Abstract
Production of a Klebsiella pneumoniae carbapenemase (KPC) is the most common mechanism of carbapenem resistance in the United States; however, until now, KPC-producing isolates have not been found in western Michigan. Molecular typing of two KPC-producing K. pneumoniae isolates from Michigan showed their similarity to other Midwestern isolates. They were also unrelated to the dominant sequence type observed throughout the United States, multilocus sequence type 258. This could represent regional dissemination of another KPC-producing K. pneumoniae strain.
Klebsiella pneumoniae carbapenemase (KPC) is an Ambler class A β-lactamase which confers resistance to all β-lactam agents, including carbapenems. KPCs occur most commonly in Klebsiella pneumoniae, although they have been identified in several other enteric bacteria, including species of Enterobacteriaceae and Pseudomonas aeruginosa (15, 16). In addition to their broad-spectrum activity, KPCs appear to be particularly mobile, making this resistance mechanism a significant public health concern. The blaKPC gene is usually located on a plasmid and within the Tn4401 transposon (11). Dissemination of this resistance mechanism by plasmid transfer between isolates and by transposition of Tn4401 has previously been described (4, 7, 13). Also, KPC-producing K. pneumoniae isolates of a common lineage, sequence type (ST) 258, have been identified in the United States, Europe, and Israel. This could represent dissemination of a resistant strain over a broad geographic area (9, 14).
Within the United States, KPC-producing bacteria are most commonly isolated in health care institutions located in the Northeast, specifically metropolitan New York City (2, 3, 10). KPC-producing bacteria have also been identified in 33 states, representing each major geographic region of the continental United States (9). Recently, KPC-producing K. pneumoniae isolates of the ST 258 lineage were identified in 10 states. Other STs were also detected in this study; however, the only evidence of another ST's dissemination was from two Midwestern isolates identified as ST 14.
We report the occurrence of two patients indentified with KPC-producing K. pneumoniae in western Michigan and the relationship of these isolates to other Midwestern isolates. Previously, only two cases of KPC-producing bacteria were identified in Michigan: a Klebsiella oxytoca and a Citrobacter freundii isolate, each identified in a central Michigan health care institution (13). The two KPC-producing K. pneumoniae isolates reported here were recovered from urine specimens of two patients staying in different extended care facilities. In both cases, the patient had no history of travel to the Northeast and no hospital stays in other regions of Michigan.
Patient A had a history of infective endocarditis with aortic valve replacement and type II diabetes and was admitted with a chief complaint of leg pain. The patient was diagnosed with a urinary tract infection but had not undergone any recent surgeries or urological procedures. A urine culture yielded a KPC-producing K. pneumoniae isolate resistant to imipenem (MIC, 16 μg/ml), meropenem (MIC, >32 μg/ml), and ertapenem (MIC, 32 μg/ml), as determined by Etest using CLSI interpretive standards (6). Initially, the patient was treated with nitrofurantoin and levofloxacin and later switched to fosfomycin, which resulted in a positive clinical outcome.
Patient B was debilitated from a closed head injury, was unable to communicate, and had a recent history of bacterial pneumonia due to Pseudomonas aeruginosa. The patient experienced fever and was also diagnosed with a urinary tract infection. A urine culture exhibited a KPC-positive K. pneumoniae isolate demonstrating an ellipse that intersected with the imipenem, meropenem, and ertapenem Etest strips at 3 μg/ml. Individual colonies occurred within the elliptical zones of inhibition for all three of these Etests, indicating resistance. Patient B's treatment included doxycycline, cefepime, fosfomycin, metronidazole, and meropenem, resulting in the patient's being discharged in stable condition. Two months later, patient B was readmitted to the same extended care facility and produced a urine culture exhibiting another KPC-positive K. pneumoniae isolate.
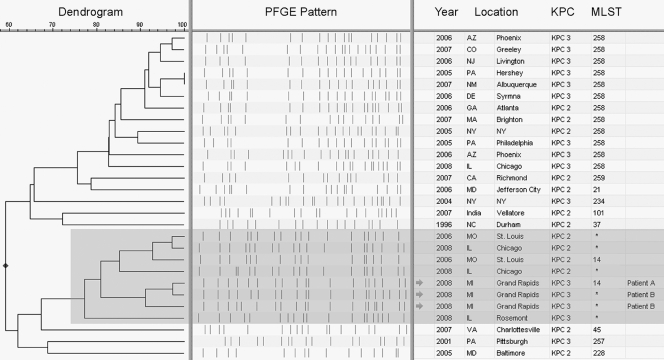
KPC-producing K. pneumoniae isolates from patient A (n = 1) and patient B (n = 2, taken 2 months apart) were sent to the Centers for Disease Control and Prevention (CDC) for further testing. Pulsed-field gel electrophoresis (PFGE) of XbaI-digested DNA was performed on the three isolates as previously described for Escherichia coli (http://www.cdc.gov/pulsenet/protocols.htm), and the results determined that the isolates from both patients were highly related, with PFGE patterns demonstrating >91% similarity. These patterns were compared to those in the CDC's PFGE database of KPC-producing K. pneumoniae isolates (n = 402) and clustered with other isolates from the Midwestern United States, demonstrating >80% similarity to isolates from Illinois (n = 3) and Missouri (n = 2). Multilocus sequence typing (MLST) was performed on the isolate from patient A, along with one of the related Missouri isolates (Fig. 1), in accordance with the protocol described on the K. pneumoniae MLST website (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html). Both isolates were identified as ST 14, which had previously been observed in an Italian blood culture isolate with high-level ceftazidime resistance (8). Since PFGE has a higher level of discriminatory power than MLST, it is likely that the other Midwestern isolates with related PFGE patterns are also ST 14 (1) (see highlighted PFGE patterns in Fig. 1).
FIG. 1.
Dendrogram based on similarity of PFGE patterns. Isolates shaded in gray (n = 8) make up a branch of the dendrogram representing related K. pneumoniae isolates that have disseminated in the Midwestern United States, thus far identified in Illinois, Missouri, and now Michigan. The other isolates (n = 20) were selected to represent the CDC's PFGE database of KPC-producing K. pneumoniae, consisting of over 400 isolates, primarily of ST 258. KPC subtyping was determined via bidirectional sequencing of the blaKPC gene by using previously described primers (13). MLST results marked with an asterisk indicate isolates that were not typed by MLST but demonstrated PFGE patterns closely related to those of known ST 14 isolates.
Previous reports suggest that KPC spread can be partly attributed to dissemination of KPC-producing isolates from patients previously hospitalized in areas where KPC-producing bacteria are common (12, 17). Based on the lack of travel for the two Michigan patients, no epidemiologic link could be associated with KPC-positive isolates from other locations. The KPC-producing K. pneumoniae isolates from these patients had MLST and PFGE results similar to those obtained for isolates from Missouri (2006) and Illinois (2008), which suggests that there may be regional dissemination of a KPC-producing strain.
Another concern is that KPC-producing K. pneumoniae appears to have persisted in patient B for at least a 2-month time period. Persistence of resistant bacteria in the host enhances the opportunity for transmission to other patients. Persistence also increases possible horizontal spread of plasmids containing blaKPC genes to other enteric bacteria constituting the host's normal flora. Fortunately, in these two cases, no transmission of these strains to other patients within the hospitals was detected. This was most likely the result of infection control measures (i.e., placing the patient in contact isolation) that were immediately instituted upon the report of the presumptive presence of a KPC-positive isolate as determined by the phenotypic tests done in the hospital's microbiology laboratory. Further recommended infection control measures can be found on the CDC's website (http://www.cdc.gov/ncidod/dhqp/guidelines.html) (5).
These findings highlight the possibility for regional dissemination of KPC-producing isolates. The spread of KPC-producing bacteria is a significant infection control problem, and prevention of dissemination will require a combination of accurate laboratory detection of resistance and good communication between microbiology laboratory and infection control officials.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Published ahead of print on 17 August 2009.
REFERENCES
- 1.Barrett, T. J., P. Gerner-Smidt, and B. Swaminathan. 2006. Interpretation of pulsed-field gel electrophoresis patterns in foodborne disease investigations and surveillance. Foodborne Pathog. Dis. 3:20-31. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, P. A., S. Bratu, C. Urban, M. Visalli, N. Mariano, D. Landman, J. J. Rahal, S. Brooks, S. Cebular, and J. Quale. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 39:55-60. [DOI] [PubMed] [Google Scholar]
- 3.Bratu, S., D. Landman, R. Haag, R. Recco, A. Eramo, M. Alam, and J. Quale. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City. Arch. Intern. Med. 165:1430-1435. [DOI] [PubMed] [Google Scholar]
- 4.Cai, J. C., H. W. Zhou, R. Zhang, and G. X. Chen. 2008. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob. Agents Chemother. 52:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2009. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb. Mortal. Wkly. Rep. 58:256-260. [PubMed] [Google Scholar]
- 6.CLSI. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Cuzon, G., T. Naas, and P. Nordmann. 2008. Functional characterization of Tn4401, a Tn3-based transposon involved in carbapenemase blaKPC gene mobilization, abstr. C1-117. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 8.Diancourt, L., V. Passet, J. Verhoef, P. A. Grimont, and S. Brisse. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitchel, B., J. K. Rasheed, J. B. Patel, A. Srinivasan, S. Navon-Venezia, Y. Carmeli, A. Brolund, and C. G. Giske. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 53:3365-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landman, D., S. Bratu, S. Kochar, M. Panwar, M. Trehan, M. Doymaz, and J. Quale. 2007. Evolution of antimicrobial resistance among Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae in Brooklyn, N.Y. J. Antimicrob. Chemother. 60:78-82. [DOI] [PubMed] [Google Scholar]
- 11.Naas, T., G. Cuzon, M. V. Villegas, M. F. Lartigue, J. P. Quinn, and P. Nordmann. 2008. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrob. Agents Chemother. 52:1257-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naas, T., P. Nordmann, G. Vedel, and C. Poyart. 2005. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob. Agents Chemother. 49:4423-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasheed, J. K., J. W. Biddle, K. F. Anderson, L. Washer, C. Chenoweth, J. Perrin, D. W. Newton, and J. B. Patel. 2008. Detection of the KPC-2 carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and Klebsiella oxytoca carrying a common plasmid. J. Clin. Microbiol. 46:2066-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuelsen, O., U. Naseer, S. Tofteland, D. H. Skutlaberg, A. Onken, R. Hjetland, A. Sundsfjord, and C. G. Giske. 2009. Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J. Antimicrob. Chemother. 63:654-658. [DOI] [PubMed] [Google Scholar]
- 15.Villegas, M. V., K. Lolans, A. Corrrea, J. N. Kattan, J. A. Lopez, J. P. Quinn, and the Colombian Nosocomial Resistance Study Group. 2007. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing β-lactamase. Antimicrob. Agents Chemother. 51:1553-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walther-Rasmussen, J., and N. Høiby. 2007. Class A carbapenemases. J. Antimicrob. Chemother. 60:470-482. [DOI] [PubMed] [Google Scholar]
- 17.Woodford, N., J. Zhang, M. Warner, M. E. Kaufmann, J. Matos, A. Macdonald, D. Brudney, D. Sompolinsky, S. Navon-Venezia, and D. M. Livermore. 2008. Arrival of Klebsiella pneumoniae producing KPC carbapenemase in the United Kingdom. J. Antimicrob. Chemother. 62:1261-1264. [DOI] [PubMed] [Google Scholar]