Invasive mucormycosis is a mycosis often lethal in immunocompromised patients with hematological malignancies and hematopoietic stem cell transplantation. Among the different genera causing mucormycosis, Cunninghamella is associated with the highest overall mortality (4).
Growth studies of filamentous fungi have shown that the composition of culture medium strongly affects growth rates and sporulation (3). It is unclear whether growth in different nutrient media affects the virulence of molds in vivo independently of the in vitro growth rate. To address this question, we used an established model of mucormycosis in Drosophila melanogaster (1) to compare the virulence of Cunninghamella bertholletiae isolates grown on nutrient-rich medium (yeast extract-agar-glucose [YAG]) with that of the same isolates cultured on nutrient-poor medium (RPMI 1640).
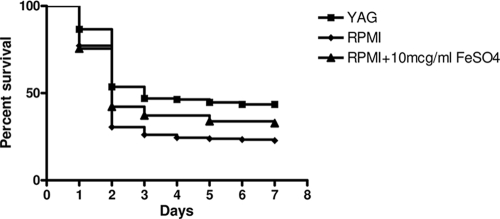
Three clinical C. bertholletiae isolates (no. 5, 12, and 17) were studied. Cunninghamella sporangiospores were collected from 2-day-old cultures and suspended in 0.85% normal saline. The suspensions were adjusted to a standardized inoculum of 5 × 107 sporangiospores/ml in 0.85% normal saline for injection into flies (at approximately 800 sporangiospores/fly) and to 104 sporangiospores/ml in yeast nitrogen base for growth rate studies. YAG plates, RPMI 1640 plates without supplemental iron, and RPMI 1640 plates supplemented with 10 μg/ml FeSO4·7H2O were used for the cultivation of sporangiospores. The growth rates of sporangiospores on poor and rich media at 29°C over 18 h were determined spectrophotometrically. Twenty flies per group were used, and each injection experiment was performed in triplicate on different days. Prism 4 software (GraphPad Software, Inc., La Jolla, CA) was used for the statistical analysis. Statistical significance was defined by a P value of <0.05. In the fly infection model, sporangiospores harvested from C. bertholletiae cultures grown on RPMI 1640 were more virulent than sporangiospores from the same isolates cultivated on YAG (P < 0.0001; median survival time, 3 versus 2 days) (Fig. 1). This effect was not dependent on differences in growth rates (data not shown). Since iron is important for growth and virulence in zygomycosis (5), we speculated that limited iron supply in RPMI 1640 might account for this difference. The addition of 10 μg/ml FeSO4 to the RPMI 1640 plates attenuated the lethality of Cunninghamella sporangiospores compared to that of sporangiospores from RPMI 1640 plates without iron, but the lethality was still greater than that of sporangiospores grown in YAG medium (P = 0.0117). This phenotype of accelerated lethality was reminiscent of results from previous studies that found that serial passage of Rhizopus oryzae on voriconazole-containing agar enhances the lethality of the isolate in fly and mouse models of mucormycosis (2).
FIG. 1.
Sporangiospores from RPMI 1640 and RPMI 1640 supplemented with iron were statistically significantly more lethal to flies than sporangiospores collected from YAG (P < 0.0001 and 0.0117, respectively). Data from three experiments per strain were pooled to obtain survival curves indicating the lethality of three Cunninghamella strains cultured in poor medium (RPMI 1640), rich medium (YAG), and RPMI 1640 supplemented with iron (RPMI + 10 mcg/ml FeSO4). Data are presented as a Kaplan-Meir plot developed using Prism 4 software (GraphPad Software, Inc., La Jolla, CA), and curves were compared by the log-rank test. Statistical significance was defined by a P value of <0.05.
In conclusion, we found that nutritional factors, such as iron availability in growth media, influence the virulence of some molds. Our preliminary data provide a conceptual framework to study the differences in growth media and possibly the contribution of a starvation phenotype to the pathogenesis of molds in experimental systems.
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Chamilos, G., R. E. Lewis, J. Hu, L. Xiao, T. Zal, M. Gilliet, G. Halder, and D. P. Kontoyiannis. 2008. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc. Natl. Acad. Sci. USA 105:9367-9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamaris, G. A., R. Ben-Ami, R. E. Lewis, G. Chamilos, G. Samonis, and D. P. Kontoyiannis. 2009. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J. Infect. Dis. 199:1399-1406. [DOI] [PubMed] [Google Scholar]
- 3.Meletiadis, J., J. F. Meis, J. W. Mouton, and P. E. Verweij. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 39:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg, B., J. Edwards, Jr., and A. Ibrahim. 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18:556-569. [DOI] [PMC free article] [PubMed] [Google Scholar]