Therefore, our therapeutic options against KPC-Kp are limited to tigecycline and colistin. However, tigecycline may not reach desired serum levels to treat bloodstream infections (19), leaving colistin as the “last choice” against infections caused by KPC-Kp (13). Unfortunately, colistin-resistant KPC-Kp isolates are also reported in the United States (1, 12). As a result of this therapeutic dilemma, new antimicrobial agents with potent activity against multidrug-resistant (MDR) K. pneumoniae need to be developed.
Recently, there has been an increased interest in developing novel aminoglycosides. This new attention is due to (i) the potent bactericidal activity of aminoglycosides against a wide spectrum of aerobic gram-positive and gram-negative pathogens, (ii) the more gradual decline in susceptibility to aminoglycosides among gram-negative bacteria than that in susceptibility to other antimicrobials, and (iii) the ability of novel aminoglycosides to bypass common mechanisms of resistance that have gradually decreased the susceptibility to clinically used aminoglycosides (e.g., gentamicin, tobramycin, and amikacin) (11, 14, 16).
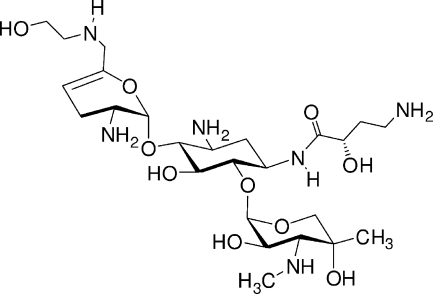
ACHN-490 (Achaogen, San Francisco, CA) is a “neoglycoside,” a next-generation aminoglycoside, currently in early clinical development (FDA, http://clinicaltrials.gov/), which has never been reported previously in the literature. The chemical structure of ACHN-490 is presented in Fig. 1.
FIG. 1.
Chemical structure of ACHN-490 [6′-(hydroxylethyl)-1-(haba)-sisomicin].
In the present work, we analyzed the in vitro activity of ACHN-490 against a collection of 102 K. pneumoniae clinical isolates collected from January 2006 to October 2007 at the University of Pittsburgh Medical Center, and three Cleveland institutions, including University Hospitals Case Medical Center, the Cleveland Clinic, and the Louis Stokes Department of Veterans Affairs Medical Center.
The 102 K. pneumoniae isolates were selected based on an MDR phenotype (i.e., resistance to ≥3 antibiotic classes). Twenty-five isolates were KPC-Kp and were part of a previous study in which the β-lactamase background and clonality were characterized (6). The remaining 77 MDR K. pneumoniae isolates were ESBL producers, according to the phenotypic results (see below).
MICs were determined by a microdilution method using cation-adjusted Mueller-Hinton broth, according to the Clinical and Laboratory Standards Institute (CLSI) criteria (2). Specific panels containing the following antibiotics were customized by Trek Diagnostics (Cleveland, OH): cefotaxime, cefotaxime-clavulanate (constant concentration of 4 mg/liter), ceftazidime, ceftazidime-clavulanate (constant concentration of 4 mg/liter), piperacillin-tazobactam, imipenem, ciprofloxacin, tigecycline, gentamicin, tobramycin, amikacin, arbekacin, neomycin, and ACHN-490. The following ATCC control strains were used: Escherichia coli 25922, Pseudomonas aeruginosa 27853, and K. pneumoniae 700603. Susceptibility results were interpreted according to the guidelines recommended by CLSI (3). Tigecycline MICs were interpreted according to the U.S. FDA criteria (i.e., susceptible at an MIC of ≤2 μg/ml). According to the CLSI criteria, isolates were defined as ESBL producers when they showed a ≥3 twofold concentration decrease in MICs for ceftazidime or cefotaxime when tested in combination with clavulanate versus their MICs when tested alone (3).
The 25 KPC-Kp isolates were analyzed by PCR for the presence of 16S rRNA methylase genes (i.e., armA, rmtA, rmtB, rmtC, rmtD, and npmA), using primers and conditions previously reported (4, 23). In addition, these strains were examined by PCR and sequencing for the presence of the most common aminoglycoside-modifying enzymes (AMEs) in gram-negative pathogens (22). In particular, the following genes were analyzed: aac(6′)-Ib, aac(6′)-Ic, aac(6′)-Id, ant(3")-Ia, ant(2")-Ia, aac(3)-Ia, aac(3)-Ib, aac(3)-IIc, aph(3′)-VIa, and aph(3′)-VIb, using primers previously reported (5, 10).
As shown in Table 1, MDR K. pneumoniae isolates were highly resistant to ceftazidime and piperacillin-tazobactam (each MIC90, >32 μg/ml). Two-thirds of the isolates were resistant to ciprofloxacin, whereas approximately 75% and 90% of strains were still susceptible to imipenem and tigecycline, respectively. Almost all KPC-Kp isolates were resistant to β-lactams and quinolones, whereas tigecycline frequently remained active in vitro (Table 1). All of these 25 isolates were colistin susceptible, as previously reported (6).
TABLE 1.
Susceptibility results of MDR K. pneumoniae isolates, including those producing KPC enzymes
| Antibiotic | All MDR K. pneumoniae isolates (n = 102)
|
KPC-producing K. pneumoniae isolates (n = 25)
|
||||
|---|---|---|---|---|---|---|
| MIC50 (μg/ml) | MIC90 (μg/ml) | S (%)a | MIC50 (μg/ml) | MIC90 (μg/ml) | S (%)a | |
| Ceftazidime | >32 | >32 | 9.8 | >32 | >32 | 0.0 |
| Imipenem | 0.5 | 8 | 75.5 | 8 | >16 | 12.0 |
| Piperacillin-tazobactam | >64 | >64 | 38.2 | >64 | >64 | 0.0 |
| Ciprofloxacin | 4 | 16 | 26.5 | >8 | >8 | 8.0 |
| Tigecyclineb,c | 1 | 2 | 90.2 | 1 | 2 | 96.0 |
| Amikacin | 2 | 32 | 78.4 | 32 | 32 | 48.0 |
| Gentamicin | 8 | ≥64 | 25.5 | 8 | 16 | 44.0 |
| Tobramycin | 32 | ≥64 | 10.8 | 32 | ≥64 | 8.0 |
| Arbekacinc | 4 | 16 | 8 | 16 | ||
| Neomycinc | 2 | 32 | 2 | 32 | ||
| ACHN-490c,d | 0.5 | 1 | 0.5 | 1 | ||
S, susceptibility according to CLSI criteria (3): ceftazidime (MIC, ≤8 μg/ml); imipenem (MIC, ≤4 μg/ml); piperacillin-tazobactam (MIC, ≤16 μg/ml); ciprofloxacin (MIC, ≤1 μg/ml); amikacin (MIC, ≤16 μg/ml); gentamicin (MIC, ≤4 μg/ml); tobramycin (MIC, ≤4 μg/ml).
Tigecycline was interpreted according to U.S. FDA criteria (susceptibility, MIC ≤ 2 μg/ml).
CLSI criteria not available.
E. coli ATCC 25922 (MICs, 0.5 to 1 μg/ml); P. aeruginosa ATCC 27853 (MIC, 4 μg/ml); K. pneumoniae ATCC 700603 (MICs, 0.25 to 0.5 μg/ml).
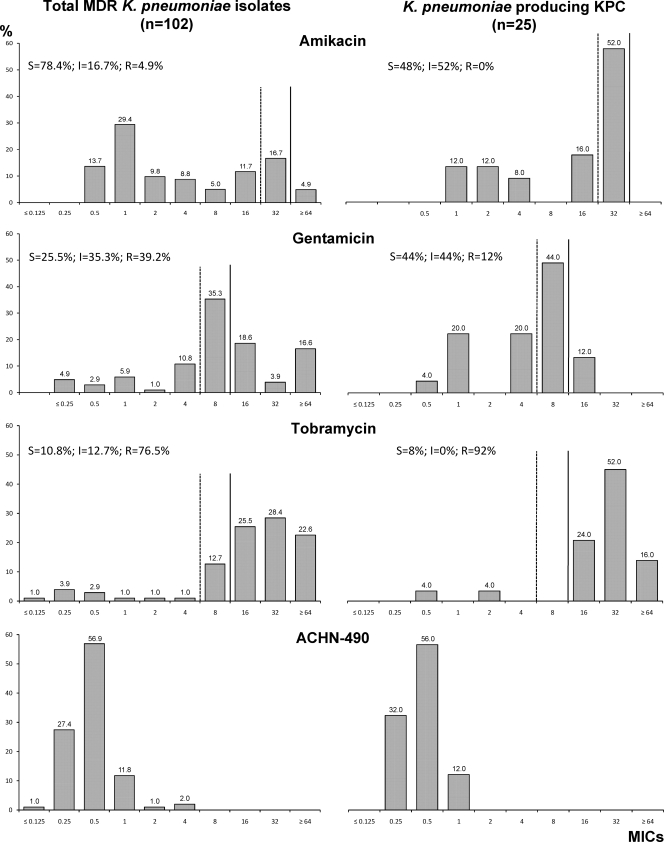
Figure 2 shows our analysis of aminoglycoside susceptibility. MDR K. pneumoniae isolates were highly resistant to gentamicin and tobramycin (less than 26% of strains were susceptible). In contrast, amikacin still maintained in vitro activity (78% of isolates were susceptible) with only five isolates being fully resistant (i.e., MICs of 64 μg/ml). The subgroup of KPC-Kp showed lower susceptibility rates for amikacin and tobramycin (48% and 8%, respectively) than did the entire group of MDR strains (Fig. 2). Notably, gentamicin was more active in vitro against KPC-Kp (44% of strains susceptible) than against the overall MDR isolate group.
FIG. 2.
MIC distributions of amikacin, gentamicin, tobramycin, and ACHN-490 against the overall collection of MDR K. pneumoniae isolates (n = 102) and the subgroup of KPC-producing strains (n = 25). S, susceptible; I, intermediate; R, resistant. Results were interpreted according to CLSI criteria (3). Dashed vertical line, susceptibility cutoff; solid vertical line, resistance cutoff.
For both MDR and KPC-Kp strains, ACHN-490 showed MIC50 and MIC90 values (i.e., 0.5 and 1 μg/ml, respectively) that were significantly lower than those for comparator aminoglycosides. The ACHN-490 MICs for all strains were ≤4 μg/ml. In particular, the MIC90 of ACHN-490 was at least 5 twofold dilutions lower than that of amikacin, which is currently the aminoglycoside with the least resistance in our armamentarium (Fig. 2).
To better understand the impact of these susceptibility data, we investigated the genetic background of KPC-Kp isolates in terms of their AMEs and methylases. All KPC-Kp strains were positive for aac(6′)-Ib and ant(3")-Ia (alternative name of aadA1) AME genes. Since neither of these AMEs modifies gentamicin, this explains the lower level of gentamicin resistance observed in the KPC-Kp strains. In contrast, the AAC(3)-II enzyme is common among Enterobacteriaceae and may be generating gentamicin resistance among the non-KPC-positive isolates (16). Two KPC-Kp strains (i.e., VA362 and VA373) were also positive for the ant(2")-Ia gene. Consistent with our MIC results (i.e., all strains with arbekacin MICs of <32 μg/ml) and the low prevalence in the clinical population, we did not find any methylase genes. An E. coli control strain in which the rmtA methylase gene was cloned had an MIC of >8 μg/ml for ACHN-490.
In conclusion, ACHN-490 possesses potent in vitro activity against MDR K. pneumoniae isolates, including those producing KPC carbapenemase. ACHN-490 represents a promising alternative to tigecycline and colistin for the treatment of isolates resistant to quinolones, β-lactam/β-lactamase inhibitor combinations, carbapenems, and existing aminoglycosides.
Acknowledgments
This research was supported by the U.S. Army Medical Research Acquisition Activity (grant W81XWH-08-0010 given to Achaogen, Inc.). R.A.B. is supported by the National Institutes of Health (grant RO1-AI063517), the Veterans Affairs Merit Review Program, and the Geriatric Research Education and Clinical Center VISN 10.
The content of this article does not necessarily reflect the position or the policy of the U.S. Government, and no official endorsement should be inferred.
We thank Louis B. Rice, David L. Paterson, Michael R. Jacobs, and Gerri S. Hall for providing K. pneumoniae isolates. We also express gratitude to Yohei Doi, University of Pittsburgh Medical Center, for the kind gift of control strains containing methylases.
Footnotes
Published ahead of print on 21 September 2009.
REFERENCES
- 1.Bratu, S., P. Tolaney, U. Karumudi, J. Quale, M. Mooty, S. Nichani, and D. Landman. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128-132. [DOI] [PubMed] [Google Scholar]
- 2.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th edition. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.CLSI. 2008. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Doi, Y., and Y. Arakawa. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88-94. [DOI] [PubMed] [Google Scholar]
- 5.Endimiani, A., L. L. Carias, A. M. Hujer, C. R. Bethel, K. M. Hujer, F. Perez, R. A. Hutton, W. R. Fox, G. S. Hall, M. R. Jacobs, D. L. Paterson, L. B. Rice, S. G. Jenkins, F. C. Tenover, and R. A. Bonomo. 2008. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicrob. Agents Chemother. 52:2680-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endimiani, A., A. M. Hujer, F. Perez, C. R. Bethel, K. M. Hujer, J. Kroeger, M. Oethinger, D. L. Paterson, M. D. Adams, M. R. Jacobs, D. J. Diekema, G. S. Hall, S. G. Jenkins, L. B. Rice, F. C. Tenover, and R. A. Bonomo. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J. Antimicrob. Chemother. 63:427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldfarb, D., S. B. Harvey, K. Jessamine, P. Jessamine, B. Toye, and M. Desjardins. 2009. Detection of plasmid-mediated KPC-producing Klebsiella pneumoniae in Ottawa, Canada: evidence of intrahospital transmission. J. Clin. Microbiol. 47:1920-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goossens, H., and B. Grabein. 2005. Prevalence and antimicrobial susceptibility data for extended-spectrum β-lactamase- and AmpC-producing Enterobacteriaceae from the MYSTIC Program in Europe and the United States (1997-2004). Diagn. Microbiol. Infect. Dis. 53:257-264. [DOI] [PubMed] [Google Scholar]
- 9.Hirakata, Y., J. Matsuda, Y. Miyazaki, S. Kamihira, S. Kawakami, Y. Miyazawa, Y. Ono, N. Nakazaki, Y. Hirata, M. Inoue, J. D. Turnidge, J. M. Bell, R. N. Jones, and S. Kohno. 2005. Regional variation in the prevalence of extended-spectrum β-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998-2002). Diagn. Microbiol. Infect. Dis. 52:323-329. [DOI] [PubMed] [Google Scholar]
- 10.Hujer, K. M., A. M. Hujer, E. A. Hulten, S. Bajaksouzian, J. M. Adams, C. J. Donskey, D. J. Ecker, C. Massire, M. W. Eshoo, R. Sampath, J. M. Thomson, P. N. Rather, D. W. Craft, J. T. Fishbain, A. J. Ewell, M. R. Jacobs, D. L. Paterson, and R. A. Bonomo. 2006. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 50:4114-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jana, S., and J. K. Deb. 2006. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 70:140-150. [DOI] [PubMed] [Google Scholar]
- 12.Lee, J., G. Patel, S. Huprikar, D. P. Calfee, and S. G. Jenkins. 2009. Decreased susceptibility of polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J. Clin. Microbiol. 47:1611-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, J., R. L. Nation, J. D. Turnidge, R. W. Milne, K. Coulthard, C. R. Rayner, and D. L. Paterson. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 6:589-601. [DOI] [PubMed] [Google Scholar]
- 14.Magnet, S., and J. S. Blanchard. 2005. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 105:477-498. [DOI] [PubMed] [Google Scholar]
- 15.Maltezou, H. C., P. Giakkoupi, A. Maragos, M. Bolikas, V. Raftopoulos, H. Papahatzaki, G. Vrouhos, V. Liakou, and A. C. Vatopoulos. 2009. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J. Infect. 58:213-219. [DOI] [PubMed] [Google Scholar]
- 16.Miller, G. H., F. J. Sabatelli, R. S. Hare, Y. Glupczynski, P. Mackey, D. Shlaes, K. Shimizu, K. J. Shaw, et al. 1997. The most frequent aminoglycoside resistance mechanisms-changes with time and geographic area: a reflection of aminoglycoside usage patterns? Clin. Infect. Dis. 24(Suppl. 1):S46-S62. [DOI] [PubMed] [Google Scholar]
- 17.Nordmann, P., G. Cuzon, and T. Naas. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228-236. [DOI] [PubMed] [Google Scholar]
- 18.Pavez, M., E. M. Mamizuka, and N. Lincopan. 2009. Early dissemination of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob. Agents Chemother. 53:2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson, L. R. 2008. A review of tigecycline—the first glycylcycline. Int. J. Antimicrob. Agents 32(Suppl. 4):S215-S222. [DOI] [PubMed] [Google Scholar]
- 20.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Baño, J., and A. Pascual. 2008. Clinical significance of extended-spectrum β-lactamases. Expert Rev. Anti Infect. Ther. 6:671-683. [DOI] [PubMed] [Google Scholar]
- 22.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wachino, J., K. Shibayama, H. Kurokawa, K. Kimura, K. Yamane, S. Suzuki, N. Shibata, Y. Ike, and Y. Arakawa. 2007. Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrob. Agents Chemother. 51:4401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]