Abstract
Bacteria utilize quorum-sensing communication to organize their behavior by monitoring the concentration of bacterial signals, referred to as autoinducers (AIs). The widespread detection of AI-2 signals and its enzymatic synthase (LuxS) in bacteria suggests that AI-2 is an inter- and intraspecies communication signal. We have previously shown that antibiotic susceptibility is affected by AI-2 signaling in Streptococcus anginosus. Since chronic infections involve persistent biofilms resilient to antibiotic treatment, we explored the role of AI-2/LuxS in Streptococcus intermedius biofilm formation and cell viability when the organism was exposed to sub-MICs of ampicillin, ciprofloxacin, or tetracycline. The S. intermedius wild type (WT) and its isogenic luxS mutant, strain SI006, were exposed to sub-MICs of ampicillin, ciprofloxacin, or tetracycline. Biofilms were formed on polystyrene discs in microtiter plates. To assess planktonic cell viability, the ATP microbial viability assay was performed and the numbers of CFU were determined. For complementation assays, the AI-2 precursor dihydroxy pentanedione (DPD) was used as a supplement for SI006. Relative luxS expression was quantified by real-time PCR. The sub-MICs of all three antibiotics increased biofilm formation in S. intermedius WT. However, biofilm formation by SI006 was either unaffected or reduced (P ≤ 0.05). Bacterial viability tests of biofilm and planktonic cell cultures indicated that SI006 was more susceptible to antibiotics than the WT. DPD complemented the luxS mutant phenotype. Real-time PCR revealed modest yet significant changes in luxS expression in the presence of antibiotic concentrations that increased biofilm formation. In conclusion, in S. intermedius, AI-2/LuxS was involved in antibiotic susceptibility and increased biofilm formation at sub-MICs of antibiotic.
Bacteria colonize biological and inert surfaces in the form of persistent, sessile, matrix-encapsulated communities referred to as biofilms. Bacterial biofilms account for the majority of chronic diseases, including gingivitis, endocarditis, and nosocomial infections (10). Biofilms represent an obstacle to clinical treatment due to their resistant nature, which shelters bacteria from the immune response and penetration by antibiotics. Bacteria may also resist the antibiotic effect through efflux pumps, antibiotic inactivation, modification of susceptible targets, or persister cell formation (13, 27). The bioavailability of antibiotics depends on the dose, drug regimen, absorption, distribution, elimination, and mode of administration (5, 15). Therefore, following antibiotic treatment, bacteria may be exposed to supra-MICs as well as sub-MICs of antibiotics. Intriguingly, several studies report that low antibiotic doses may promote the formation of bacterial biofilms (4, 11, 18).
Bacterial microorganisms exist in large cooperative populations by employing communications systems necessary for their virulence and survival. Quorum sensing is one such system that enables bacteria to coordinate their gene regulation and trigger collective population behaviors. Through this quorum-sensing system, bacteria are able to monitor their population by releasing autoinducer (AI) signals and consequently responding to a specific threshold accumulation of AI signals. AI-2 is a collective term given to describe cyclic derivatives of 4,5-dihydroxy-2,3-pentanedione (DPD), a highly reactive metabolic by-product of the activated methyl cycle. The AI-2 signal and its enzymatic synthase LuxS are broadly encountered in gram-positive and gram-negative bacteria, suggesting that AI-2 is an inter- and intraspecies communication signal (7, 26). This communication system has been shown to play a role in the vital functions, including virulence and biofilm formation, of several bacteria (26).
Streptococcus intermedius, a commensal bacterium and a member of the Streptococcus anginosus group, has been isolated from patients with periodontitis and fatal purulent infections, especially brain and liver abscesses (6, 28). In both Streptococcus anginosus and S. intermedius, AI-2 has been shown to be involved in biofilm formation (2, 16). We have also demonstrated that AI-2/LuxS affects susceptibility to ampicillin and erythromycin in S. anginosus (1). Since chronic infections involve persistent biofilms resilient to antibiotic treatment, we investigated the role of AI-2/LuxS in biofilm formation by S. intermedius in the presence of sub-MICs of antibiotics. Three antibiotics with distinct modes of action and different bacterial targets were chosen to study the role of AI-2 in biofilm formation and antibiotic susceptibility: ampicillin, a β-lactam antibiotic that targets protein cell wall synthesis; ciprofloxacin, a fluoroquinolone that targets DNA gyrase; and tetracycline, a protein inhibitor that acts on peptidyl transferase (27). S. intermedius has shown moderate susceptibility to ampicillin, ciprofloxacin, and tetracycline, although resistant strains are developing (20, 22, 25).
We show that certain low, sub-MICs of antibiotics with distinct modes of action may increase biofilm formation in the S. intermedius wild type (WT) but not in its luxS mutant. These results may implicate AI-2/LuxS in the antibiotic response mechanisms of S. intermedius biofilms.
MATERIALS AND METHODS
Bacterial strains and culture media.
The two S. intermedius strains used in this study were WT strain NCTC 11324 and its luxS isogenic mutant, strain SI006 (2). Insertional inactivation by plasmid chromosomal integration vector pSF151 carrying a kanamycin resistance cassette was used to obstruct SI006 luxS expression (24). The bacterial strains were stored at −20°C. The bacteria were first cultured on Todd-Hewitt agar plates (Difco Laboratories, Detroit, MI) for 24 h at 37°C in air supplied with 5% CO2. Before each experiment, the bacteria were cultured overnight in tryptone soy broth (TSB; Oxoid, United Kingdom). luxS mutant SI006 was supplied with 0.5 μg/ml kanamycin (Sigma-Aldrich, St. Louis, MO) during the first overnight growth.
Biofilm formation.
The MICs of ampicillin, ciprofloxacin, and tetracycline were determined by the broth microdilution method in ultra-low-binding 96-well plates (Corning). S. intermedius WT and SI006 were inoculated to a final concentration of ≈4 × 105. The growth of S. intermedius WT and its luxS mutant was measured at 595 nm in a KC4 V 3.4 spectrophotometer (Bio-Tek Instruments, Inc., Winooski, VT). The MIC was considered the lowest concentration of antibiotic that inhibited bacterial growth.
We then investigated the role of AI-2 in biofilm formation in the presence of several antibiotics at sub-MICs. TSB without or with ampicillin (Roche, Switzerland), ciprofloxacin (Fluka, Sigma-Aldrich), or tetracycline (Sigma-Aldrich) added at time zero over concentration ranges of 0.02 to 0.10 μg/ml, 0.10 to 0.28 μg/ml, and 0.05 to 0.30 μg/ml, respectively, was dispensed into 24-well microtiter plates containing sterile Thermanox plastic coverslips (Nunc, Copenhagen, Denmark). Since maximum biofilm formation in S. intermedius was reached at stationary phase (10 to 12 h of growth; data not shown), the microtiter wells were incubated for 12 h at 37°C in a 5% CO2 aerobic atmosphere. Biofilm formation was measured as described previously (2). The experiments were repeated three times in three parallels.
The biofilms that formed on the coverslips were prepared for and visualized by scanning electron microscopy (model XL 30 ESEM; Philips, Eindoven, The Netherlands), as described previously (16).
To evaluate biofilm viability, the biofilms were formed in 12-well microtiter plates and scraped with a disposable cell scraper (BD Falcon) into fresh TSB medium, and appropriate dilutions were plated on TSB agar.
Planktonic cell ATP levels and CFU.
Since spectrophotometric growth values may represent both viable and nonviable bacteria, we assessed viability in planktonic cell cultures using the BacTiter-Glo microbial cell viability assay (Promega, Madison, WI) (3). TSB without or with ampicillin, ciprofloxacin, or tetracycline at concentrations of 0.02 to 0.14, 0.10 to 0.35, and 0.03 to 0.20 μg/ml, respectively, was dispensed in ultra-low-binding plates (Corning). S. intermedius produced approximately 72% less biofilms in ultra-low-binding plates than in microtiter plates (data not shown). S. intermedius WT and SI006 were inoculated to a final volume of ≈4 × 105 CFU/ml and incubated for 12 h at 37°C in a 5% CO2 aerobic atmosphere. The bacterial cells were thoroughly resuspended, diluted 10% in TSB, and combined with the BacTiter-Glo reagent in black-walled, optical-bottom 96-well plates (Nunc). Luminescence was measured in a KC4 V 3.4 spectrophotometer (Bio-Tek Instruments, Inc.). TSB medium was used to measure the background luminescence, and the levels of ATP (Sigma) were calculated against a standard curve. The experiments were repeated three times in four parallels. The CFU counts obtained with and without antibiotic supplementation were determined parallel to the BacTiter-Glo microbial cell viability assay. The experiments were repeated three times in two parallels.
AI-2 supplementation.
To confirm the role of AI-2/LuxS in biofilm formation and antibiotic susceptibility, pre-AI-2 molecules in the form of DPD (Omm Scientific Inc.) were added to TSB prior to SI006 inoculation at an optimum complementation concentration of 0.8 nM, as described previously (2).
Real-time PCR.
S. intermedius WT planktonic cells from exponential growth phase (5 h) were incubated in the absence or the presence of ampicillin (0.04 μg/ml), ciprofloxacin (0.10 μg/ml), or tetracycline (0.10 μg/ml) for 60 min. During this period, optical density measurements were unaffected by the antibiotic concentrations used (data not shown). Total RNA was isolated from harvested S. intermedius WT cells by use of a High Pure isolation kit (Roche, Switzerland), as described by the manufacturer, except that the cells were incubated at 37°C for 20 min in 200 μl extraction buffer containing 20 mg/ml lysozyme, 1 M Tris-HCl (pH 8), and 100 U/ml mutanolysin. The RNA concentrations were adjusted to 100 ng/μl, and the samples were stored at −70°C. cDNA templates were synthesized from 10 ng/μl RNA with a RevertAid first-strand synthesis kit (Fermentas, Ontario, Canada). Real-time reverse transcription-PCR was run in a thermal cycler Mx3005P QCR system (Stratagene) with quantitative PCR FastStart SYBR green PCR master mixture (Rox; Roche). The thermal cycling program was as follows: 50°C for 2 min; 95°C for 10 min; and then 40 cycles consisting of denaturation at 95°C for 15 s, primer annealing at 59°C for 30 s, and primer extension at 72°C for 30 s. The thermal cycle was finalized with a cycle of 95°C for 1 min, 55°C for 30 s, and 95°C for 30 s. Dissociation curves were prepared immediately after the last PCR cycle by plotting the fluorescence intensities against temperatures as the set-point temperature (55°C) was increased by 1°C for 30 s (41 cycles). Gene-specific primers FP162 (GCCGGACTGGCTTTCACAT) and FP163 (GGTTGTGCCAGGGACATCAG) were designed to amplify luxS in S. intermedius 11324. The 16S rRNA and tuf genes simultaneously served as reference (housekeeping) genes to normalize the level of luxS gene expression. Primer pair FP116 (TGAAGAAGGTTTCGGATCG) and FP117 (CGCTCGGGACCTACGTATTA) was used to amplify 16S rRNA, while primer pair FP331 (GTATCCGCGAGGAAATCCA) and FP332 (CGAGCAACCTTCGTCCAA) amplified the tuf genes. The relative levels of expression, based on the expression ratio between the target gene and reference genes, was calculated by using the relative expression software tool (REST 2008, version 2.0.7) (17). The reaction mixture without a template was run as a control. Linearity and amplification efficiency were determined for each primer pair. Real-time PCR was repeated twice in triplicate parallel experiments.
Statistical analysis.
The Wilcoxon signed-rank test and the paired t test (SigmaStat, version 3.1) were used for statistical comparisons between groups. The level of statistical significance was set at a P value of ≤0.05.
RESULTS
Antibiotics at sub-MICs increased biofilm formation by the S. intermedius WT.
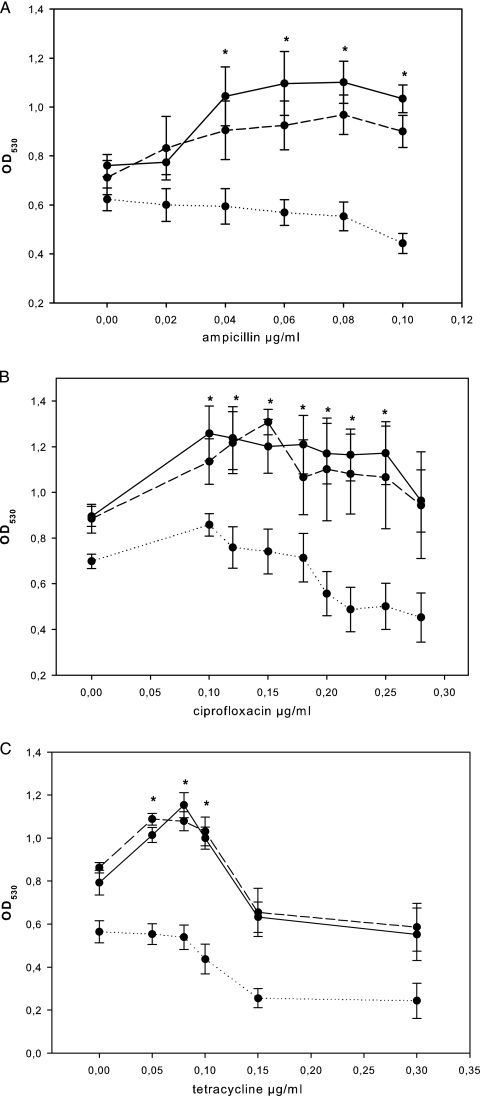
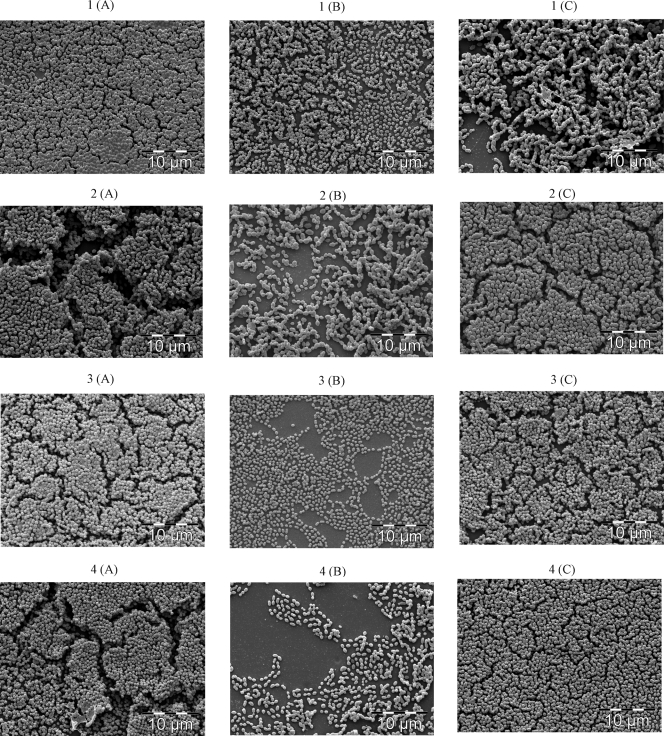
The growth of the S. intermedius WT was inhibited by 0.16 μg/ml of ampicillin, 0.50 μg/ml of ciprofloxacin, and 0.70 μg/ml of tetracycline (data not shown). There were no significant differences in MICs between the WT and the luxS mutant. In the absence of antibiotics, the luxS mutant displayed an approximately 25% reduction in the level of biofilm formation compared to that of the S. intermedius WT. The level of biofilm formation by the S. intermedius WT significantly increased by 20 to 30% in the presence of 0.04 to 0.10 μg/ml ampicillin, 0.10 to 0.25 μg/ml ciprofloxacin, or 0.05 to 0.10 μg/ml tetracycline (Fig. 1A to C). At a corresponding range of sub-MICs, the biofilm formation by the luxS mutant either remained unaffected or was reduced, independent of the antibiotic tested (Fig. 1A to C). Scanning electron microscopy displayed multilayer aggregations in S. intermedius WT biofilms, whereas the biofilms of the luxS mutant showed monolayer distributions (Fig. 2).
FIG. 1.
Biofilm formation by S. intermedius WT (continuous line), luxS mutant SI006 (dotted line), and luxS mutant SI006 supplemented with 0.8 nM DPD (interrupted line) following 12 h of incubation in the presence of sub-MICs of ampicillin (A), ciprofloxacin (B), and tetracycline (C). The data points represent mean values (n = 9) with the standard errors of the means. *, significant increase (P ≤ 0.05) in biofilm formation by S. intermedius WT (continuous line) but not luxS mutant SI006 (dotted line); OD530, optical density at 530 nm.
FIG. 2.
Scanning electron microscopy images of biofilms of the S. intermedius WT (A), luxS mutant SI006 (B), and luxS mutant SI006 supplemented with 0.8 nM DPD (C) following 12 h of incubation in the absence of antibiotics (control) (panels 1) and in the presence of 0.04 μg/ml ampicillin (panels 2), 0.15 μg/ml ciprofloxacin (panels 3), or 0.08 μg/ml tetracycline (panels 4).
We investigated whether changes in biofilm formation were associated with bacterial viability. The biofilm CFU counts revealed a lower level of viability (23% reduction) in the luxS mutant biofilms than in the S. intermedius WT biofilm in the absence of antibiotics (Table 1). In the presence of antibiotics at sub-MICs, the S. intermedius WT biofilms displayed an increase in biofilm mass and contained higher CFU counts than biofilms formed in the absence of the antibiotics (Table 1). The S. intermedius WT displayed the highest biofilm CFU with 0.10 μg/ml of ciprofloxacin and 0.05 μg/ml of tetracycline, followed by a gradual decline in biofilm viability (Table 1). In a reversed pattern, higher sub-MICs of ampicillin increased biofilm CFU, with the highest present at a sub-MIC of 0.06 μg/ml. However, unlike the WT, the luxS mutant biofilms exhibited a steady decline in CFU at the corresponding sub-MICs of ampicillin, ciprofloxacin, and tetracycline compared to their controls.
TABLE 1.
Biofilm CFU of S. intermedius WT, luxS mutant SI006, and DPD-supplemented luxS mutant SI006 cultured in medium with or without antibiotics at sub-MICs
| Drug and concn (μg/ml) | CFU (105/ml)a
|
||
|---|---|---|---|
| WT | SI006 | SI006 + DPD | |
| 0.00 (control) | 402.9 (26.88) | 310.8 (17.10) | 413.7 (24.47) |
| Ampicillin | |||
| 0.02 | 486.2 (64.04) | 324.1 (25.43) | 459.5 (29.33) |
| 0.04 | 621.6 (64.77) | 268.6 (28.65) | 686.0 (65.32) |
| 0.06 | 894.7 (182.65) | 162.1 (36.63) | 819.2 (43.61) |
| Ciprofloxacin | |||
| 0.10 | 768.1 (42.63) | 324.1 (24.96) | 814.7 (47.58) |
| 0.15 | 663.8 (36.30) | 333.0 (25.04) | 768.1 (44.00) |
| 0.20 | 577.2 (32.39) | 131.0 (19.28) | 519.5 (36.40) |
| Tetracycline | |||
| 0.05 | 779.2 (61.02) | 333.0 (16.49) | 828.1 (25.83) |
| 0.07 | 637.1 (66.99) | 253.1 (17.20) | 708.2 (53.33) |
| 0.10 | 610.5 (76.86) | 108.8 (37.27) | 486.2 (48.24) |
Data are for six isolates of each strain and condition. Values in parentheses are standard errors of the means.
Planktonic luxS mutant cultures were more susceptible than WT cultures to antibiotics at sub-MICs.
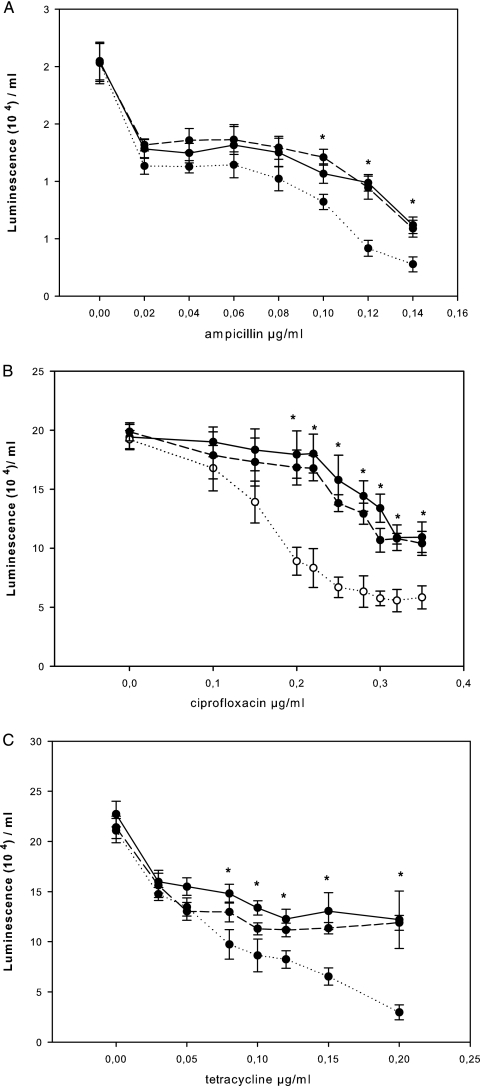
Since optical density measurements may consist of values for both viable and nonviable bacteria, we assessed cell viability in planktonic cell cultures incubated with or without the antibiotics at sub-MICs. The BacTiter-Glo microbial assay measures ATP as an indicator of metabolic activity and cell viability. The BacTiter-Glo microbial cell viability assay revealed similar levels of ATP production in the S. intermedius WT and the luxS mutant in the absence of antibiotics both during exponential growth (data not shown) and at stationary phase (Fig. 3). However, the luxS mutant displayed significantly lower levels of ATP production in the presence of ampicillin, ciprofloxacin, or tetracycline at several sub-MICs (Fig. 3). To confirm the findings of the ATP-dependent microbial assay, bacterial viability in the presence or absence of the antibiotics at several sub-MICs was further evaluated by determination of the CFU counts. The luxS mutant displayed significantly lower viable colony counts in the presence of the antibiotics at several sub-MICs in a pattern similar to that seen in the ATP-dependent viability assay (Table 2).
FIG. 3.
Luminescence (ATP level) as a measure of metabolic activity in planktonic cell cultures determined by the BacTiter-Glo viability assay following 12 h of incubation of the S. intermedius WT (continuous line), luxS mutant SI006 (dotted line), and luxS mutant SI006 supplemented with 0.8 nM AI-2 (interrupted line) in the presence of sub-MICs of ampicillin (A), ciprofloxacin (B), and tetracycline (C). The data points represent mean values (n = 12) with the standard errors of the means. *, significantly higher (P ≤ 0.05) ATP levels in the S. intermedius WT (continuous line) than in the luxS mutant SI006 (dotted line).
TABLE 2.
CFU in planktonic cultures of S. intermedius WT, luxS mutant SI006, and DPD-supplemented luxS mutant SI006 cultured with or without antibiotics at sub-MICs
| Drug and concn (μg/ml) | CFU (108/ml)a
|
||
|---|---|---|---|
| WT | SI006 | SI006 + DPD | |
| Ampicillin | |||
| 0.00 (control) | 771.7 (35.14) | 798.3 (37.09) | 793.3 (42.40) |
| 0.06 | 61.2 (4.01) | 41.8 (3.43) | 48.7 (55.06) |
| 0.08 | 43.3 (3.45) | 16.4 (1.7) | 39.1 (6.31) |
| 0.10 | 45.3 (6.72) | 10.3 (4.22) | 39.6 (6.31) |
| 0.12 | 20.7 (1.79) | 8.5 (1.39) | 18.4 (2.48) |
| Ciprofloxacin | |||
| 0.00 (control) | 756.9 (62.98) | 708.1 (47.64) | 768.6 (46.24) |
| 0.20 | 5.87 (0.52) | 2.60 (0.51) | 5.30 (0.21) |
| 0.25 | 5.14 (0.77) | 1.16 (0.26) | 4.72 (0.67) |
| 0.30 | 3.12 (0.38) | 0.74 (0.17) | 3.1 (0.37) |
| 0.35 | 2.6 (0.34) | 0.4 (0.13) | 2.3 (0.18) |
| Tetracycline | |||
| 0.00 (control) | 791.7 (44.10) | 757.5 (80.14) | 790.8 (90.09) |
| 0.08 | 61.9 (6.58) | 11.2 (2.88) | 38.4 (4.87) |
| 0.10 | 44.5 (8.52) | 5.7 (1.16) | 25.2 (2.72) |
| 0.12 | 32.0 (5.92) | 3.0 (0.94) | 16.3 (1.86) |
| 0.15 | 24.2 (5.23) | 2.0 (0.73) | 10.1 (1.75) |
Data are for six isolates of each strain and condition. Values in parentheses are standard errors of the means.
AI-2 supplementation restored phenotypic defects in the luxS mutant.
Supplementation of the culture with the 0.8 nM AI-2 precursor (DPD) significantly enhanced biofilm formation by the luxS mutant in the presence of antibiotics at sub-MICs (Fig. 1). The levels of biofilm formation by the DPD-supplemented luxS mutant were similar to those by the S. intermedius WT. DPD-supplemented luxS mutant biofilms also exhibited significantly increased numbers of CFU, similar to the S. intermedius WT biofilms (P ≤ 0.05) (Table 1). DPD supplementation had no effect on biofilm formation by the S. intermedius WT in the presence or the absence of antibiotics (data not shown).
In the absence of antibiotics, AI-2 supplementation affected neither planktonic cell ATP levels nor viability in S. intermedius WT and its luxS mutant (Fig. 3; Table 2). However, in the presence of antibiotics, AI-2 supplementation increased the bacterial viability of the luxS mutant to WT levels, as indicated by the findings of the ATP-dependent viability assay and the viable colony counts (Fig. 3; Table 2).
Effects of antibiotics at sub-MICs on luxS expression.
Real-time PCR displayed a modest, yet significant upregulation in the level of luxS expression by S. intermedius cells from planktonic, exponential-phase cultures. In the presence of 0.04 μg/ml ampicillin, 0.1 μg/ml ciprofloxacin, or 0.1 μg/ml tetracycline, the level of luxS expression increased by mean factors of 1.25, 1.24, and 1.31, respectively. At these sub-MICs, the level of biofilm formation by S. intermedius was significantly increased but the optical density measurements were not affected (data not shown).
DISCUSSION
In this study, we found that independent of the antibiotic mode of action, certain sub-MICs increased the level of biofilm formation by the S. intermedius WT but not by the S. intermedius luxS mutant. At these sub-MICs, the S. intermedius WT biofilms displayed increased viable counts, while the luxS mutant biofilm counts decreased. In planktonic cell cultures, the luxS mutant exhibited increased susceptibility to the antibiotics at several sub-MICs compared to that of the WT planktonic cell cultures.
Several bacteria have been reported to display increased biofilm formation either as a specific response to certain antibiotics or as a result of a broader antibiotic stress reaction (11, 18). In our study, the level of biofilm formation by S. intermedius increased upon exposure to antibiotics with diverse targets, indicating the involvement of a general response. This is in accordance with current findings indicating that stress conditions may promote biofilm formation (12). Adoption of a protective biofilm lifestyle may thus provide a survival advantage to bacterial cells.
Notably, the increase in the S. intermedius biofilm mass and viable counts in the presence of the antibiotics at certain sub-MICs was observed in the WT but not the luxS mutant. This supports the assumption that the mechanisms leading to increased biofilm formation in the WT depends on the AI-2/LuxS system. Another possibility is that the increased susceptibility of the luxS mutant to the antibiotics resulted in the availability of fewer viable cells to form biofilms. It is interesting, however, that in the WT, increased levels of biofilm formation were observed at concentrations that affected planktonic cell viability. The results indicated, therefore, that both increased and decreased biofilm formation may be observed concomitantly with an inhibitory effect on bacterial planktonic cell viability. In accordance with this finding, various antibiotics show either a restrictive or a provocative effect on biofilm viability, in addition to their inhibitory effects on growth (8, 11).
Since LuxS possesses an integral function in the methyl-activated cycle, increased antibiotic susceptibility and enhanced biofilm formation could also arise from metabolic defects following luxS inactivation (26). We therefore investigated whether the AI-2 precursor DPD may complement the altered antibiotic response of the luxS mutant. DPD restored the defective biofilm formation and the viability counts of the luxS mutant to the WT levels at all sub-MICs tested. Our results indicate, therefore, that AI-2 molecules are implicated in the biofilm-forming and susceptibility responses observed.
In Streptococcus pneumoniae, penicillin at the MIC50 significantly upregulates luxS expression (19). We therefore explored the possibility that S. intermedius luxS expression would be affected upon exposure to lower sub-MICs. Our results revealed a modest upregulation of luxS expression in response to sub-MICs that increased the level of S. intermedius WT biofilm formation. These subtle changes may be crucial to the synthesis of threshold AI-2 levels. It is difficult to ascertain whether such a modest effect would play a significant role in the biofilm outcomes observed. However, since LuxS is engaged in the global regulatory genetic networks of several bacteria (21, 23), complex mechanisms may be involved in antibiotic stress responses. The diverse role of AI-2/LuxS in bacteria suggests that numerous pathways may be involved in biofilm formation (9, 14, 29). Nonetheless, the molecular basis for the role of AI-2/LuxS in increased biofilm formation by S. intermedius in the presence of sub-MICs of various antibiotics calls for further investigation.
In conclusion, the AI-2/LuxS system in S. intermedius was involved in increased biofilm formation and susceptibility to various antibiotics at several sub-MICs. Our results support the significance of intercellular signaling in bacterial survival strategies and emerging views on interference with bacterial signaling as a novel means of fighting infections.
Acknowledgments
We thank Steinar Stølen for his assistance with the scanning electron microscopy images.
The present study was supported by research funds from the Faculty of Dentistry, University of Oslo, Oslo, Norway.
Footnotes
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Ahmed, N. A., F. C. Petersen, and A. A. Scheie. 2007. AI-2 quorum sensing affects antibiotic susceptibility in Streptococcus anginosus. J. Antimicrob. Chemother. 60:49-53. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, N. A., F. C. Petersen, and A. A. Scheie. 2008. Biofilm formation and autoinducer-2 signaling in Streptococcus intermedius: role of thermal and pH factors. Oral Microbiol. Immunol. 23:492-497. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2007. BacTiter-Glo™ microbial cell viability assay. Technical bulletin no. TB337. Promega, Madison, WI.
- 4.Bagge, N., M. Schuster, M. Hentzer, O. Ciofu, M. Givskov, E. P. Greenberg, and N. Hoiby. 2004. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and beta-lactamase and alginate production. Antimicrob. Agents Chemother. 48:1175-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner, M., U. Hollenstein, S. Delacher, D. Jager, R. Schmid, E. Lackner, A. Georgopoulos, H. G. Eichler, and M. Muller. 1999. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob. Agents Chemother. 43:1307-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claridge, J. E., III, S. Attorri, D. M. Musher, J. Hebert, and S. Dunbar. 2001. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin. Infect. Dis. 32:1511-1515. [DOI] [PubMed] [Google Scholar]
- 7.De Keersmaecker, S. C., K. Sonck, and J. Vanderleyden. 2006. Let LuxS speak up in AI-2 signaling. Trends Microbiol. 14:114-119. [DOI] [PubMed] [Google Scholar]
- 8.Di Bonaventura, G., I. Spedicato, D. D'Antonio, I. Robuffo, and R. Piccolomini. 2004. Biofilm formation by Stenotrophomonas maltophilia: modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob. Agents Chemother. 48:151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eboigbodin, K. E., J. R. Newton, A. F. Routh, and C. A. Biggs. 2006. Bacterial quorum sensing and cell surface electrokinetic properties. Appl. Microbiol. Biotechnol. 73:669-675. [DOI] [PubMed] [Google Scholar]
- 10.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman, L. R., D. A. D'Argenio, M. J. MacCoss, Z. Zhang, R. A. Jones, and S. I. Miller. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171-1175. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson, K. K. 2004. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 236:163-173. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, K. 2007. Persister cells, dormancy and infectious disease. Nature 5:48-56. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., C. Attila, L. Wang, T. K. Wood, J. J. Valdes, and W. E. Bentley. 2007. Quorum sensing in Escherichia coli is signaled by AI-2/LsrR: effects on small RNA and biofilm architecture. J. Bacteriol. 189:6011-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McColm, A. A., and D. M. Ryan. 1985. Penetration of beta-lactam antibiotics into cardiac vegetations, aorta and heart muscle in experimental Staphylococcus aureus endocarditis: comparison of ceftazidime, cefuroxime and methicillin. J. Antimicrob. Chemother. 16:349-358. [DOI] [PubMed] [Google Scholar]
- 16.Petersen, F. C., N. A. Ahmed, A. Naemi, and A. A. Scheie. 2006. LuxS-mediated signalling in Streptococcus anginosus and its role in biofilm formation. Antonie van Leeuwenhoek 90:109-121. [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers, P. D., T. T. Liu, K. S. Barker, G. M. Hilliard, B. K. English, J. Thornton, E. Swiatlo, and L. S. McDaniel. 2007. Gene expression profiling of the response of Streptococcus pneumoniae to penicillin. J. Antimicrob. Chemother. 59:616-626. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz, F. J., R. Sadurski, A. Stattfeld, A. Kray, J. Verhoef, and A. C. Fluit. 1999. Cross-resistance analyses and molecular typing of Staphylococcus aureus and Streptococcus spp. isolates resistant to quinupristin/dalfopristin. J. Antimicrob. Chemother. 44:847-849. [DOI] [PubMed] [Google Scholar]
- 21.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 183:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streit, J. M., J. N. Steenbergen, G. M. Thorne, J. Alder, and R. N. Jones. 2005. Daptomycin tested against 915 bloodstream isolates of viridans group streptococci (eight species) and Streptococcus bovis. J. Antimicrob. Chemother. 55:574-578. [DOI] [PubMed] [Google Scholar]
- 23.Sztajer, H., A. Lemme, R. Vilchez, S. Schulz, R. Geffers, C. Y. Yip, C. M. Levesque, D. G. Cvitkovitch, and I. Wagner-Dobler. 2008. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J. Bacteriol. 190:401-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120:105-110. [DOI] [PubMed] [Google Scholar]
- 25.Tracy, M., A. Wanahita, Y. Shuhatovich, E. A. Goldsmith, J. E. Clarridge III, and D. M. Musher. 2001. Antibiotic susceptibilities of genetically characterized Streptococcus milleri group strains. Antimicrob. Agents Chemother. 45:1511-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vendeville, A., K. Winzer, K. Heurlier, C. M. Tang, and K. R. Hardie. 2005. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 3:383-396. [DOI] [PubMed] [Google Scholar]
- 27.Walsh, C. 2000. Molecular mechanisms that confer antibacterial drug resistance. Nature 406:775-781. [DOI] [PubMed] [Google Scholar]
- 28.Whiley, R. A., D. Beighton, T. G. Winstanley, H. Y. Fraser, and J. M. Hardie. 1992. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J. Clin. Microbiol. 30:243-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, L., H. Li, C. Vuong, V. Vadyvaloo, J. Wang, Y. Yao, M. Otto, and Q. Gao. 2006. Role of the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. Infect. Immun. 74:488-496. [DOI] [PMC free article] [PubMed] [Google Scholar]