Abstract
We report the isolation and identification of a new quassinoid named simalikalactone E (SkE), extracted from a widely used Amazonian antimalarial remedy made out of Quassia amara L. (Simaroubaceae) leaves. This new molecule inhibited the growth of Plasmodium falciparum cultured in vitro by 50%, in the concentration range from 24 to 68 nM, independently of the strain sensitivity to chloroquine. We also showed that this compound was able to decrease gametocytemia with a 50% inhibitory concentration sevenfold lower than that of primaquine. SkE was found to be less toxic than simalikalactone D (SkD), another antimalarial quassinoid from Q. amara, and its cytotoxicity on mammalian cells was dependent on the cell line, displaying a good selectivity index when tested on nontumorogenic cells. In vivo, SkE inhibited murine malaria growth of Plasmodium vinckei petteri by 50% at 1 and 0.5 mg/kg of body weight/day, by the oral or intraperitoneal routes, respectively. The contribution of quassinoids as a source of antimalarial molecules needs therefore to be reconsidered.
This study of the antiplasmodial properties of a new quassinoid from Quassia amara L. leaves results from our ongoing work on traditional antimalarial remedies in French Guiana. Through a “knowledge, attitudes, and practices” survey focused on malaria and its treatments in this French overseas department, we showed that Q. amara leaf tea was the most frequently used antimalarial remedy (35). Q. amara belongs to the Simaroubaceae, a family known to contain quassinoids, secondary metabolites characteristic of the Sapindale order, that display a wide range of biological activities, among them antiparasitic activity and cytotoxicity (11, 17).
Simalikalactone D (SkD) was identified as one of the compounds responsible for the activity of Quassia amara juvenile leaf tea (6), but the small amount present in the traditional preparation, made out of mature leaves (5), could not fully explain the activity seen in vitro and in vivo (4). This is why we looked for other active ingredients responsible for the antiplasmodial activity and isolated a new quassinoid, named simalikalactone E (SkE). We report here our detailed studies of the antimalarial and cytotoxic properties of this new compound.
MATERIALS AND METHODS
Plant material.
Leaves of Quassia amara were collected in Rémire-Montjoly, French Guiana. A sample specimen (GB3012) was deposited in the Cayenne Herbarium (CAY), and a specialist confirmed the botanical identification.
Chemistry.
Detailed protocol for the isolation of SkE from the aqueous decoction or the methanolic extract can be found in the supplemental material.
Structural data for SkE.
Structural data for simalikalactone E follow: APCIMS, 579 (MH+), 561 (MH+-H2O), 1H nuclear magnetic resonance (1H NMR) (CDCl3, 500 MHz), δ 6.19 (m, 1H, H-15), 6.17 (s, 1H, H-3), 5.19 (dd, J = 2.6 Hz, J = 11.7 Hz, 1H, H-6), 4.75 (d, J = 5.1 Hz, 1H, H-11), 4.70 (d, J = 2.6 Hz, 1H, H-7), 4.65 (d, J = 7.4 Hz, 1H, H-17a), 4.19 (s, 1H, H-1), 3.83 (s, 1H, H-12), 3.70 (d, J = 7.4 Hz, 1H, H-17b), 3.37 (d, J = 11.5 Hz, 1H, H-5), 2.51 (m, 1H, H-24), 2.48 (m, 1H, H-19), 2.45 (m, 1H, H-14), 2.43 (m, 1H, H-9), 2.08 (s, 3H, H-30), 1.79 (m, 2H, H-21a, H-26a), 1.61 (m, 1H, H-26b), 1.53 (m, 1H, H-21b), 1.45 (s, 3H, H-28), 1.35 (s, 3H, H-29), 1.21 (d, J = 7.0 Hz, 3H, H-20), 1.19 (d, J = 7.0 Hz, 3H, H-25), 1.01 (t, J = 7.4 Hz, 3H, H-22), 0.99 (t, J = 7.4 Hz, 3H, H-27). 13C NMR (CDCl3, 125 MHz), δ 196.5 (C-2), 176.2 (C-23), 175.2 (C-18), 166.5 (C-16), 163.0 (C-4), 126.5 (C-3), 82.8 (C-7), 81.8 (C-1), 80.0 (C-13), 79.8 (C-12), 74.2 (C-11), 70.9 (C-17), 69.1 (C-6), 67.3 (C-15), 52.7 (C-14), 50.4 (C-10), 46.1 (C-8), 45.9 (C-5), 41.3 (C-24), 41.3 (C-19), 41.1 (C-9), 27.2 (C-26), 26.7 (C-21), 26.1 (C-30), 22.8 (C-28), 16.7 (C-20), 15.6 (C-25), 12.5 (C-29), 11.7 (C-22), 11.5 (C-27). Infrared (KBr, cm−1): 2,965, 2,926, 2,855, 1,766, 1,738, 1,722, 1,667. [α]D26 = +94° (c = 0.35, CHCl3).
CCDC 739477 contain the supplementary crystallographic data for SkE. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre [CCDC], 12 Union Road, Cambridge CB2 1EZ, United Kingdom. Fax: 44 1223 336033. E-mail: deposit@ccdc.cam.ac.uk).
Biological tests. (i) Activity against erythrocytic stages of cultured Plasmodium falciparum.
SkE antiplasmodial activity was determined against three strains of P. falciparum: chloroquine (CQ)-sensitive F32 Tanzania (CQ 50% inhibitory concentration [IC50], 36 ± 3 nM) and chloroquine-resistant FcB1 Colombia and W2 Indochina (CQ IC50s, 167 ± 32 nM and 196 ± 16 nM, respectively).
Parasites were cultured by the method of Trager and Jensen (32) with modifications (34). The cultures were synchronized by a combination of magnetic enrichment and 5% d-sorbitol lysis (Merck, Darmstadt, Germany) (22, 27). In vitro antimalarial activity testing was performed by the method of Desjardins et al. (12) with modifications (34).
The sensitivity of the different asexual erythrocytic stages of P. falciparum to SkE was determined on the FcB1 strain, synchronized on a 4-hour period as previously described (19). Cultures in 24-well plates (1% parasitemia, 2% hematocrit) were subjected to 8-hour pulses of SkE at 8.6 and 86.5 nM. After the pulses, the cultures were washed three times with culture medium and returned to normal culture conditions. At the end of the experiment (70 hours, young trophozoite stage of the next erythrocytic cycle), parasitemia was evaluated in each well by visual examination by counting Giemsa-stained smears (5,000 erythrocytes/smear). Results are expressed as the percentage of inhibition of parasitic growth.
(ii) In vitro activity against cultured P. falciparum gametocytes.
P. falciparum gametocytogenesis was induced on day 0 with the W2 strain by the method of Ifediba and Vanderberg (18) with modifications (3, 31). At day 13 (3 days after the addition of N-acetylglucosamine), increasing dilutions of SkE were added to the culture medium. At day 15, a thin smear corresponding to each concentration was made and Giemsa stained. Visual estimation of the gametocytemia was carried out by counting at least 10,000 erythrocytes. Negative and positive controls were performed without SkE and with primaquine, respectively.
(iii) Cytotoxicity.
Cytotoxicity was evaluated with three cell lines: (i) Vero, a monkey kidney cell line; (ii) MCF7, a human breast cancer cell line; and (iii) THP1, a human leukemia cell line. All the cell lines were cultured under the same conditions as used for P. falciparum, except for the 5% human serum, which was replaced by 10% fetal calf serum (Boehringer). Cell growth was measured by [3H]hypoxanthine (Amersham-France) incorporation after a 48-hour incubation with serial drug dilutions. The amount of [3H]hypoxanthine incorporated in the presence of drugs was compared with that of control cultures without the test compounds (30).
(iv) In vivo antimalarial activity.
In vivo antimalarial activity of SkE was tested with the 4-day suppressive test performed on Plasmodium vinckei petteri-infected CD female mice (8). Mice (mean body weight, 20 ± 2 g) were infected with 106 infected red blood cells in RPMI on day 0. Groups of five mice were treated intraperitoneally (i.p.) or orally (p.o.) from days 0 to 3 with increasing doses (0.5 to 20 mg/kg of body weight) of the drugs. On day 4, Giemsa-stained blood smears (tail blood) were made for each mouse, and parasitemia was estimated by visual counting of at least 5,000 erythrocytes. The survival time was also monitored until day 21. Chloroquine was used as the reference drug. The percentage of inhibition was calculated with the following formula: (control parasitemia − parasitemia with test drug)/(control parasitemia) × 100. All the procedures involving animals conformed to European regulations (European Economic Community [EEC] directive 86/609).
Statistical analysis.
A survival analysis was performed. Each treatment was tested by the log rank test for survivor functions, in which the analysis time was the survival time. Failure was defined as death. The mean survival time reported was calculated as the area under the Kaplan-Meier survivor function. As some mice were still alive at the end of the study, the longest analysis time was not available. If the observation with the longest analysis time was not available, the survivor function did not go to zero. Consequently, the area under the curve underestimated the mean survival time. If the longest observed analysis time was not available, the extended mean reported was calculated as the area under the extension of the survivor function from the last observed time to zero using an exponential function. All tests were performed using Stata 9.2 (Intercooled Stata 9.2 for Windows; StataCorp, College Station, TX).
RESULTS
The chloroform extract of a tea made with defatted dry and mature leaves, which retained biological activity, was depigmented and further fractionated by countercurrent chromatography (see Scheme S1 in the supplemental material). Seven fractions were obtained (fraction 1 [F1] to F7). Previous purification with the same protocol but on a smaller quantity allowed us to identify two active fractions (IC50 < 1 μg/ml) with a similar composition to F1, F2, and F6 (according to their thin-layer chromatography profiles). F1, F2, and F6 were therefore further purified. From F6, we were not able to isolate any pure compounds. SkD was isolated from F2 (0.0002% yield [i.e., 2 mg from 1 kg {dry weight} of plant]). More interestingly, a new quassinoid structurally related to SkD (that we called SkE) was isolated from F1 and showed a very good activity in vitro on the different P. falciparum strains (Table 1) . However, the yield obtained for this active compound was very low (0.00035% [i.e., 3.5 mg from 1 kg {dry weight} of plant]) and precluded any further investigation of its antiplasmodial properties.
TABLE 1.
Antiplasmodial activity against asexual (F32, FcB1, and W2 strains) and sexual (W2 Indochina strain) stages and cytotoxicity of SkE
| Druga | Antiplasmodial activity (IC50)b against P. falciparum strain:
|
Cytotoxicityb against cell line:
|
|||||
|---|---|---|---|---|---|---|---|
| F32 Tanzania | FcB1 Colombia | W2 Indochina | W2 Indochina (gametocytes) | Vero | MCF7 | THP1 | |
| SkE | 68 ± 12 | 45 ± 32 | 24 ± 10 | 1,120 ± 400 | 6,574 ± 264 | 47 ± 2 | 33 ± 5 |
| SkD | NT | 1 | NT | NT | 58 ± 11 | <20c | <2c |
| CQ | 36 ± 3 | 167 ± 32 | 196 ± 16 | NT | >500d | >500d | >500d |
| PMQ | 6.17 ± 1.2 | 8.9 ± 1.1 | 7.14 ± 0.9 | 8.9 ± 2.3 | 340 ± 29 | NT | NT |
SkE, simalikalactone E; SkD, simalikalactone D; CQ, chloroquine; PMQ, primaquine.
Values are expressed in nanomolar (except for PMQ, which is shown in micromolar). Each value corresponds to the mean ± standard error of the mean from at least three independent experiments. NT, not tested.
Lower concentration tested.
Higher concentration tested.
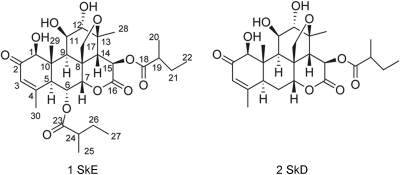
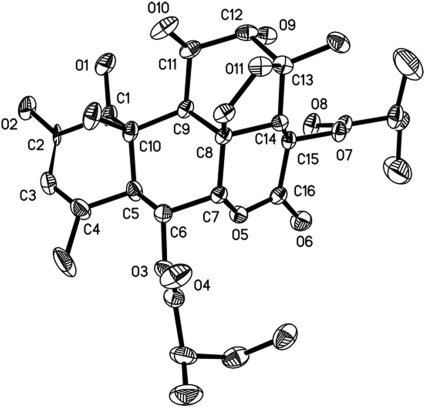
We then set up an improved extraction procedure from the methanol extract of the mature dry leaves of Q. amara and increased the yield to 0.004% (40 mg from 1 kg of plant). Careful analysis of infrared, optical rotation, and mass and NMR spectra enabled us to establish the structure of SkE. Crystals were also obtained from deuterated methanol, and X-ray diffraction confirmed the established structure and its relative stereochemistry (Fig. 1 and 2).
FIG. 1.
Chemical structures of simalikalactone E (compound 1) and simalikalactone D (compound 2).
FIG. 2.
Structure of SkE obtained from X-ray diffraction experiment.
Other already known quassinoids could be also identified: quassin (from fraction F3), picrasin H (from F4), picrasin B (from F5), and picrasin J (from F7). These compounds showed no significant antiplasmodial activity (D. Stien, S. Bertani, G. Bourdy, E. Deharo, E. Houël, V. Jullian, A. Valentin, and S. Chevalley, presented at the ZingConference on Natural Products Chemistry, Antigua, 10 to 13 January 2008).
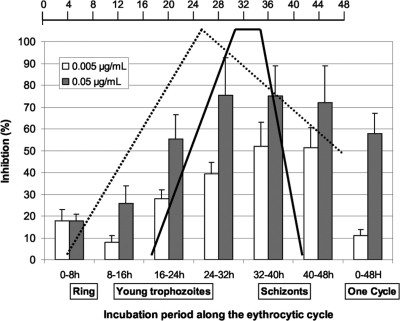
The antiplasmodial activity of SkE was determined on three strains of P. falciparum and gave IC50s ranging from 24 to 68 nM (Table 1). When tested on highly synchronized parasite cultures, SkE had a maximal activity beginning at the second half of the erythrocytic cycle (Fig. 3). This peak decreased at the 40- to 48-h pulse for the lowest concentration tested (about 1/10 of the IC50) (2).
FIG. 3.
Impact of SkE on the eythrocytic cycle of P. falciparum. A parasite culture synchronized on a 6-h period was subjected to 8-h pulses of SkE at the IC50 (gray bars) and 1/10 the value (white bars). After the pulse, the culture was washed and returned to normal culture conditions until the beginning of the second erythrocytic cycle, and then parasitemia was determined. The scale bar at the top of the figure shows time (in hours). Major events along the eythrocytic cycle are shown. The dotted line shows protein synthesis, and the solid black line shows DNA synthesis. This figure was adapted from the Annals of Tropical Medicine and Parasitology (2) with the permission of the publisher.
SkE reduced gametocytemia by 50% at a dose sevenfold lower than primaquine, a reference molecule for the elimination of gametocytes (Table 1) (33).
The cytotoxicity of SkE varied, with an IC50 from 6 μM (Vero cells) to 33 nM (THP1 cells) (Table 1).
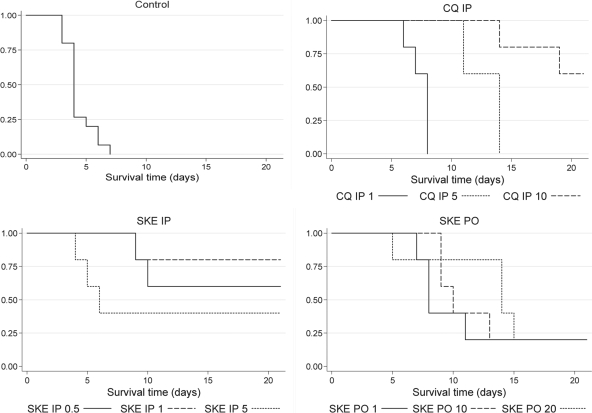
SkE was then orally and intraperitoneally administered to P. vinckei petteri-infected mice. The i.p. route (50% effective dose [ED50], 0.5 mg/kg/day) was almost twice as effective as the p.o. route (ED50, 1 mg/kg/day), the control being chloroquine given i.p. (ED50, 3 mg/kg/day) (Table 2). The survival of mice was monitored for 3 weeks, and the mean survival time of the mice was evaluated (Table 3). The highest values were obtained with mice treated with SkE i.p. at 1 mg/kg/day (18.6 days; extended mean, 93.89 days) and with CQ at 10 mg/kg/day (19.20 days; extended mean, 43.87 days). Lower survival means were obtained with SkE given i.p. at a higher dose or by oral route. However, all the treated mice had a significantly higher survival time than that of control mice (P < 0.05 for CQ given i.p. [1 mg/kg/day] and P < 0.01 for the other treatments). The Kaplan-Meier curves (Fig. 4) showed similar aspects for SkE given 0.5 and 1 mg/kg/day i.p. and for CQ given 10 mg/kg/day i.p.
TABLE 2.
Inhibition of parasitemia at day 5a
| Treatment and routeb | % Inhibition of parasitemia |
|---|---|
| DMSO | 0 |
| CMC | 0 |
| CQ i.p. | |
| 1 | 0 |
| 5 | 100 |
| 10 | 100 |
| SkE | |
| i.p. | |
| 0.5 | 59 |
| 1 | 98 |
| 5 | 100 |
| p.o. | |
| 1 | 55 |
| 10 | 95 |
| 20 | 100 |
The control mice treated with dimethyl sulfoxide alone showed 100% parasitemia.
Treatment and routes were as follows: chloroquine (CQ) (1, 5, or 10 mg/kg/day for 4 days); simalikalactone E (SkE) (0.5, 1, 5, 10 or 20 mg/kg/day); p.o., oral route; i.p., intraperitoneal route. DMSO, dimethyl sulfoxide, control for i.p. route; CMC, carboxymethylcellulose, control for p.o. route.
TABLE 3.
Mean survival time of the treated mice and statistical significance
| Treatment and routea | No. of mice | Mean survival time (days) [95% CI]b | Extended mean survival time (days) | Statistical significance (P value) compared to the following group:
|
|
|---|---|---|---|---|---|
| Control | CQ (i.p., 1 mg/kg/day) | ||||
| Control | 15 | 4.33 [3.76-4.91] | |||
| CQ | 5 | 7.40 [6.70-8.10] | 0.013 | ||
| i.p. | |||||
| 1 | |||||
| 5 | 5 | 12.80 [11.51-14.09] | <0.001 | 0.026 | |
| 10 | 5 | 19.20 [16.82-21.58]c | 43.87 | <0.001 | 0.002 |
| SkE | |||||
| i.p. | |||||
| 0.5 | 5 | 16.40 [11.45-21.35]c | 41.07 | <0.001 | 0.005 |
| 1 | 5 | 18.60 [14.39-22.81]c | 93.89 | <0.001 | 0.003 |
| 5 | 5 | 11.40 [4.51-18.29]c | 20.57 | <0.001 | 0.047 |
| p.o. | |||||
| 1 | 5 | 11.00 [6.46-15.54]c | 13.61 | <0.001 | NSd |
| 10 | 5 | 12.40 [8.42-16.38]c | 15.01 | <0.001 | 0.041 |
| 20 | 5 | 13.80 [9.32-18.28]c | 16.41 | <0.001 | 0.014 |
Treatment and routes were as follows: chloroquine (CQ) (1, 5, or 10 mg/kg/day for 4 days); simalikalactone E (SkE) (0.5, 1, 5, 10 or 20 mg/kg/day); p.o., oral route; i.p., intraperitoneal route.
95% CI, 95% confidence interval.
As some mice were still alive at the end of the study, the largest observed analysis time was not available, and therefore, the mean is underestimated.
NS, not significant.
FIG. 4.
Bivariate analysis (Kaplan-Meier curves and log rank tests) for survival time. Survival of treated mice was monitored for 21 days (inoculation at day 0). The antimalarial treatment and route were as follows: chloroquine (CQ) (1, 5, or 10 mg/kg/day for 4 days); simalikalactone E (SKE) (0.5, 1, 5, 10, or 20 mg/kg/day); PO, oral route; IP, intraperitoneal route. When the line does not reach 0, the mice were still alive at day 21.
DISCUSSION
Biodiversity is clearly a source of new drugs (26). When biodiversity analysis combines with traditional treatments, there is the hope of finding some promising candidate molecules for pharmaceutical development. Here, we describe a new quassinoid, obtained after bioguided fractionation of a widely used Amazonian traditional remedy for malaria (35).
This quassinoid, named simalikalactone E, exhibited very good antiplasmodial activity against the three P. falciparum strains tested, whatever their geographic origin or chloroquine sensitivity. The IC50s obtained were in the range of most commercially available antimalarial drugs tested under similar conditions (16) and varied from 24 to 68 nM. These data clearly indicate that SkE does not interfere with CQ resistance pathways.
Against P. falciparum gametocytes, a stage which is fundamental for transmission to mosquitoes, SkE was more active (IC50, 1.2 μM) than the reference compound primaquine (IC50, 8.9 μM). In a previous study, Benoit-Vical et al. (3) showed that an artemisinin derivative, artesunate, inhibited gametocyte growth at a 10-fold-lower dose (around 0.1 μM).
Regarding cytotoxicity, the SkE IC50s were similar to those obtained against P. falciparum when using cancer-derived cell lines (MCF7 and THP1). However, when tested on Vero cells (which are of primate origin and are not cancer-derived cells) SkE displayed an IC50 around 100 times higher than that observed on the asexual stages of the parasite, and is less toxic than SkD (but more toxic than CQ). This particularly good selectivity index prompted us to perform in vivo experiments.
In vivo, at day 5, the lowest tested doses (0.5 mg/kg/day i.p. and 1 mg/kg/day p.o.) were close to the ED50. Higher doses led to a complete cure of the mice at day 5 (Table 2). However, when the survival of mice was evaluated over a longer period, clear differences appeared between the i.p. and p.o. routes. The analysis of survival times showed that the i.p. route, at doses in the same range, seemed to be more efficient than the p.o. route as illustrated by the Kaplan-Meier curves (Fig. 4). It is to be noted that SkE administered by the i.p. route was considerably more active than CQ (about 10-fold more efficient when looking at SkE [1 mg/kg/day i.p.] and CQ [10 mg/kg/day i.p.], Table 3 and Fig. 4). On the other hand, at the higher i.p. dose (5 mg/kg/day), the survival time was shorter than for the other drugs, which could be explained by toxicity. The statistical analysis of the survival times (Table 3) showed better activity of SkE compared with the control, and at the doses tested, a better activity than that of CQ at 1 mg/kg/day, except for the highest dose (5 mg/kg/day, i.p. route) where a toxic effect was probably emerging. It is also to be noted that there were no significant differences between the SkE-treated mice given 1 and 0.5 mg/kg/day (i.p.) and the CQ-treated mice given 10 mg/kg/day (i.p.). Taken together, these data demonstrated that SkE showed good antimalarial activity in mice, with the best dose for a complete cure being around 1 mg/kg/day.
This activity was in the same range as in vivo activities previously reported for other quassinoids. When administered by the i.p. route, sergeolide (13), glaucarubinone (23), cedronine (24), and bruceolide and its carbonate derivatives (25) had ED50s between 0.2 mg/kg/day and 1.8 mg/kg/day. We also showed that SkD inhibits 50% of Plasmodium yoelii yoelii rodent malaria parasite growth at 3.7 mg/kg/day in vivo by the oral route (6).
Toxicity was also monitored, and at the higher dose of SkE (5 mg/kg/day given i.p., 10 times the IC50), an immediate toxicity close to the LD50 was observed (three deaths out of five mice in 3 days), while at lower doses, the antimalarial activity was clear.
The study of the activity of SkE on the different stages of the P. falciparum life cycle showed that SkE had a better inhibitory effect on stages where DNA synthesis occurred, but our results do not enable a distinction to be made between an SkE-DNA interaction and an inhibition of proteins implicated in DNA synthesis. DNA and protein synthesis inhibition in P. falciparum has been reported for several quassinoids. In general, the inhibition of DNA synthesis was less pronounced and seemed to be a consequence of protein synthesis inhibition (15, 21, 28). Recent reinvestigation of the antineoplastic activity of various quassinoids showed that NF-κB activation (9) and downregulation of c-myc (10) were implicated in the cell differentiation and apoptosis induced by quassinoids. Mitochondrial membrane depolarization and caspase 3 activation also played a role in this process (29). It has also been shown that 6α-tigloyloxychaparrinone was an inhibitor of hypoxia-inducible factor 1 (20). Ailanthinone, glaucarubinone, and 6α-senecionylchaparrin were also identified as inhibitors of the transcription factor AP-1, but this function did not correlate with cytotoxicity or protein synthesis inhibition (7). Those new findings suggest that the antiplasmodial mechanism of action of quassinoids merits further detailed investigation.
The structural requirements for the antimalarial activity of quassinoids are well-documented, and both SkD and SkE meet them: they have an α,β-unsaturated lactone on ring A and an oxymethylene bridge between C-8 and C-11 or between C-8 and C-13. The isolation of SkE allowed us to compare the antiplasmodial potencies of SkE and SkD and thus evaluate the effect of the carboxylate group in the C-6 position. C-6 substitution occurs sometimes in quassinoids. In a review describing 230 quassinoids, 10% were shown to be substituted on C-6, and hydroxy or carboxy groups were the only substituents reported (11). For one of these quassinoids, 15-desacetylundulatone, isolated from Quassia undulata (1) and Hannoa chlorantha (14), a good antiplasmodial activity was reported. However, there is no clear evidence in the literature of the influence of a substituent on the C-6 position on antiplasmodial activity. We have shown here that this activity in vitro is lower for SkE than for SkD, but the selectivity index when using Vero cells is better for SkE (the selectivity index of SkE is 111, while the selectivity index for SkD is 58). The C-6 carboxylation could contribute to lowering the cytotoxicity of the quassinoid.
The present report demonstrated that despite their reputation as toxic molecules, quassinoids remain potentially interesting as antimalarials, and further research should be done on rationalizing the effect of the C-6 substitution to improve their efficacy as drugs and lower their toxicity. Because quassinoids are the active ingredients of many traditional antimalarial preparations all over the world, this type of research would be of great interest for people living in places where malaria is endemic and relying on these preparations.
Supplementary Material
Footnotes
Published ahead of print on 10 August 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Adesanwo, J. K., O. Ekundayo, F. O. Shode, V. C. O. Njar, A. J. J. van den Berge, and O. A. T. Oludahunsi. 2004. Eniotorin, an anti-malarial coumarin from the root bark of Quassia undulata. Niger. J. Nat. Prod. Med. 8:69-73. [Google Scholar]
- 2.Arnot, D. E., and K. Gull. 1998. The Plasmodium cell cycle: facts and questions. Ann. Trop. Med. Parasitol. 92:361-365. [DOI] [PubMed] [Google Scholar]
- 3.Benoit-Vical, F., J. Lelievre, A. Berry, C. Deymier, O. Dechy-Cabaret, J. Cazelles, C. Loup, A. Robert, J.-F. Magnaval, and B. Meunier. 2007. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob. Agents Chemother. 51:1463-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani, S., G. Bourdy, I. Landau, J. C. Robinson, P. Esterre, and E. Deharo. 2005. Evaluation of French Guiana traditional antimalarial remedies. J. Ethnopharmacol. 98:45-54. [DOI] [PubMed] [Google Scholar]
- 5.Bertani, S., E. Houël, G. Bourdy, D. Stien, V. Jullian, I. Landau, and E. Deharo. 2007. Quassia amara L. (Simaroubaceae) leaf tea: effect of the growing stage and desiccation status on the antimalarial activity of a traditional preparation. J. Ethnopharmacol. 111:40-42. [DOI] [PubMed] [Google Scholar]
- 6.Bertani, S., E. Houël, D. Stien, L. Chevolot, V. Jullian, G. Garavito, G. Bourdy, and E. Deharo. 2006. Simalikalactone D is responsible for the antimalarial properties of an Amazonian traditional remedy made with Quassia amara L. (Simaroubaceae). J. Ethnopharmacol. 108:155-157. [DOI] [PubMed] [Google Scholar]
- 7.Beutler, J. A., M.-I. Kang, F. Robert, J. A. Clement, J. Pelletier, N. H. Colburn, T. C. McKee, E. Goncharova, J. B. McMahon, and C. J. Henrich. 2009. Quassinoid inhibition of AP-1 function does not correlate with cytotoxicity or protein synthesis inhibition. J. Nat. Prod. 72:503-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chance, M., H. Momen, D. Warhurst, and W. Peters. 1978. The chemotherapy of rodent malaria. XXIX. DNA relationships within the subgenus Plasmodium (Vinckeia). Ann. Trop. Med. Parasitol. 72:13-22. [DOI] [PubMed] [Google Scholar]
- 9.Cuendet, M., J. J. Gills, and J. M. Pezzuto. 2004. Brusatol-induced HL-60 cell differentiation involves NF-κB activation. Cancer Lett. 206:43-50. [DOI] [PubMed] [Google Scholar]
- 10.Cuendet, M., and J. M. Pezzuto. 2004. Antitumor activity of bruceantin: an old drug with new promise. J. Nat. Prod. 67:269-272. [DOI] [PubMed] [Google Scholar]
- 11.Curcino Vieira, I. J., and R. Braz-Filho. 2006. Quassinoids: structural diversity, biological activity and synthetic studies. Stud. Nat. Prod. Chem. 33:433-492. [Google Scholar]
- 12.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fandeur, T., C. Moretti, and J. Polonsky. 1985. In vitro and in vivo assessement (sic) of the antimalarial activity of sergeolide. Planta Med. 51:20-23. [DOI] [PubMed] [Google Scholar]
- 14.Francois, G., C. Diakanamwa, G. Timperman, G. Bringmann, T. Steenackers, G. Atassi, M. Van Looveren, J. Holenz, J.-P. Tassin, L. A. Assi, R. Vanhaelen-Fastre, and M. Vanhaelen. 1998. Antimalarial and cytotoxic potential of four quassinoids from Hannoa chlorantha and Hannoa klaineana, and their structure-activity relationships. Int. J. Parasitol. 28:635-640. [DOI] [PubMed] [Google Scholar]
- 15.Fresno, M., A. Gonzalez, D. Vazquez, and A. Jimenez. 1978. Bruceantin, a novel inhibitor of peptide bond formation. Biochim. Biophys. Acta 518:104-112. [DOI] [PubMed] [Google Scholar]
- 16.Garavito, G., S. Bertani, J. Rincon, S. Maurel, M. C. Monje, I. Landau, A. Valentin, and E. Deharo. 2007. Blood schizontocidal activity of methylene blue in combination with antimalarials against Plasmodium falciparum. Parasite 14:135-140. [DOI] [PubMed] [Google Scholar]
- 17.Guo, Z., S. Vangapandu, R. W. Sindelar, L. A. Walker, and R. D. Sindelar. 2005. Biologically active quassinoids and their chemistry: potential leads for drug design. Curr. Med. Chem. 12:173-190. [DOI] [PubMed] [Google Scholar]
- 18.Ifediba, T., and J. P. Vanderberg. 1981. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294:364-366. [DOI] [PubMed] [Google Scholar]
- 19.Jacquemond-Collet, I., F. Benoit-Vical, A. Valentin, E. Stanislas, M. Mallié, and I. Fourasté. 2002. Antiplasmodial and cytotoxic activity of galipinine and other tetrahydroquinolines from Galipea officinalis. Planta Med. 68:68-69. [DOI] [PubMed] [Google Scholar]
- 20.Jin, X., H. R. Jin, D. Lee, J.-H. Lee, S. K. Kim, and J. J. Lee. 2008. A quassinoid 6α-tigloyloxychaparrinone inhibits hypoxia-inducible factor-1 pathway by inhibition of eukaryotic translation initiation factor 4E phosphorylation. Eur. J. Pharmacol. 592:41-47. [DOI] [PubMed] [Google Scholar]
- 21.Kirby, G. C., M. J. O'Neill, J. D. Phillipson, and D. C. Warhurst. 1989. In vitro studies on the mode of action of quassinoids with activity against chloroquine-resistant Plasmodium falciparum. Biochem. Pharmacol. 38:4367-4374. [DOI] [PubMed] [Google Scholar]
- 22.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 23.Monjour, L., F. Rouquier, C. Alfred, and J. Polonsky. 1987. Therapeutic trials of experimental murine malaria with the quassinoid, glaucarubinone. C. R. Acad. Sci. III 304:129-132. [PubMed] [Google Scholar]
- 24.Moretti, C., E. Deharo, M. Sauvain, C. Jardel, P. T. David, and M. Gasquet. 1994. Antimalarial activity of cedronin. J. Ethnopharmacol. 43:57-61. [DOI] [PubMed] [Google Scholar]
- 25.Murakami, N., M. Sugimoto, M. Kawanishi, S. Tamura, H. S. Kim, K. Begum, Y. Wataya, and M. Kobayashi. 2003. New semisynthetic quassinoids with in vivo antimalarial activity. J. Med. Chem. 46:638-641. [DOI] [PubMed] [Google Scholar]
- 26.Newman, D. J., and G. M. Cragg. 2007. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 70:461-477. [DOI] [PubMed] [Google Scholar]
- 27.Ribaut, C., A. Berry, S. Chevalley, K. Reybier, I. Morlais, D. Parzy, F. Nepveu, F. Benoit-Vical, and A. Valentin. 2008. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malaria J. 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Fonseca, C., R. Amils, and R. A. Garrett. 1995. Fine structure of the peptidyl transferase centre on 23 S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J. Mol. Biol. 247:224-235. [DOI] [PubMed] [Google Scholar]
- 29.Rosati, A., E. Quaranta, M. Ammirante, M. C. Turco, A. Leone, and V. De Feo. 2004. Quassinoids can induce mitochondrial membrane depolarisation and caspase 3 activation in human cells. Cell Death Differ. 11(Suppl. 2):S216-S218. [DOI] [PubMed] [Google Scholar]
- 30.Roumy, V., G. Garcia-Pizango, A. L. Gutierrez-Choquevilca, L. Ruiz, V. Jullian, P. Winterton, N. Fabre, C. Moulis, and A. Valentin. 2007. Amazonian plants from Peru used by Quechua and Mestizo to treat malaria with evaluation of their activity. J. Ethnopharmacol. 112:482-489. [DOI] [PubMed] [Google Scholar]
- 31.Sall, C., A.-D. Yapi, N. Desbois, S. Chevalley, J.-M. Chezal, K. Tan, J.-C. Teulade, A. Valentin, and Y. Blache. 2008. Design, synthesis, and biological activities of conformationally restricted analogs of primaquine with a 1,10-phenanthroline framework. Bioorg. Med. Chem. Lett. 18:4666-4669. [DOI] [PubMed] [Google Scholar]
- 32.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 33.Vale, N., R. Moreira, and P. Gomes. 2009. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 44:937-953. [DOI] [PubMed] [Google Scholar]
- 34.Valentin, A., F. Benoit-Vical, C. Moulis, E. Stanislas, M. Mallie, I. Fouraste, and J. M. Bastide. 1997. In vitro antimalarial activity of penduline, a bisbenzylisoquinoline from Isopyrum thalictroides. Antimicrob. Agents Chemother. 41:2305-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vigneron, M., X. Deparis, E. Deharo, and G. Bourdy. 2005. Antimalarial remedies in French Guiana: a knowledge attitudes and practices study. J. Ethnopharmacol. 98:351-360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.